Abstract
Background
Gastric duplication cysts are a very rare variant of all enteric duplications, and their isolated forms are much rarer developmental anomalies in the form of case reports only. In this study, a 4-month-old male patient, who was diagnosed with an intra-abdominal cystic mass in his antenatal examination and laparoscopic excision of the isolated gastric duplication was presented.
Case presentation
A 4-month-old male patient was born at 39 weeks of gestation, 3180 g. When a 37 × 17mm intra-abdominal cystic mass was detected in the detailed ultrasonography (USG) performed at the 20th week of his antenatal examination, he was followed up in another center with the preliminary diagnosis of mesenteric cyst and intestinal duplication cyst. It was learned that the patient had transient constipation and vomiting in his history. Physical examination revealed a mobile, smooth-surfaced 5-cm mass on palpation in the lower midline of the abdomen. Control ultrasonography revealed a lobulated contoured cystic lesion measuring 59 × 30 × 23 mm, with a multilayered wall structure and debris inside. In laparoscopy, the mass is mobile, thick-walled, cystic in appearance, isolated from surrounding tissues. It was observed that it was attached to the sigmoid colon mesentery with a handle. The thick peduncle containing the feeding vessels was closed and cut, preserving the mesentery. Thick mucoid cyst contents were aspirated with a percutaneous needle. The shrinking cyst was removed from the abdomen by enlarging the working opening of 5 mm. The macroscopic appearance was consistent with intestinal duplication cyst. The patient was fed orally at the 2nd hour postoperatively and was discharged at the 10th hour. In the pathological examination, gastric duplication cyst was diagnosed due to the type of gastric lining epithelium. No recurrence or additional pathology was detected in the control ultrasonography in the 6-month follow-up postoperatively.
Conclusion
Laparoscopic exploration should be considered as the first surgical option in asymptomatic, growing intra-abdominal cystic masses with a preliminary diagnosis of duplication cysts. The advantages of laparoscopy can make important contributions to patient management.
Similar content being viewed by others
Background
Gastrointestinal duplication cysts are rare congenital anomalies that can be located from the mouth to the anus and appear during embryological development. Recently, it is detected from early intrauterine periods by radiologic investigation and usually give clinical symptoms mostly in the first days of life [1]. Gastric duplication cysts are rare duplications. Isolated gastric duplication cysts, on the other hand, are a much rarer form reported in the literature as case reports.
Case presentation
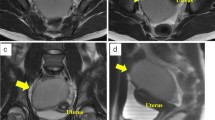
A 4-month-old male patient was born at 39 weeks of gestation, 3180 g. He was followed up in another center with the prediagnosis of mesenteric cyst and intestinal duplication cyst due to a 37 × 17mm intra-abdominal cystic mass detected in his detailed ultrasonography (USG) at the 20th week of antenatal examination. In this detailed examination, there was no need for further research since there was no additional anomaly or suspicion in the fetus. He admitted to our clinic because of an increase in cyst size on USGs performed 1 month apart after birth. In the patient’s history, it was learned that he had temporary constipation and vomiting. When he admitted to us, he had stool 3–4 times a day, and he did not have vomiting or feeding problems. The weight percentile for age was 50–75%. On physical examination, a mobile, smooth-surfaced 5 cm mass was detected on palpation in the lower midline of the abdomen. Control ultrasonography revealed a lobulated contoured cystic lesion in the right lateral part of the bladder, starting from the pelvic region and extending to the level of the umbilicus, 59 × 30 × 23 mm in size, with a stratified wall structure and debris inside (Fig. 1). Due to its stratified wall structure and location, intestinal duplication cyst was considered first and the operation decision was made without the need for additional imaging examination. During the operation, a 5-mm camera port was placed in the umbilicus, a 5-mm working port in the right lower quadrant, and a 3-mm working port in the left lower quadrant. The mass was mobile, thick-walled, cystic in appearance, isolated from surrounding tissues; It was seen that it was connected to the sigmoid colon mesentery by a peduncle (Fig. 2a). The root of the cyst was reached by following the peduncle, preserving the mesentery; the thick peduncle containing the feeding vessels was sealed and cut. The contents of the thick mucoid cyst were aspirated with the percutaneous needle (Fig. 2b). The cyst, which shrank when its contents were emptied, was taken out of the abdomen by expanding the 5 mm working port. The macroscopic appearance was compatible with intestinal duplication cyst. The patient was fed orally in the 2nd postoperative hour and was discharged at the 10th hour. The mass was diagnosed as gastric duplication cyst due to the gastric type of lining epithelium as a result of histological and immunohistochemical examination. No recurrence or additional pathology was detected in the control ultrasonography in the 6-month postoperative follow-up.
