Abstract
Background
L-arginine (Arg) is an amino acid that contributes to several aspects of human biochemistry. Individuals with malnutrition and certain physical conditions could benefit from arginine intake. However, as Arg is required by certain viruses, it is advised to avoid it in one's diet and supplementation during viral illnesses. New studies have emerged during the COVID-19 pandemic, and pioneering research has been reviewed.
Main body of the abstract
The purpose of this review is to determine when and why Arg depletion, supplementation, or avoidance is advisable, considering the divergent results. A narrative review was conducted by surveying scientific publications indexed in electronic databases. Studies published from 1960 up to 2024, with no language restrictions, were included. Arg comes from proteins in the human diet. The kidney is the main site of endogenous Arg synthesis and also responsible for the overall metabolism of this amino acid, participating in synthesis, degradation and reabsorption. The liver can synthesize Arg, but since this is completely recycled in the urea cycle, it contributes little or no to the Arg plasma flux. Arg present in diet is passively absorbed in the small intestine and also transformed into urea and ornithine via urea cycle in hepatocytes. It is associated with macrophage metabolism, vasomotor control, intracellular signaling, memory formation, immune response, and an important messenger of the bronchopulmonary, cardiovascular and neural systems. Thus, excessive or decreased Arg concentration could impair health condition. High Arg concentrations stimulated rapid reactivation and resumption of protein synthesis in some viruses.
Conclusion
According to research, caution should be exercised when supplementing or depleting the amino acid arginine. Individuals who are carriers of latent viruses, such as herpesviruses, and/or who have been exposed to other viruses studied, should avoid arginine supplements and the consumption of foods rich in arginine. However, as prophylaxis or antiviral therapy, control of arginine intake as well as the use of lysine supplements, its antagonist, is recommended for short periods starting after a possible viral exposure, or in face of stimuli that can remove viruses from their latent state and/or at the very beginning of the viral manifestation, in order to avoid a large viral multiplication and consequently control the infection. Long-term arginine depletion can significantly affect cellular metabolism and its use as supplemental therapy needs case-by-case evaluation.
Graphical abstract

Similar content being viewed by others
Background
L-arginine (Arg) is an amino acid that is involved in a variety of human biochemistry processes, including ammonia detoxification, hormone secretion, and immune modulation. It acts as an endogenous messenger molecule involved in various endothelium-dependent physiological effects in the cardiovascular system, as well as a precursor to nitric oxide (NO), a critical component of endothelium-derived relaxing factor. Arg is a precursor to symmetrically and asymmetrically (NG)-dimethylated guanidine derivatives, as well as L-homoarginine (L-hArg), the methylene homolog of Arg. It plays an important role in the urea cycle and it is prescribed for treating diseases such as preeclampsia, intermittent claudication, erectile dysfunction, and diabetes mellitus [1]. In addition, Arg has cardiovascular protective effects by improving endothelial function and lowering blood pressure, and is used in the treatment of angina pectoris, congestive heart failure, and coronary artery disease [2].
Controlling Arg intake as an antiviral measure has been extensively studied over the years. However, new studies have emerged during the COVID-19 pandemic, revealing groundbreaking findings. Arg is an essential amino acid for certain viruses and should, therefore, be avoided in the diet and supplementation during viral illness. Although there is evidence that amino acid balance is important in viral control, more studies are needed, particularly regarding emerging viruses such as SARS-CoV-2. Arg depletion via the antagonistic amino acid L-lysine (Lys) or recombinant arginases should be considered parsimoniously, as Arg plays a vital role in metabolic functions and should not be suppressed for long periods [3].
Several enzymes that deplete arginine showed to be safe and effective in lowering Arg levels. Clinical trials have suggested that this therapy could be used in COVID-19 patients, reinforcing arginine's viral activity in SARS-CoV-2 [4].
Given these contradictory effects, the purpose of this review is to provide information on when, how, and why Arg should be avoided, depleted, or supplemented.
Main text
Methodology
A narrative review was conducted by surveying scientific publications indexed in electronic databases such as PubMed, Web of Science, Scielo, Medline, and Google Scholar. It was included studies published from 1960 up to 2024, with no language restrictions. The article selection was based on analyzing abstracts containing descriptors such as “L-arginine,” “nitric oxide,” “virus,” “herpesvirus,” “coronavirus,” “SARS-CoV-2,” “viral infection,” “viral replication,” “antivirals,” “amino acids,” “pharmacology of amino acids,” and “arginase,” as well as their interrelationships. The search was supplemented with a manual review of references cited in the selected articles.
Discussion
Biochemistry
There are approximately 20,000 unique genes that are responsible for coding more than 100,000 unique proteins in the human body. Despite the fact that nature contains hundreds of amino acids, only about 20 amino acids are required to produce all the proteins found in the human body and most other forms of life [5]. These amino acids feature a carboxyl group (COOH) and an amino group (NH2) attached to the same carbon atom (C). The amino acids differ from one another in their side chains, known as R groups, which can be classified into five main classes based on structural, size, and electric charge properties, that affect their solubility in water. R group polarity varies from nonpolar and hydrophobic to highly polar and hydrophilic at a biological pH of about 7. The polar R group is then categorized as uncharged, negatively charged (acidic polar), or positively charged (basic polar). L-arginine (R) is a 2-amino-5-guanidino-pentanoic acid (C6H14N4O2), a polar basic amino acid with pKa above 10 in the R chair assuming a protonated (cationic) condition at neutral pH [6].
