Abstract
Background
Citrus species are among the most important and widely consumed fruit trees in the world and are subjected to increasing global cultivation. Sweet orange (Citrus sinensis L. Osbeck) is one of 30 species of citrus which is cultivated in different regions of Iran. In this study, 80 trees of 13 sweet orange cultivars of Mazandaran province were studied for genetic diversity and fingerprinting by five short simple repeat (SSR) marker.
Results
The studied cultivars showed a high degree of genetic variability with an average genetic polymorphism of 98.46%. Behshahr and Jadeh Ghadim2 genotypes had the highest and lowest values in Nei genetic diversity, number of effective alleles, and Shannon index, respectively. Based on k-means clustering, the studied genotypes were divided into two main different groups. The high magnitude of genetic similarity between replicates of different cultivars indicated a potential case of homonymy or synonymy. DAPC analysis showed genetic admixture among some of the cultivars. The heatmap plot illustrated the alleles involved in cultivar differentiation. The CAPs analysis of monomorphic alleles of SSR loci indicated that these alleles differ in their sequences which add up to the genetic variability of citrus germplasm.
Conclusion
In general, SSR markers, due to their codominant nature and abundance in genome, are a good indicator for cultivar fingerprinting and hybrid prediction in orange cultivars. The present results showed the high diversity of sweet orange trees in different cultivars in the north of the country.
Similar content being viewed by others
Background
Citrus is one of the most important and abundant fruit crops in the world [20], with over the approximately 157 million tons of production in 159 countries until 2019 (http://www.fao.org/faostat/en/#data/SC).
Citrus belongs to the Citrineae subtribe of the Aurantioideae subfamily, which is one of the seven subfamilies of Rutaceae family, and it consists of two tribes (Clauseneae and Citreae), six subtribes, and 33 genera [32].
Citrus fruits are widely grown in areas with tropical, subtropical, and borderline subtropical/temperate climates [2]. The exact origin of many citrus species is not well known, but Southeast Asia is considered to be its source [22].
Citrus phylogeny and taxonomy are complicated due to the occurrence of bud mutations, sexual compatibility between citrus and related genera, wide dispersion, and long history of cultivation [18].
Climatic conditions especially in the southern and northern provinces of Iran create suitable conditions for citrus production [8]. Therefore, in 2019, Iran is ranked as the 10th largest producer of citrus in the world (http://www.fao.org/faostat/en/#data/SC). However, little is known about the genetic diversity of the Iranian citrus germplasm [6, 8, 9, 20].
Among citrus species, sweet oranges (Citrus sinensis) with 2n = 2× = 18 [20] are very popular among citrus fruits due to their many properties, such as their ability to prevent atherosclerosis, cancer, kidney stones, stomach ulcers, cholesterol levels, and high blood pressure [23].
In order to identify citrus species, various molecular techniques have been reported such as random amplified polymorphic DNA (RAPD, [13, 14, 17, 26]), restriction fragment length polymorphisms (RFLP, [6]), amplified fragment length polymorphism (AFLP, [12, 25]), sequence-related amplified polymorphism (SRAP, [3]), start codon targeted (SCoT) polymorphisms [9], inter-simple sequence repeats (ISSRs, [28,29,30]), LTR-IRAP, LTR-REMAP [5], and SSR [3, 4, 7, 8, 12, 16, 20, 30, 32].
Most of the sweet orange accessions showed a narrow genetic basis [24, 25]. It is suggested that the observed morphological polymorphism must be associated with somatic mutations, which were not detected by some molecular markers.
SSR marker is known as a reliable genetic marker for genetic variation assay and fingerprinting in sweet oranges (C. sinensis) cultivars along with several tangerines (C. reticulata Blanco, [7, 8]). On the other hand, the homoplasy of SSR microsatellites showed that SSR markers rate in DNA fragments by size. Using characterized amplified polymorphism (CAPS) markers in plant species is a suitable method to reveal sequence variations without using cost-consuming sequencing methods [15].
The present study aims are as follows: (1) genetic fingerprinting of sweet orange (C. sinensis) cultivars using SSR molecular markers and (2) evaluation of monomorph allele sequences by using CAPs-SSR method.
Methods
Plant materials
The fresh leaves of 80 trees from 13 different cultivars were collected from Mazandaran province during 2018–2019. The studied cultivars and their locations are given in Table 1.
DNA extraction and molecular marker assay
Genomic DNA was extracted based on CTAB method with some modifications [10, 19]. DNA qualification was checked by using 0.8% agarose gel electrophoresis.