Discussion
Gastrointestinal duplication cysts are rare congenital anomalies that can be found anywhere along the gastrointestinal tract; it is most common in the ileum (33%), followed by the esophagus (20%), colon (13%), jejunum (10%), stomach (7%), and duodenum (5%). It occurs in 1:4500 live births and is found in 0.2% of all children [1,2,3,4]. While 80–90% of gastrointestinal duplication cysts are in cystic form, a few of them are tubular [5]. They usually have an epithelial lining that is tightly attached to at least one point of the digestive tract, has a common blood supply with the adjacent part of the intestine, and resembles a portion of the digestive tract [5]. Duplications are usually in the form of a single lesion. They rarely communicate with the adjacent intestinal lumen, generally they are noncommunicating. Multiple duplications, including isolated forms, have also been reported [2].
Interestingly, the anatomical location does not always correlate with the type of epithelial lining [3].
Approximately one third of patients with enteric duplications have been reported to have associated malformations such as spinal defects, pulmonary sequestration, congenital cystic adenomatoid malformation, and cardiac malformations, and therefore detailed examination of other systems is recommended [1].
Isolated duplications have also been described like in our case rarely. In this form, the duplication is suspended on a vascular stalk and does not exhibit luminal communication with adjacent alimentary segments [3].
Various radiological methods such as plain radiographs, US, CT, and MRI, can be used in the diagnosis of enteric duplication cysts. There are 2 sonographic signs suggesting intestinal duplication in ultrasound examinations. These are the “double wall” sign and the presence of peristalsis. The “double wall” sign consists of an inner hyperechoic rim associated with the mucosa-submucosa and an outer surrounding hypoechoic layer reflecting the muscularis propria [1, 4, 6, 7]. In our case, since the preliminary diagnosis was probably interpreted in favor of a duplication cyst with only US, no additional examination was requested and possible exposure to radiation was prevented.
Due to their settlements and their typical or atypical features, many theories have been put forward, and a single theory does not cover all formations. Some of these theories are as follows: split notochord theory [8], vascular accident theory [3], abnormal recanalization of the lumen, and abortive twinning theories [8].
The split notochord theory suggests that abnormal separation of the notochord from the endoderm causes enteric duplications [9].
Abnormal recanalization of the lumen and abortive twinning theory covers the mechanisms of duplication cyst in the mouth, upper GIS, hindgut, and lower genitourinary system [8].
The vascular accident theory was first proposed by Steiner and Mogilner and explains the duplication cysts whose nutrition is from another vascular structure. In theory, it suggests that in case of vascular accident during the formation of the intestinal segment in the embryological period, a duplication cyst with separate vascular nutrition may occur [3]. Although our case is more in line with this theory, the biggest gap in the theory is that the epithelial characteristics of the adjacent intestinal segment and the cyst are different.
Intrauterine ischemic damage to the gut appears to be an attractive potential explanation for isolated duplications. Some authors hypothesize that the pathological events in these cases may have progressed through torsion or a vascular accident at the proximal end of the diverticulum. This event may have separated it from the intestinal wall and a separate duplication cyst may have emerged [3].