Amino acids classified as nonessential (produced in the body) are necessary for the health of adult humans, but must be supplemented in the face of various diseases and also during growth. Based on this statement, they can be classified as semi-essential or conditionally essential [7, 8].
Arginine endogenous and exogenous
Arg is derived from three sources: tissue protein breakdown as an alternative source of fuel during fasting or in uncontrolled diabetes mellitus, endogenous “de novo” production from both normal cellular protein breakdown (recycling) and dietary proteins [6, 9, 10].
Three amino acids are primarily involved in this biosynthesis process: glutamine, ornithine, and citrulline. The enzymes may exist at multiple tissues and subcellular levels, implying that endogenous arginine production can occur in all cells in some tissues [10].
Researchers identified arginine production in kidney tissue in 1981. Citrulline enters the bloodstream via enzymatic action in the mitochondrial matrix of the enterocytes in the small intestine during the metabolism of glutamine, a semi-essential amino acid produced in the body but also obtained from food. Citrulline so is converted in the kidney to arginine, which is then released into the circulation via the renal vein [7, 9, 11, 12].
The kidney is the main site of endogenous Arg synthesis and is also responsible for the overall metabolism of this amino acid, participating in synthesis, degradation, and reabsorption [8, 9, 13]. The liver, on the other hand, can synthesize significant amounts of Arg, but since this is completely recycled in the urea cycle, the liver contributes little or no to the plasma flux of Arg [14].
There is a balance between arginine synthesis and breakdown. Kidney and liver are not the only organs involved in this synthesis. Other sources have not yet been fully identified, but arginine can be produced from citrulline released during NO synthesis, in both endothelial cells and macrophages, explaining the endogenous production in tissues lacking the urea cycle [8, 9].
In mammals and other ureotelic organisms, the ammonia produced during protein catabolism is toxic. This nitrogen is deposited in the mitochondria of hepatocytes and converted to urea before being excreted. This occurs during the urea cycle, which includes hepatocytes cytosol as well as mitochondria. Another proteinogenic amino acid, ornithine, enters hepatocyte mitochondria and is degraded into citrulline, which leaves the mitochondria and enters the cytosol, where it is degraded to arginine. Arg is degraded again in the cytosol by the enzyme arginase 1 (Arg1) to ornithine and urea. The cycle is restarted by ornithine, and urea is excreted. This process also occurs in the kidney, but it involves the enzyme arginase 2 (Arg 2) [4, 6, 15].
Arg is a natural component of the human diet that is found in all proteins of animal and plant origin and is considered proteinogenic [2]. Some foods have high concentrations of arginine, such as chocolate, peanuts, cereals, cashews, and almonds [3]. The average intake of arginine from a Western-style diet is about 4–5 g/day, and its normal plasma level is 100–200 µM [6, 9, 10].
Some foods also contain significant amounts of the amino acid citrulline in its free form, such as watermelon fruit (Citrullus vulgaris), which contains approximately 1 g citrulline/kg. Citrulline and arginine have nutritional equivalence, and in the absence of exogenous arginine, citrulline can be converted to arginine via the renal pathway. As previously discussed [8, 9], it is also very likely that the extrarenal pathways that convert citrulline to arginine are important for Arg supply.
Arginine metabolism, like that of all amino acids, is primarily influenced by the amount of protein consumed. In addition, different species have different capacities for endogenous arginine synthesis, and dietary amino acid requirements vary greatly, particularly during growth. The effects of dietary arginine on endogenous synthesis, from deficiency to excess, are also highly species-dependent [16].
Pharmacokinetics and pharmacodynamic
In the human body, like all amino acids, arginine present in food or supplements is absorbed in the small intestine, specifically in the brush border region of enterocytes, through active sodium-dependent transport. In addition, arginine is passively absorbed in the paracellular region of the intestinal mucosa. Enterocytes use the absorbed arginine for their functions, while the excess is transported via hepatic portal circulation. Transport of amino acids from enterocytes to the splanchnic circulation occurs through the basement membrane region, hydrophilic channels, or the paracellular region. Six amino acid transport systems across the cell membrane have been identified, only two of which are exclusive to basic amino acids such as Arg [4, 6, 15].
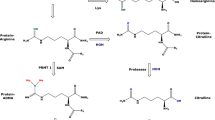
In the liver, specifically within hepatocyte's urea cycle, arginine is transformed into urea and ornithine via Arg1 [4]. In the renal tubules, arginine is metabolized into ornithine and urea by Arg2. Ornithine is then recycled back into the urea cycle, and urea is ultimately excreted in urine (Fig. 1) [17,18,19].
An extensive review of relevant literature [3] reveals that while some studies suggest that “arginase increases arginine excretion” [20,21,22,23] in humans, arginine is not excreted in the urine, except in certain pathologies [3].
In the kidneys, arginine shares a transporter with lysine, ornithine, and cystine on the apical membrane of the proximal tubular cells, to enter the cells. It uses another heteromeric transporter which can also be occupied by lysine and ornithine to leave the proximal tubular cells and go to the basolateral membrane [9].
It is important to remember that amino acids in their natural state are only excreted in the event of metabolic malfunction. Excess amino acids are broken down, leaving only the carbon chains and the amino group, which then bind to CO2 and are converted into metabolites (urea) to be excreted by the human body [19].
The information “arginase increases arginine excretion” in humans [20,21,22,23,24,25] has been erroneously transcribed.
Studies in chickens [26, 27] were poorly written and misinterpreted by some authors [21] which was then cited by other authors in new studies over the years [20, 22,23,24,25].