In present study, five high polymorphic SSR loci were used to investigate the genetic diversity and fingerprinting of orange accessions (Table 2). These loci were selected based on a comprehensive study by Liu et al. [16] and verified by alignment at the NCBI information database.
DNA amplification was carried out in 20 μl reactions containing 12 ng of template DNA, 1.15 μM each of forward and reverse primers, and 10 μl of 1X Master Mix (ParsTous Biotech, Iran).
Thermal program consisted of 94 °C for 5 min; 38 cycles of 94 °C for 40 s, with annealing temperatures for each locus; TAA27 for 52 °C, CAC15 for 52 °C, AG14 for 58 °C, CAT01 for 53 °C, and TC26 for 55 °C for 40 s; and extension segment at 72 °C for 1 min. Final extension was at 72 °C for 5 min. PCR reaction was performed by using Techne Prime thermocycler.
The PCR products were visualized on 3% high-resolution ultrapure agarose gel (UltraPure™ Agarose, Invitrogen, Iran).
For CAPS-SSR assay, BamHI restriction enzyme was used due to the high abundance of its site in the genome. For that, monomorph SSR-PCR products (130, 140, 160, and 180 bps for TAA27; 140, 150, and 170 bps for TC26; 140, 150, 180, 200 bps for AG14; 150, 170, 200 bps for CAT01; and 160, 180, 190, and 200 bps for TC26 locus) were mixed with 0.4 μl of 10 \(U\left/\mu l\right.\) Thermo BamHI enzyme (Generay, China), 0.5 μl of the buffer, and 4.5 μl of distilled water. The reaction was incubated at 37 °C for 8–10 h. Digestion products were visualized on 3% UltraPure Agarose gel.
Data analysis
Each band was scored as present (1) or absent (0). Genetic parameters such as polymorphism percentage (P%), Nei genetic diversity (He), Shannon index (I), and AMOVA test were estimated by using GenAlEx 4.6 software. We used different clustering methods for grouping of the studied genotypes. These dendrograms were constructed by PAST software ver 3.01. Details of genetic grouping by the SSR markers were studied by constructing a heatmap plot. The genetic admixture and assignment of the studied citrus trees were determined by DAPC (discriminant analysis of principal components). These analyses were performed in R package 4.1.
Results
SSR amplification
In total, 5 SSR loci produced 84 alleles ranging from 100 to 300 bps (Fig. S1, S2). The lowest and highest number of alleles were observed in CAC15 and TAA27 loci respectively. All loci produced polymorphic bands (Fig. 1). The highest number of alleles (Na) and effective allele belonged to Behshahr cultivar (no. 9).
Band pattern of studied cultivars and genetic parameters obtained for each cultivar. Cultivar number according to Table 1. Na, no. of different alleles; Ne, no. of effective alleles = 1/(Sum pi^2); I, Shannon’s information index = −1* Sum (pi × Ln (pi)); He, expected heterozygosity = 1 — Sum pi^2
The highest and lowest observed heterozygosity (Ho) belonged to cultivars Fereydoon kenar (no. 8) and Jadeh Ghadim 2 (no. 13) with a mean of 0.45 and 0.067, respectively.
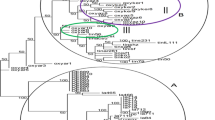
Based on Nei’s genetic distance, the highest distance was observed between Behshahr cultivars (no. 9) and local orange (no. 4). According to the Ward clustering (Fig. 2), genotypes were divided into 2 main groups. Group A includes Thomson Novel, local orange, Cara Cara orange, Jooybar, Sangrin navel and Baed az bazar, Valencia, Jadeh Ghadim 2, and Jadeh Nazami cultivars, while group B includes Baed az bazar, Jadeh Ghadim 1, Beirut, Jadeh Nazami, Jooybar and Behshahr, Beirut, and Fereydoon kenar.
WARD grouping method based on SSR data, cultivars are divided into two main groups: A and B. Number of individuals are according to Table 1
The AMOVA analysis showed that 70% of variance attributed among individuals and groups, while 30% of variance attributed within individuals. This analysis revealed a significant difference between groups (cultivars, P-value = 0.001) with Fst = 0.110. (Table S1, S2).
The heatmap plot showed cultivars genetic affinity and the SSR loci which group the cultivars alike to each other (Fig. 3). In heatmap analysis, areas with dark color indicate the high resolution of SSR alleles.