Duplications are divided into types 1 and 2 according to their vascular support, as defined by Li et al. [6], and while additional anomalies are seen less frequently in type 1 cases, it is possible to remove them without resecting the adjacent segment. It is possible to define our case and similar ones as type 1.
In general, it has been reported that the cyst tends to grow gradually after intrauterine detection. It has also been stated that for isolated cysts, the contents can be absorbed, and the cyst shrinks due to the lack of connection with the adjacent intestine [3].
Gastrointestinal duplication cysts are present symptomatically or asymptomatically throughout life due to size, location, and epithelialization of the cyst. Symptoms of abdominal duplication cysts mainly depend to the site that is located and are usually vomiting, abdominal distension, palpable mass, ileus, volvulus, and acute abdominal findings due to perforation or ulceration [1, 10, 11]. The most serious complications such as ulceration, bleeding, and perforation are seen in cysts lined with the gastric mucosa. Malignant changes are also reported in the mucosa of the duplication regardless of the anatomical location or type of mucosa in adult ages [1, 12, 13]. For all these reasons, when an enteric duplication cyst is detected, it should be resected electively [1, 3, 12].
In general, congenital cysts larger than 5 cm, those with complex content and septation, and those with persistent cysts and symptoms should be excised to avoid catastrophic complications [12].
Gastric duplication cysts are most commonly located near the greater stomach curvature and pancreas, while isolated gastric duplication cysts are rare cases in the literature [14]. Isolated cases of retroperitoneal, thoracic, and colonic mesentery have been reported [2, 15, 16]. Isolated duplications are usually single cysts, but the coexistence with a second classic enteric duplication cyst also has been reported [2, 8]. Retroperitoneal enteric duplications with a polycystic imaging appearance has been previously reported [17].
According to the authors’ testimony, the first isolated enteric duplication cyst was published in 1999 by Steiner et al. [3]. The cyst epithelium of this patient contained gastric mucosa and other features of the cyst were similar to our case.
According to Tatsuya’s report in 2008, the isolated enteric duplication cases published up to that date were 8 together with their own cases, and most of them, except one, contained gastric mucosa. In addition, one of these cases was reported in adulthood [16].
It has been reported that some of the isolated duplication cysts are retroperitoneal. According to the reports of Pachl et al. in 2011, 6 isolated retroperitoneal duplication cysts have been reported up to that date. Three of them were in the adult age group, and 3 of them were in the pediatric age group, and they were detected as cystic lesion in their antenatal examinations [12].
In their 2013 report, Souzaki et al. reviewed 14 isolated retroperitoneal enteric duplication cases, including adults. Six, including his own cases, were detected in childhood. Gastric mucosa was detected in 4 of these pediatric 6 cases and classified as retroperitoneal gastric duplication [17].
Except that the mucosa it contains consists of enteric structures, its relations with the intestinal system have not been determined. With these features, their classification in the same category as intraperitoneal cysts or other enteric duplications is controversial [12]. Possible adrenal and renal mass and cysts should be considered in the differential diagnosis of retroperitoneal duplications [15].
Since the first child case was described in 1992, a total of 16 isolated gastric duplication cysts, (First)intraperitoneal [3] and (first)retroperitoneal [18], have been reported in childhood in the literature as a result of our review [2, 3, 7,8,9, 12, 14,15,16,17,18,19,20,21,22] (Table 1). Retroperitoneal localization was seen in only 7 of these 16 cases. Five of these 7 cases were located in the left retroperitoneal area.
Of all the cases, only three retroperitoneal cysts were excised laparoscopically [14, 17, 22]. The case we presented in our article is the first case in which an intraperitoneal isolated gastric duplication cyst was excised laparoscopically. In fact, laparoscopic excision or approach by any minimally invasive technique has been reported in cases with intestinal duplication cysts other than isolated gastric duplication cysts [1, 23].