It is interesting to note that there are still studies where some errors of wording or interpretation are maintained, such as the studies by Jones [26] or Austic and Nesheim [28]. Chickens supplemented with the amino acid L-lysine, an arginine antagonist, showed depletion of arginine in the blood plasma due to an increase in renal arginase and, consequently, an increase in urea excretion [26]. Another study corroborates the previous study by also inferring that arginase promotes the breakdown of arginine into urea, which is then excreted [28], but this information is also incorrect and may have already been described in other articles.
The correct information on arginine metabolism is that humans (ureotelic) mainly excrete urea and chickens (uricotelic) excrete uric acid [29, 30].
Arg is excreted in urine only in the case of rare metabolic disorders, lysinuria and cystinuria, which must be controlled due to the risk of death, particularly in newborns [19, 31]. In most diseases, plasma levels of Arg are not considerably decreased, except in end-stage renal failure treated with hemodialysis. High doses of Arg, provided intravenously or orally through diet, have demonstrated to increase the NO formation. NO is produced by a group of enzymes called nitric oxide synthases (NOSs), where the substrate is arginine. In the brain, NO functions as a neurotransmitter. In the immune system, it mediates host defense, while in the cardiovascular system, it acts as a vasodilator and an endogenous antiatherogenic molecule that mediates the protective effects of intact endothelium. Long-term L-Arg administration has been shown to improve cardiovascular disease symptoms in some patients, but for others, it was not effective. In conclusion, Arg supplementation seems to have multifactorial and dose-related effects on human physiology. Administration of doses ranging between 3 and 8 g/day appears to be safe and does not induce immediate pharmacological effects in humans [32].
The pharmacology of arginine is complex and depends on several factors, including the current stage of the disease and the age of the patient. Research is needed to understand the pharmacokinetic–pharmacodynamic relationship of supplement administration, which is essential to move from preclinical to clinical trials studies [33].
Arginine: role in human physiology
Exons are DNA sequences responsible for protein synthesis, and the entire set of exons is called the exome. In the human exome, there are 64 different combinations (triads) which will form 64 different codons. Of these codons, 61 will code for the 20 different types of amino acids that exist [34]. Arginine is one of the amino acids with the highest number of triads (codons) for its synthesis, with six in the human exome (CGU, CGC, CGA, CGG, AGA, and AGG) [35].
Like the other 19 amino acids, Arg has a proteinogenic function, and even though it is produced endogenously, it must be acquired exogenously through the diet [4, 15], thus being characterized as a conditional or semi-essential amino acid. For the development of infants, children, or adults in some conditions, it is considered an essential amino acid that must be in the diet or must be supplemented [36, 37].
Arg is a substrate for several enzymatic reactions and is metabolized through three primary pathways: arginases which convert Arg into ornithine and urea, Arg decarboxylase which results in agmatine metabolites, and nitric oxide synthase (NOS) which uses Arg to produce NO and citrulline (Fig. 2) [38, 39].
Arginine is also at the center of the different functions associated with M1 and M2 macrophages, being called “a small amino acid for macrophage metabolism, a giant controller for mammalian pathophysiology [8]. Macrophages are highly versatile cells and important for tissue homeostasis in their “default or recovery and healing mode,” phagocytizing cellular debris and in their “kill/fight mode,” controlling infections [40].
Given this enormous complexity, macrophages have been classified according to their two main functions, “kill/fight or heal/repair.” Within this classification, macrophage biology is guided by two phenotypes (M1—kill/fight versus M2—heal/repair). Dominant M1 or M2 macrophages then direct T lymphocytes to produce Th1 or Th2 responses, respectively, to further amplify M1 or M2-type responses. It is interesting to note that M1 and M2 macrophages also metabolize the amino acid arginine through two different pathways: M1—via NOS into NO and citrulline and M2—via arginase into ornithine and urea (Fig. 2) [41].
Both NOS and arginase are enzymes that catalyze a multienzyme reaction that will produce other physiologically important products dependent on the concentration of arginine [8].
Among the various physiological processes dependent on arginine include vasomotor control, intracellular signaling, memory formation, immune response [11], messenger of the bronchopulmonary, cardiovascular, neural and renal systems [42], homeostatic processes, regeneration, and its metabolic pathways. All arginine-dependent processes are potential therapeutic targets for supplementation or depletion [43], opening up new avenues for some diseases’ treatment.
Arginine: a potential role in some diseases’ treatment and physical performance
Supplemental arginine has demonstrated potential as a safe therapeutic choice for enhancing endogenous NO regulation. For supplemental use, it is available as an intravenous or oral supplement. Regarding doses, they vary widely depending on the disease and protocol for each patient, ranging from 500 mg/day to 500 mg per kg/day, according to some scientific studies. Citrulline, an arginine precursor, is available in oral forms, and an intravenous preparation is being studied in clinical trials in children undergoing cardiopulmonary bypass surgery [33].
Arginine in cancers
Arg is essential for the survival of all cell types and serves as an opportunistic fuel in some cancers that are auxotrophic for certain amino acids. Its restriction has been used as a therapeutic strategy in these cases [4, 44]. Its depletion by enzymes is a rational strategy in the fight against arginine-dependent cancers. Future clinical practice should include studies of arginine deprivation therapy in cancer patients [45].
On the other hand, it was discovered that amino acids act as “immunonutrients,” modulating cellular homeostasis processes and interrupting the progression of cancer. Thus, amino acid deficiencies in cancer patients can impair immune functions, increasing mortality and morbidity rates. Arg may be an immunonutrient acting as a nodal regulator of immune responses linked to carcinogenesis processes through its versatile signaling molecule, NO. The amount of NO generated directly influences the cytotoxic and cytostatic processes of cell cycle arrest, apoptosis, and senescence [46].