Cluster based on heatmap, analysis based on SSR data, horizontal axis representing the studied loci and vertical graph of the number of cultivars studied according to Table 1
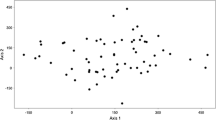
The assignment test as illustrated in DAPC plot (Fig. 4) showed close genetic affinity of the studied sweet orange trees due to genetic admixture. In general, the studied genotypes were grouped in four genetic groups. Some of the trees within these cultivars were genetically similar to the other genotypes. This may be due to either mislabeling of the cultivars by locals or due to extensive genetic admixture of the plants.
DAPC diagram of genetic structure of sweet orange cultivars based on SSR data. Number of individuals is based on Table 1
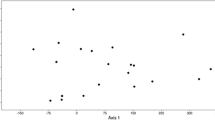
K-means cluster also showed high admixture of sweet orange trees. Although high similarity has been reported between cultivars, genetic variations were observed among trees of each cultivar (Fig. 5).
K-means cluster based on SSR alleles. Each cluster is showing with a color. Number of individuals are according to Table 1
Based on Ward dendrogram, the heatmap clustering and k-means clustering results, we considered four major genetic groups for the studied sweet orange cultivars. K-means clustering also shows some degree of overlaps if more than four groups are taken.
CAP-SSR assay
CAPS-SSR (Fig. 6) and alignment of some sequences of the CAC15 locus (Fig. 7) were also investigated for allele homoplasy. The results showed that although products had the same locus in length, they were different in sequences. These data may indicate that studied sweat orange trees which are grouped close to each other may still differ genetically in details of sequences. Therefore, these difference adds up to genetic variability present in citrus germplasm.
Discussion
The present study showed almost low degree of genetic variability among orange genotypes studied. It has been stated that morphological difference in sweat orange cultivars is due to the occurrence of the somatic mutations in the ancestral trees; hence, orange cultivars though show some degree of morphological variation but have a high level of genetic similarity [11].
The polymorphism percentage of this study was different from studies that were reported by different countries [6, 17] or even in Iran [9]. This controversial differences may come from either nature of markers used or type and number of studied cultivars. However, the PIC value in current study (PIC = 0.76) was almost the same with Golein’s study (PIC = 0.7 [7]. In general, high genetic similarity in SSR genetic fingerprinting of sweat oranges was also reported by others [21].
The earlier studies performed on the rate of polymorphism in different molecular markers, utilized in genetic fingerprinting of sweat oranges [8, 12], indicate that relatively a higher level of polymorphism is present in SSR markers. For example, Kumar Biswas et al. [12] investigated genetic diversity of thirty-four citrus genotypes from the National Citrus Breeding Center of China by different molecular markers like AFLP, SSAP, SAMPL, and SSR, and reported that SSRs show a higher polymorphism rate compared to the other markers studied. They obtained the mean polymorphism value of 98.46%. In a similar study on the sweat orange genotypes of Iran, Jannati et al. [8] used 15 SSR loci and reported the high polymorphism percentage for three loci: CAT01, TAA27, and AG14.This high level of SSR marker polymorphisms is expected to be due to amplification slip. The codominant common nature of SSR markers also allows the detection of large numbers of alleles in each location and contributes to higher levels of expected heterozygosity [12]. Genetic parameters like He, Shannon index, and Ne also supported high heterozygosity among trees studied. Therefore, it can be concluded that the SSR marker may be more useful for studies of genome segregation and mapping in citrus than other markers.
The present study revealed a high degree of genetic admixture in sweet orange trees, and individuals studied from different cultivars were genetically similar. This may be partly due to the presence of synonyms, homonyms, and mislabeling within citrus germplasm. It seems that mislabeling is almost a common phenomenon in many cultivated plant species like fig [1], grapevine [5], and grape ([1].
Analysis of SSR loci are based on size of DNA fragments. Length variation is usually the only and most obvious criteria for describing allelic diversity [15]. This may indicate that although monomeric alleles do not appear to be distinct in individuals, they have different sequences.
In order to investigate the homoplasy of microsatellite alleles, CAPs method was performed on monomorphic alleles in cultivars studied. The first report on CAPSs markers was on Arabidopsis [31]. Since then, this method has been repeatedly adapted and used in different plants with different changes to suit specific plant species. Therefore, CAPs have important applications in the analysis of genetic and phylogenetic polymorphisms, especially in closely related species [27, 31]. Therefore, it can be concluded that although a locus has the same allele and size, the sequences are different. Our finding also showed variation among monomorph SSR alleles which may helpful to differentiate genotypes and provide more polymorphism among sweet oranges with high similarities.