The occurrence of a retroperitoneal duplication with an integrated wall is extremely rare. On reviewing the literature from 1953 to 2008, only 14 such cases have been reported and most of them were female [1, 11, 18, 19]. Nearly all of them did not share a common muscular wall with the native bowel. Only 1 of the 14 cases had a communication with the adjacent colon [3]. The most common histologic type of duplication cyst is the small intestine. Most were spherical or nearly spherical in shape, and only 2 had a dumbbell configuration [12, 19]. Unlike previously reported retroperitoneal duplications, the lining of the duplication cyst in the present case was gastric mucosa. Retroperitoneal duplications are commonly located behind the right mesocolon and in the left suprarenal region [12, 14, 15, 17, 18, 22] and may be confused with pancreatic cysts or adrenal masses on imaging studies [22]. More than 75% of enteric duplications are usually found within the first 2 years of life [5], but two thirds of reported retroperitoneal duplications were diagnosed in adulthood [14, 16]. Except for 4 cases of prenatal or incidental detection [9, 16, 20, 22], most patients presented with vague abdominal complaints when complications such as obstruction [10], inflammation [2], bleeding [10], pancreatitis [9], and neoplasia [13] developed. At the time of presentation, the duplications were usually large, with the diameter ranging from 5 to 31 cm [2, 3, 15, 19, 22].
Conclusion
Duplication cysts have an important place in the differential diagnosis of prenatal cystic mass. In cases with special ultrasonographic findings of duplication cysts, additional examination may not be required. The ideal treatment for gastrointestinal duplications is complete resection, usually with resection of the adjacent intestines and mesentery. However, it can be performed safely without the need for bowel resection in completely isolated duplication cysts. Awareness of this form of duplication cysts will help surgeons choose the optimal surgical procedure. Laparoscopic exploration should be considered as the first surgical option in asymptomatic, growing intra-abdominal cystic masses with a preliminary diagnosis of duplication cysts. The advantages of laparoscopy can make important contributions to patient management.
Availability of data and materials
There is no conflict of interest in this article. Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Abbreviations
- MRI:
-
Magnetic resonance imaging
- CT:
-
Computed tomography
- US or USG:
-
Ultrasonography
- GIS:
-
Gastrointestinal system
- ND:
-
Not defined
References
Laje P, Flake AW, Adzick NS. Prenatal diagnosis and postnatal resection of intraabdominal enteric duplications. J Pediatr Surg. 2010;45:1554–8 W.B. Saunders.
Mirza B, Ahmad S, Wasti AR, Mirza MA, Talat N, Saleem M. Our experience with unusual gastrointestinal tract duplications in infants. Afr J Paediatr Surg. 2014;11(4):326–9.
Steiner Z, Mogilner J. A rare case of completely isolated duplication cyst of the alimentary tract. J Pediatr Surg. 1999;34(8):1284–6.
Kangarloo H, Sample WF, Hansen G, Robinson JS, Sarti D. Ultrasonic evaluation of abdominal gastrointestinal tract duplication in children. Radiology. 1979;131(1):191–4.
Li L, Jin-Zhe Z, Yan-Xia W. Vascular classification for small intestinal duplications: experience with 80 cases. J Pediatr Surg. 1998;33(8):1243–5.
Segal SR, Sherman NH, Rosenberg HK, Kirby CL, Caro PA, Bellah RD, et al. Ultrasonographic features of gastrointestinal duplications. J Ultrasound Med. 1994;13(11):863–70.
Udiya AK, Shetty GS, Chauhan U, Singhal S, Prabhu SM. Multiple isolated enteric duplication cysts in an infant - a diagnostic dilemma. J Clin Diagn Res. 2016;10(1):TD15–6.
Pant N, Grover JK, Madan NK, Chadha R, Agarwal K, Choudhury SR. Completely isolated enteric duplication cyst associated with a classic enterogenous duplication cyst. J Indian Assoc Pediatr Surg. 2012;17(2):68–70 [cited 2021 Jan 9]. Available from: /pmc/articles/PMC3326825/?report=abstract.