However, as with anticancer therapies, arginine is sometimes depleted [44, 45] and must sometimes be supplemented [46].
As previously discussed, arginine is one of the most common residues involved in the formation of protein/DNA and protein/RNA complexes with the highest number of triads, six in the human exome [35].
Methylation of arginine in proteins is a common posttranslational modification that regulates a variety of cellular processes, including gene transcription, mRNA splicing, DNA repair, protein cellular localization, cell fate determination, and cell signaling [47]. Like other histone modifications, this process may contribute to the pathogenesis of several diseases, including cancer, inflammatory disorders, and neurodegenerative diseases [48].
Posttranslational modification of arginine residues is a key mechanism for epigenetic control of gene expression, which plays a significant role in disease pathophysiology [49].
Significant progress has been made in recent years on the physiological role of individual arginine modifications and its effects on chromatin function [50]. Epigenetic regulation by arginine modification is an emerging feature of eukaryotic organisms, and manipulation of the underlying enzymes is promising for intervention in diseases ranging from cancer to rheumatoid arthritis [51].
Arginine exerts a global influence on the expression of metabolic genes through histone acetylation. In studies of prostate cancer cells, arginine was observed to act as an epigenetic regulator, modulating histone acetylation resulting in global regulation of oxidative phosphorylation genes encoded in the nucleus [52].
Modification of arginine residues can occur in a variety of ways, leading to a form of antagonism since different types of modifications on arginine are mutually exclusive. Furthermore, the introduction of modifications on arginine can create or compromise a substrate recognition site for other histone-modifying enzymes, even spanning different histone tails. The molecular details of this communication between modifications are the subject of intense research [51].
The effect of arginine on tumor appears to be bidirectional. This contradiction sometimes promoting, other times controlling diseases such as cancer, is also found in relation to COVID-19 which will be discussed in another subchapter.
Arginine in arterial hypertension
The restricted amount of NO is critical to the development and maintenance of arterial hypertension, endothelial dysfunction, and organ damage. Endothelial NO synthesis is dependent on the concentration of extracellular Arg, which acts as a substrate for endothelial NO synthesis. Supplementing with Arg can improve the overall transportation of Arg within cells and increase the bioavailability of endothelial NO, resulting in reduced blood pressure [53].
As NO is a potent vasodilator, arginine-deficient individuals with hypertension may be advised to take an Arg supplement in safe amounts, since it has protective effects on the cardiovascular system, reducing blood pressure [2]. In patients with known endothelial dysfunction, Arg supplementation (6–8 g/day) has been shown to improve endothelial function and ultimately decrease arterial blood pressure [54].
Arginine deficiency syndrome (ADS)
A syndrome is defined as a “set of signs or symptoms that characterize a specific disease or condition” and pathological arginine deficiency can be referred to as ADS. The signs or symptoms related to ADS are arginine plasma depletion, consequently lower NO production and abnormal T-cell function associated with a pathological increase in Arg1 [55].
Several diseases appear to be associated with ADS, including renal cell and prostate cancer, tuberculosis, and infections following surgery or trauma [55, 56]. There are a growing number of diseases in which the pathological release of intracellular arginase from erythrocytes and hepatocytes can deplete arginine [57, 58]. In these cases, arginase, which is constitutively expressed, is released through hepatocyte necrosis [57] or erythrocyte destruction, observed during hemolysis [58].
Blocking Arg1 expression with COX-2 inhibitors is a therapy that can also prevent tumor growth [59]. Arg1 function can also be blocked with pharmacological inhibitors such as nor-hydroxy-L-arginine [55].
In high-risk surgical patients, dietary arginine supplementation results in a consistent and significant reduction in postoperative infections. Citrulline, a precursor of arginine, has recently emerged as an intriguing alternative because it is well tolerated even under conditions of high Arg1 activity [60]. All of these therapies aim to increase plasma arginine concentrations [55].
Arginine in hyperlysinemia/saccharopinuria syndrome
Arginine also appears to have an antagonistic effect on the amino acid Lys. In terms of biochemical properties, both amino acids are structurally very similar (Fig. 3) [3] and, as previously discussed, arginine and lysine share a transporter. Defects in this heteromeric transporter cause cystinuria and lysinuric which are dangerous dysfunctions characterized by urinary loss of arginine, lysine, and ornithine [9].
Patients with lysine-related inborn metabolic errors, such as pyridoxine-dependent epilepsy (PDE) and glutaric aciduria type 1 (GA1), follow an arginine-fortified lysine-free amino acid formula. Because of the lysine antagonism, oral arginine supplementation is the most recent therapy for PDE [19, 31].
Arginine in physical performance
Spaceflight exerts an extreme and unique influence on human physiology, as astronauts are exposed to long or short periods of microgravity which causes a variety of physiological changes, including loss of skeletal muscle mass, bone resorption, oxidative stress, decreased blood flow, and a significant increase in amino acid metabolism. Arginine consumption becomes increasingly important during spaceflight not only for NO synthesis, but also for protein synthesis, cardioprotection, and increasing the synthesis of bioactive molecules. In addition, supplementation with other protein sources should not be neglected [61].
Arg intake has shown potential for therapeutic use in a variety of situations. Its use in the fitness community is widespread because its inherent properties can improve performance and muscle hypertrophy, especially when combined with other nutrients [36, 37].