Conclusion
According to present study, the sweet orange genotypes were divided into two main groups. However, the genotypes were genetically very similar due to genetic admixture. In general, all SSR loci used in this study showed high levels of polymorphism (mean 98.46%), which confirmed the high genetic diversity of sweet orange trees in different genotypes in the northern part of the country. Sequencing and CAPS-SSR studies have also provided more variation among monomorph alleles of the same locus can be sequentially different. SSR and CAPs could be helpful for differentiation of sweet orange genotypes.
Availability of data and materials
Raw data are available in request.
Abbreviations
- CAP:
-
Characterized amplified polymorphism
- CTAB:
-
Cetyltrimethylammonium bromide
- DAPC:
-
Discriminant analysis of principal components
- SSR:
-
Short simple repeat
References
Achtak H, Oukabli A, Ater M, Santoni S, Kjellberg F, Khadari B (2009) Microsatellite markers as reliable tools for fig cultivar identification. J Am Soc Hort Sci 134:624–631
Ahmed S, Rattanpal HS, Kumari P, Singh J (2017) Study of genetic variability in citrus fruit crop by molecular markers-a review. Int J Pure App Biosci 5:111–128
Amar MH, Kumar Biswas M, Zhang Z, Guo WW (2011) Exploitation of SSR, SRAP and CAPS-SNP markers for genetic diversity of citrus germplasm collection. Sci Hortic 128:220–227
El Zayat AMS, Ahmed HH, Nishawy E, Ali M, Amar MH (2021) Patterns of genetic structure and evidence of Egyptian citrus rootstock based on informative SSR, LTR-IRAP and LTR-REMAP molecular markers. J Genet Eng Biotechnol 19:1–14
Fossati T, Labra M, Castiglione S, Failla O, Scienza A, Sala F (2001) The use of AFLP and SSR molecular markers to decipher homonyms and synonyms in grapevine cultivars: the case of the varietal group known as “Schiave”. Theoret Appl Genet 102:200–205
Golein B, Bigonah M, Azadvar GM (2012a) Analysis of genetic relationship between ‘Bakraee’ (Citrus sp.) and some known citrus genotypes through SSR and PCR-RFLP markers. Sci Hortic 148:147–153
Golein B, Talaie A, Zamani Z, Ebadi A, Biol Behjatnia A (2005) Assessment of genetic variability in some Iranian sweet oranges (Citrus sinensis [L.] Osbeck) and mandarins (Citrus reticulata Blanco) using SSR markers. J Int J Agric 7:169–170
Jannati MR, Fotouhi A, Abad P, Salehi Z (2009) Genetic diversity analysis of Iranian citrus varieties using micro satellite (SSR) based markers. J Hort Forest 1:120–125
Juibary Laame P, Seyedmehdi FS, Sheidai M, Noormohammadi Z, Koohdar (2021) Genetic structure analysis and genetic finger printing of sweet orange cultivars (Citrus sinensis (L.) Osbeck) by using SCoT molecular markers. Genet Resour Crop Evol 68:1645–1654
Križman M, Jakše J, Baričevič D, Javornik B, Slovenica Prošek M (2006) ‘Robust CTAB-activated charcoal protocol for plant DNA extraction. Acta Agri 87:427–433
Krueger RR, Navarro L (2007) In: Khan IA (ed) Citrus germplasm resources. CAB International 2007. Citrus Genetics, Breeding and Biotechnology. https://doi.org/10.1079/9780851990194.0045
Kumar Biswas M, Chai L, Amar MH, Zhang X, Deng XX (2011) Comparative analysis of genetic diversity in citrus germplasm collection using AFLP, SSAP, SAMPL and SSR markers. Sci Hortic 129:798–803
Lamine M, Chebaane A, Mliki A (2015a) Genetic diversity analysis in Tunisian Maltaise orange (Citrus sinensis L.). J New Sci 14: 38-448
Lamine M, Mliki A (2015b) Elucidating genetic diversity among sour orange rootstocks: a comparative study of the efficiency of RAPD and SSR markers. Appl Biochem Biotechnol 175:2996–3013
Li D, Lewis RS, Jack AM, Dewey RE, Bowen SW, Miller RD (2012) Development of CAPS and dCAPS markers for CYP82E4, CYP82E5v2 and CYP82E10 gene mutants reducing nicotine to nornicotine conversion in tobacco. Mol Breed 29:589–599
Liu S-R, Li W-Y, Long D, Hu C-G, Zhang J-Z (2013) Development and characterization of genomic and expressed SSRs in citrus by genome-wide analysis. PLoS One 8:e75149