Nakazawa N, Okazaki T, Miyano T. Prenatal detection of isolated gastric duplication cyst. Ped Surgery Int. 2005;21:831–4.
Jancelewicz T, Simko J, Lee H. Obstructing ileal duplication cyst infected with salmonella in a 2-year-old boy: a case report and review of the literature. J Pediatr Surg. 2007;42(5):19–21.
Norris RW, Brereton RJ, Wright VM, Cudmore RE. A new surgical approach to duplications of the intestine. J Pediatr Surg. 1986;21(2):167–70.
Pachl M, Patel K, Bowen C, Parikh D. Retroperitoneal gastric duplication cyst: a case report and literature review. Pediatr Surg Int. 2012;28:103–5.
Nakashima S, Yamada T, Sato G, Sakai T, Chinen Y, Itakura H, et al. A case of completely isolated advanced enteric duplication cyst cancer performed partial pancreatectomy. Int J Surg Case Rep. 2019;54:83–6.
Chen PH, Lee JY, Yang SF, Wang JY, Lin JY, Chang YT. A retroperitoneal gastric duplication cyst mimicking a simple exophytic renal cyst in an adolescent. J Pediatr Surg. 2010;45(10):e5–8.
Castillo-Fernández AL, Vázquez-Rueda F, Cañete MD, Caballero-Villarraso J. Retroperitoneal gastric duplication mimicking a prenatal adrenal cyst. Congenit Anom (Kyoto). 2017;58(4) [cited 2021 May 12]. Available from: https://pubmed.ncbi.nlm.nih.gov/28815737/.
Okamoto T, Shigeru AE, Ae T, Yokoi A, Shiiki AE, Ae S, et al. Completely isolated alimentary tract duplication in a neonate; 2008.
Souzaki R, Ieiri S, Kinoshita Y, Nishie A, Koga Y, Kuda M, et al. Laparoscopic resection of an isolated retroperitoneal enteric duplication in an infant. J Pediatr Surg Case Rep. 2013;1(7):167–70.
Duncan BW, Scott Adzick N, Eraklis A. Retroperitoneal alimentary tract duplications detected in utero. J Pediatr Surg. 1992;27(9):1231–3.
May DA, Spottswood SE, Ridick-Young M, Nwomeh BC. Case report: prenatally detected dumbbell-shaped retroperitoneal duplication cyst. Pediatr Radiol. 2000;30(10):671–3.
Menon P, Rao KLN, Vaiphei K. Isolated enteric duplication cysts. J Pediatr Surg. 2004;39(8):5–7.
Sinha A, Ojha S, Sarin YK. Completely isolated, noncontiguous duplication cyst. Eur J Pediatr Surg. 2006;16(2):127–9.
Gupta V, Javaid U, Jaber G, Mohd D, AlMarzouqi M. Laparoscopic resection of isolated retroperitoneal gastric duplication cyst in an infant. J Coll Physicians Surg Pak. 2019;29:S141–3.
Ren HX, Duan LQ, Wu XX, Zhao BH, Jin YY. Laparoscopic resection of gastric duplication cysts in newborns: a report of five cases. BMC Surg. 2017;17(1):1–4.
Acknowledgements
This article was presented as a poster presentation at the 38. Ulusal Çocuk Cerrahisi Kongresi, Antalya, Türkiye, 25 - 28 Kasım 2021.
Funding
The authors declare that this study received no financial support.
Author information
Authors and Affiliations
Contributions
ME: designed, literature searched, collected the data, wrote and critically reviewed the manuscript. AC: designed, literature searched, collected the data, wrote and critically reviewed the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics approval is not required.
Consent for publication
Consent was obtained from the patient’s relatives.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Erman, M., Celik, A. Laparoscopic excision of a very rare isolated gastric enteric duplication cyst: case report and literature review. Ann Pediatr Surg 18, 71 (2022). https://doi.org/10.1186/s43159-022-00210-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43159-022-00210-2