Ergogenic aids such as Arg supplementation have been shown to have a positive effect on athletic performance through various physiological and metabolic mechanisms. The results are still controversial due to the physiological demands of aerobic and anaerobic exercise. Studies comparing Arg supplementation with placebo in identical aerobic and anaerobic situations have shown that Arg supplementation can improve performance in tests. It has been suggested that to improve immediate aerobic and anaerobic performance, 0.15 g/kg of body weight should be taken 60–90 min before exercise. As a chronic supplement, the dose would be 1.5–2 g/day for 4–7 weeks to improve aerobic [62].
Oral Arg supplementation also has been associated with improved physical performance due to a possible reduction in muscle fatigue caused by nitric oxide-induced vasodilation in skeletal muscles. During an eight-week resistance training program, 20 male subjects were randomly assigned in two groups: A and B. Group A used 3 g of Arg plus vitamin C, while group B used only vitamin C (control group). Group A showed significantly higher body weight and lean mass, lower body fat percentage, and higher lower limb strength (p < 0.05), while group B had no significant differences for the same period. The Arg oral administration (3 g/day) appears to enhance the effects of weight training, providing greater gains in strength and muscle mass, while additionally aiding in the reduction of body fat percentage [63]. But its use for these purposes must be monitored due to the risks of pancreatitis, which will be discussed in another subchapter.
Arginine in disturbed carbohydrate and lipid metabolism
In humans and animals, obesity is caused by a chronic imbalance between energy intake and expenditure, as well as the development of brown adipose tissue. Adult humans can benefit from Arg supplementation at a recommended dose of 50–120 mg Arg/kg body weight per day to reduce adiposity and improve insulin sensitivity. The therapeutic mechanisms involved in combating the global obesity epidemic are complex and operate at the molecular and cellular levels. These mechanisms may include stimulation of mitochondrial biogenesis and regulation of gene expression and cellular metabolic pathways and arginine supplementation has the potential to play a critical role in this effort [64] reducing obesity which may be useful in the treatment of several metabolic disorders, including type 2 diabetes mellitus (T2DM). The mechanisms underlying the regulatory effects of Arg may result from its contribution to improving insulin sensitivity and glucose metabolism, stimulating lipolysis or endocrine functions, all of which may mitigate T2DM [65].
Experimental clinical data indicate that Arg supplementation may be useful in metabolic control of the obesity, relief of the T2DM symptoms, and blood pressure regulation, but the mechanisms underlying these effects have not been sufficiently clarified. The Arg therapeutic potential, as evidenced by its effects on the metabolism of NO, carbohydrates, and lipids, may have beneficial effects on human health; however, an Arg increase in the diet has been associated with the worsening of some existing pathology and may be a potential risk factor for the development of other diseases [66].
The effectiveness of the amino acid arginine in therapeutic practice, especially in conditions where hypoxia, of different origins, may be present, was recently discussed. The genetic and epigenetic mechanisms of adaptation to hypoxia and the clinical efficacy of Arg in diseases such as those of the cardiovascular system and stress disorders associated with these diseases were also cited. The review demonstrates a favorable effect of supplementation with Arg [67]; however, it is important to discuss the negative effects of arginine supplementation in other physiological situations.
Arginine: negative role in some diseases
Argininemia
The primary function of the urea cycle is to remove nitrogen from the body caused by protein consumption and to metabolize nitrogen-containing compounds such as adenosine monophosphate. The same cycle is an endogenous source of arginine, citrulline, and ornithine synthesis. Disruption of the urea cycle due to an unexpressed or mutated enzyme results in ammonia accumulation and associated symptoms, as well as elevated levels of circulating arginine [68, 69].
Arg1 deficiency, also known as hyperargininemia or argininemia, an autosomal recessive disease of the urea cycle, is associated with accumulation of free arginine in the blood. Arg dietary control and drug therapies for nitrogen depletion are the standard of care for patients with harmful Arg1 gene mutations. Around 25% of patients may experience severe mental deficits and loss of gait. It has been shown that even with standard therapy, 75% of these patients continue to experience neurocognitive deficits. In vitro and in vivo administration of Arg1 increases mRNA Arg1 enzyme expression and subsequently increases urea, indicating a potential treatment option to be developed for patients with this dysfunction [44, 68].
Children with hyperargininemia may experience brain damage because of increased ammonia and decreased urea levels in the urine, both of which are toxic to the body, as well in the brain. Another possible therapy is Lys supplementation, which has shown promise due to its antagonistic potential with Arg and its role in the production of renal arginase. This triggers a significant reduction in plasma ammonia, and urinary orotic acid to normal levels. Furthermore, the significant increase in arginine associated with lower levels of ornithine and lysine in the brain may play an important role. Administering lisine supplements effectively maintained plasma Lys, plasma ammonia and urinary orotic acid within acceptable limits, resulting in manageable disease and satisfactory patient growth. These patients should be given dietary control in order to avoid arginine overload [70].
Oxidative stress and inflammatory processes
In aerobic organisms, free radicals (FRs) are constantly produced during the normal functioning of cells, mainly in the form of reactive oxygen species (ROS). Most FRs are removed by the cell's antioxidant defenses, which include enzymes and non-enzymatic molecules. Maintaining a balance between the production of ROS and antioxidant defenses is an essential condition for the normal functioning of the organism [71].
In addition to ROS, there are also nitrogen-containing radicals, known as reactive nitrogen species (RNS), of which NO is the main radical and is produced by NOS in biological tissues during the metabolism of arginine [72].
These radicals suffer the action of endogenous defenses, but it is important to note that there are several natural or synthetic molecules with antioxidant properties that can provide an exogenous defense system. Some natural products with antioxidant activity may be useful to support the endogenous defense system and can be used as nutraceuticals [73].