Malik SK, Rohini MR, Kumar S, Choudhary R, Pal D, Chaudhury R (2012) Assessment of genetic diversity in sweet orange [Citrus sinensis (L.) Osbeck] cultivars of India using morphological and RAPD markers. Agric Res 1:317–324
Marlykynti H, Kumar Malik S, Rao SR (2014) Genetic diversity and phylogenetic analysis of Citrus (L) from north-east India as revealed by meiosis, and molecular analysis of internal transcribed spacer region of rDNA. Meta Gene 2:237–251
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8:4321–4326
Nematollahi AK, Golein B, Vahdati K (2013) Analysis of the genetic diversity in citrus (Citrus spp.) species using SSR markers. J Plant Physiol Breed 3:39–47
Novelli Valdenice M, Cristofani M, Souza AA, Machado MA (2006) Development and characterization of polymorphic microsatellite markers for the sweet orange (Citrus sinensis L. Osbeck). Genet Mol Biol 29:90–96
Rajput NA, Atiq M, Tariq H, Saddique WM, Hameed A (2016) Citrus gummosis: a formidable challenge to citrus industry: a review
Roussos PA (2016) Orange (Citrus sinensis (L.) Osbeck). In: Nutritional composition of fruit cultivars Academic Press: Cambridge, UK, pp. 469-496. https://doi.org/10.1016/B978-0-12-408117-8.00020-9
Saddoud Debbabi O, Bouhlal R, Abdelaali N, Mnasri S, Mars M (2013) Pomological study of sweet orange (Citrussinensis L. Osbeck) cultivars from Tunisia. Intel J Fruit Sci 13:274–284
Saddoud Debbabi O, Mezghani N, Madini M, Ben Abedelaali N, Bouhlel R, Ksia A, Mars M (2014) Genetic diversity of orange fruit (Citrus sinensis L.) cultivars in Tunisia using AFLP markers. Intl J Agro Agri Res 5:7–15
Sankar TG, Gopi V, Deepa B, Gopal K (2014) Genetic diversity analysis of sweet orange (Citrus sinensis Osbeck) varieties/clones through RAPD markers. Int J Curr Microbiol App Sci 3:75–84
Scarabel LS, Panozzo W, Savoia M, Sattin M (2014) Target-site ACCase-resistant Johnsongrass (Sorghum halepense) selected in summer dicot crops. Weed Technol 28:307–315
Shahsavar AR, Izadpanah K, Tafazoli E, Tabatabaei BS (2007) Characterization of citrus germplasm including unknown variants by inter-simple sequence repeat (ISSR) markers. Sci Hortic 112:310–314
Sharafi AA, Abkenar AA, Sharafi A, Masaeli M (2016) Genetic variation assessment of acid lime accessions collected from south of Iran using SSR and ISSR molecular markers. Physiol Mol Biol Plants 22:87–95
Sharafi AA, Sharafi A, Abkenar AA (2017) Molecular genetic diversity assessment of citrus species grown in Iran revealed by SSR, ISSR and CAPS molecular markers. J Sci Res: Rev Ciencia e Investig 2:22–27
Shavrukov YN (2016) CAPS markers in plant biology. Russian J Genet: Appl Res 6:279–287
Uzun A, Yesiloglu T (2012) Genetic diversity in citrus. In: In Tech pp. 213–230. https://doi.org/10.5772/32885
Acknowledgements
The authors gratefully acknowledge the Science and Research Branch, Islamic Azad University (IAU), and Shahid Beheshti University. Also, we thank Prof. Behrooz Golein for providing samples.
Funding
None.
Author information
Authors and Affiliations
Contributions
NSh, data collection and lab work and writing; ZN, conceptualization of the project, data analyses, and manuscript writing; MSh, analyses of data and manuscript writing; and FK, lab work and collecting samples. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
I testify on behalf of all co-authors that our article is submitted to the Journal of Genetic Engineering and Biotechnology.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1
. AMOVA table based on SSR alleles for 13 cultivars. Table S2. Pairwise groups Fst value between cultivars. Fig. S1. Allele patterns of CAC15 SSR locus. 50bps Ladder. Fig. S2. Allele patterns of TAA27 SSR locus. 50bps Ladder.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shahnazari, N., Noormohammadi, Z., Sheidai, M. et al. A new insight on genetic diversity of sweet oranges: CAPs-SSR and SSR markers. J Genet Eng Biotechnol 20, 105 (2022). https://doi.org/10.1186/s43141-022-00393-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43141-022-00393-6