From this perspective, the antioxidants present in our diet are possible protective agents that help the body to reduce oxidative damage. Among them are phytochemicals, bioactive compounds from different parts of plants that have been associated with reducing the risk of various chronic diseases caused by oxidative stress [74].
Aggregations of active phytochemicals have been studied in the production of herbal medicines that will influence cellular metabolism for disease control. Supramolecular strategies are the focus of current research, and interestingly, one study showed that some supermolecules significantly enhanced the anti-inflammatory activity of compounds by affecting the metabolism of arginine in inflammatory cells [75].
An alternative source of NO is the nitrate–nitrite–NO pathway, and this pathway and the L-arginine–NOS pathway are interconnected. Plasma nitrate is derived from nitrate formed as a product of NO metabolism, as well as from dietary nitrate, mainly from green leafy vegetables and beets [76]. It is possible that phytochemical compounds can interfere with cellular metabolism and interfere with arginine metabolism, but more studies need to be done to confirm this hypothesis.
Arg is a substrate for both arginase and NOS. As a result, it has been proposed that Arg2 could limit the bioavailability of Arg and consequently NO, thereby aiding in the control of oxidative stress and inflammatory processes [17, 77].
Arg, as a precursor of NO, has powerful antioxidant and anti-inflammatory properties and is considered a natural antioxidant in the prevention of various diseases. According to some authors, NO has antioxidant properties, scavenging free radicals and reducing oxidative stress. Arg supplementation has shown therapeutic potential in preventing and ameliorating various health conditions such as cardiovascular disease, neurodegenerative disease, metabolic disorders, immune function, and antiaging effects, and improve endothelial function; however, more research is needed to fully elucidate the mechanisms of action and the optimal dosage for specific conditions [78].
NO plays an important role in macrophages response to inflammatory processes, but there is a fine line between the non-toxic NO concentrations for host cells and the toxicity required for its antimicrobial action. It can be beneficial or potentially toxic depending on the concentration and tissue in which it acts. Some authors refer to NO as a “double-edged sword,” and arginine, as a precursor of NO, may have both a positive effect against the inflammatory process and also a negative one, potentially damaging tissues indirectly [42].
In diseases with an inflammatory potential, arginine should be avoided or even suppressed because of its role in NO formation. A significant number of human diseases have an inflammatory component, and a key mediator of immune activation and inflammation is inducible nitric oxide synthase (iNOS), whose substrate is arginine [79]. NO inhibitors represent a significant therapeutic advance in the treatment of inflammatory diseases characterized by tissue damage [80].
Pancreatitis
In the first individual studies of acute pancreatitis, specific supplements added to enteral nutrition, such as arginine, glutamine, omega-3 polyunsaturated fatty acids, and probiotics, have been associated with a beneficial effect on this disease, but the studies are too few to make solid treatment recommendations [81]. The role of supplementing enteral nutrition with potential immunomodulatory agents, including arginine, is still questionable and more research is needed in this area, as there is little or no evidence for the effects of immunonutrition on efficacy and safety outcomes [82].
As previously discussed, arginine has been used by millions of athletes over the last 20 years to increase the production of human growth hormone, of fat burning and muscle building. However, in 2004 a report was published of acute pancreatitis (AP) in a young male taking 500 mg/day of arginine for muscle gain. The patient was hospitalized after 5 months of daily use of the arginine supplement along with 10 mg/day of zinc. He had severe abdominal pain, nausea and increased serum and urine amylase. Zinc can also cause pancreatitis depending on the dose, but in the case reported, the authors attributed the AP to the use of the arginine supplement, warning doctors and trainers to be cautious when prescribing this supplement [83].
In vivo studies showed that a single injection of arginine induced AP [84,85,86]. Analyses revealed a neutrophil infiltrate in the pancreas as well as rising levels of serum amylase and lipase, transaminases (ALT and AST) activities, and IL-6. Cytokines induce oxidative stress through the generation of oxygen free radicals, the expression of iNOS, and consequently the production of NO and superoxide. The cytokine resulted in a large increase in iNOS protein levels. Damage to the pancreas and other organs is associated with an increase in NO concentration, and these reactions are directly related to an increase in arginine [84]. The administration of Lys, an antagonistic competitor of Arg [3], reduced the levels of iNOS as well as NO, resulting in the suppression of pancreatitis. This suppression can also be attributed to a decrease in the superoxide production [84].
In the human pancreas, the role of NO is still controversial. In pancreatitis, the entire NO signaling machinery was increased, as were levels of oxidative stress markers. There is a direct link between oxidative stress and the enzymatic control of NO bioavailability at cellular level, and there are fundamental mechanisms underlying pancreatic diseases associated with Arg–NO signaling cascade disruptions [87].
Viral infection
Viruses are intracellular parasites that are unable to replicate without a host cell [88]. Studies have already confirmed that viral proteins contain more arginine [89] and that an infected cell absorbs far more arginine from the culture medium than an uninfected one [20, 90].
According to Sanchez et al. [89], arginine is essential for the replication and expression of genetic material (DNA, RNA) in a virion. Its absence can result in nucleic acid-free virions [91, 92] or a defective capsid that exposes DNA to nuclear enzymes [93].
In vitro studies have demonstrated the necessity of arginine for the replication and reactivation of herpes simplex virus (HSV). The absence of this amino acid did not reduce viral DNA synthesis, but it prevented the formation of complete viroids. However, high concentrations of the amino acid stimulated rapid viral reactivation and resumption of protein synthesis. It is important to note that these findings showcase the significance of amino acids in viral reactivation and protein synthesis [91, 93].
Human herpes viruses (HHV), with their lifelong latency characteristic, can remain in their inactive form for years, but a decline in the immune system, a trigger such as sun exposure, frostbite, menstrual periods, and immune dysregulation promoted by vaccines, particularly those immunized against COVID-19, can cause the virus to emerge from its latent form and allow lesions to reactivate [94, 95]. It has also been discussed that increased dietary or supplemental arginine availability may promote rapid reinfection. When this amino acid is not available, viral synthesis is impaired and the formation of complete viral particles is inhibited; however, when it is present or reintroduced, rapid reinfection occurs with an increase in viral plaque-forming units (PFU) [91].
Other viruses, both RNA and DNA, have also been studied by analyzing their expression in the presence and absence of arginine. Positive results have been observed in several studies with arginine depletion in cytomegalovirus (CMV) [96], H1N1 [97], vaccinia [98], adenovirus 1 and 2 [99,100,101], SV-40 [102], measles virus [103], reovirus [104], Marrek's disease (MDHV), hepatitis C (HCV) [4].
When arginine was suppressed in reoviruses (RNA) of the reoviridae family (rotaviruses), electron microscopy revealed a rupture in the capsid, resulting in defective particles exposed to the DNases of the nucleus and consequently without DNA [104], as observed by Becker and collaborators [93].
In human CMV, arginine is required at the beginning of infection, in replication, promoting an increase in viral cytopathogenicity. Removal of arginine from the culture medium 24 or 48 h after infection reduced virus production, indicating that the continued presence of the amino acid is required for virus production [96]. This fact supports studies indicating that arginine suppression, whether by diet or supplementation, should be initiated at the onset viral symptoms, during the prodromal phase, which is the phase of viral replication [95].
In the case of adenoviruses, the viral proteins produced in the absence of arginine are defective, impairing capsid formation and proper encapsulation of viral DNA [101]. It has also been suggested that arginine is important for the elongation of the viral DNA strand itself, as well as being necessary for capsid formation. Its absence during the first 15 h after infection would produce defective viruses with no viral power [99]. The suggestion of deprivation early in infection was strengthened by the conclusion that arginine must be suppressed within the first 20 h of infection to interfere with the production of all viral proteins [100].
The influenza virus (RNA) has arginine residues that are present in the viral NS1 N-terminal domain, which is necessary for binding to host cells [105]. This has been suggested by others to also be the case for the arginine-rich binding terminus. These regions have been proposed as a therapeutic target for arginine depletion and virus–host binding disruption [106,107,108].
Vaccinia virus has been shown to require arginine both for DNA production at an early stage of replication and for virus encapsidation at a late stage of replication [98]. Studies of arginine depletion in polyomavirus (simian virus 40) show that DNA and viral proteins can be synthesized but not encapsulated, similar to what has been found in adenoviruses [102]. Measles morbillivirus (RNA) does not depend exclusively on arginine, but low levels of this amino acid reduce viral progeny, that is, complete viruses [103].
Pegylated recombinant human arginase I (Peg-rhArgI) was used in an in vitro study to determine whether the selection of host arginine-associated pathways would inhibit HSV replication. Cells continuously treated with Peg-rhArgI for 48 h showed no evidence of cytotoxicity or loss of cell viability, but were able to potently inhibit HSV-1 and HSV-2 viral replication, cell-to-cell spread/transmission, production of mature and infectious virus, and virus-mediated cytopathic effects. Due to its host-directed mechanism of action, Peg-rhArgI demonstrated comparable efficacy to other drugs in controlling the replication of drug-resistant viruses and greater antiviral activity compared to acyclovir. These results demonstrate that targeting host arginine-associated pathways is an effective means of controlling viral replication processes. Arginine catabolic enzymes can modulate the deleterious sequelae associated with viral diseases by controlling viral replicative processes and represent a novel pharmacological approach [89].
Arg plasma depletion mediated by ADI-SS PEG 20, another recombinant arginase, was also applied to patients with HCV infection, and concomitant in vitro analyses were performed showing selective inhibition of HCV replication. Laboratory testing on 15 HCV patients revealed that viral titers were reduced by up to 99% in 5 of them. In the others, with HCV serotype 1b, the reduction in viral load was 50%. These patients also showed significant improvements in liver function and inflammatory potential, possibly due to the reduction in serum NO [43, 109].
The use of autologous arginase, an enzyme that metabolizes arginine, had been studied in rabbits with ocular herpes as early as 1979. An increased arginine concentration was observed in tears, which allowed the herpes hominis virus to replicate in the squamous epithelium of the infected cornea, the main source of arginine, and after abrasion, the tear arginine content was reduced, similar to the tears of healthy rabbits. A low level of arginase was also observed in the tears of rabbits with ocular herpes, suggesting that to reduce local arginine produced by herpes-infected squamous epithelium, an eye drop supplemented with arginase could be used to control the herpetic process [110].
A supporting experiment showed that macrophage arginase release also inhibits HSV-1 replication, suggesting that control of local arginine levels is an antiviral mechanism [111], as well as the potential additive or synergistic effects of combining an arginine depletion approach with nucleotide analog therapies, such as remdesivir [4].
Exploration of Lys-Arg antagonism has been used as a therapeutic strategy against viral infections (HHV-1/6/7) that depend on arginine for viral replication. Lys inhibits the effect of Arg on viruses by competing with it and also promotes arginine catabolism by increasing the production of renal arginase [112].
Another study also observed promising results in suppression the viral manifestation of herpes labialis virus type 1 when arginine was reduced, either alone by reducing the consumption of arginine-rich foods or in combination with supplementation with Lys, a competitive antagonist of arginine. The number of viral lesions was recorded monthly for 26 patients over a 24-month period. The reference period was the first 12 months. Group A, consisting of 15 patients, suppressed arginine during the first 6 months of treatment by avoiding foods high in this amino acid and taking capsules of Lys (1 g) on an empty stomach. Group B, with 11 patients, used dietary arginine suppression + placebo. In the second semester, while still avoiding arginine-rich foods, group A received a placebo and group B received 1 g of Lys. Both placebo and lysine resulted in a significant reduction in lesions. The number of lesions was lower in the 6 months where the protocol was arginine suppression + lysine, but only with dietary arginine suppression was there also a decrease in the number of lesions [113], reinforcing how important arginine can be for viral replication.
A study with Lys supplementation associated with Arg dietary control showed positive results in herpetic epithelial keratitis, controlling reactivation as well as healing time in an elderly woman, reinforcing active viral replication associated with high levels of Arg, often hindering the action of antivirals [114].
Pedrazini et al. also followed patients with recurrent cold sores for 8 years using the L-lysine 500 mg fasting + dietary arginine suppression protocol, and in the first year of treatment there was an average 49% reduction in lesion healing time and an average 63% reduction in the number of annual lesions [115, 116]. In theory, the consumption of arginine-rich foods such as peanuts, cashews, almonds, granola, and chocolate should be avoided [35, 95].
Antiviral therapies often focus on a single viral protein or a single step in a virus’s replication process, ultimately limiting the therapeutic efficacy and applicability of these drugs. Because of their viral specificity, many of them result in the development of drug-resistant strains and the inability to control harmful host-mediated inflammation. As obligate intracellular parasites, viruses are dependent on the host's metabolic and macromolecular synthesis pathways, as well as the resulting amino acids for these processes. Many viruses, including the HSV, are dependent on the Arg bioavailability. This amino acid, as previously stated, is closely linked to the performance of physiological and pathophysiological processes associated with the facilitation of viral replication or disease progression [89].
Arginine in COVID-19—SARS-CoV-2
SARS-CoV-2, like other viruses, depends on chemical reactions of nutrients and amino acids from the host to synthesize macromolecules and viral proteins. Deprivation of essential nutrients, an approach already used in oncology to treat tumors, may also interfere with viral replication. Although this metabolic starvation approach has not yet been clinically tested to viral control, preclinical studies support the concept. Arginine is a key nutrient that has been shown in vitro to be essential in the life cycle of many DNA and RNA viruses. According to the results of the studies reviewed, arginine appeared to be an important metabolite for successful viral replication of SARS-CoV-2. In addition, arginine is also a key substrate in the host inflammatory response, and reducing serum arginine levels in plasma could plausibly attenuate the severe inflammatory response of SARS-CoV-2 infection. Arginine depletion by arginase-like enzymes would reduce systemic arginine levels and is a promising therapy against COVID-19, but further studies are needed [4].
Arg is involved in various biological processes, and recently several reports suggested that it also plays a critical role in viral illnesses. The COVID-19 pandemic, caused by the SARS-CoV-2 coronavirus, has led to severe acute respiratory syndrome (SARS) and studies on the effect of arginine and its ester derivative on viral expression revealed a significant increase in expression. In the absence of more extensive studies, it has been recommended that supplementary and excess dietary arginine intake should be avoided for both the prevention and treatment of SARS-CoV-2 infections [97].
Only in SARS-CoV-2, the spike protein facilitates the virus's entry into host cells, which possess a crucial S1/S2 cleavage site necessary for cell-to-cell pulmonary infection. This mechanism differs from that of other coronaviruses. The significance of this specific site for cell-to-cell infection is arginine-dependent and yet to be confirmed in other coronaviruses [106, 108]. Considering that the S1/S2 multibasic cleavage site is arginine-dependent and crucial for SARS-CoV-2 infection in humans, it is advisable to investigate this region further as a potential therapeutic target [107].
However, recent reports have suggested that Arg may also play a positive role in COVID-19 [38] going against studies that suggested that exogenous arginine should be reduced and that arginine depletion through arginases would be beneficial for patients with SARS-CoV-2 infection [4].
For some researchers, patients hospitalized with severe or long-term COVID-19 would have reduced plasma arginine levels, which could explain the severity of the disease through several mechanisms. Given these findings, arginine supplementation would be beneficial in these patients, improving the prognosis [38, 117,118,119].
Studies have compared the bioavailability of Arg in different patient groups, including normosystemic adults and adults and children hospitalized with COVID-19. These patients exhibited low levels of arginine in their plasma, as well as a reduced ratio of Arg to ornithine due to increased arginase activity in severe cases of COVID-19 [120]. In other words, as the disease becomes more severe, including an increased frequency of thrombotic events due to the massive activation of platelets, plasma arginine levels decrease [121].
However, another explanation for the drop in plasma arginine levels in critically ill patients would be increased NO production through the iNOS pathway [79]. Arg serves as a substrate for NOS [17]. Higher levels of NO expression may lead to increased disease severity, as evidenced by some studies [4, 79].
The decline in the patients' condition also may have resulted from an increase in producing ON (Arg-iONS-ON axis), which could be the reason for the respiratory illness's severity via various mechanisms [122].
Acute respiratory distress syndrome (ARDS) with prolonged mechanical ventilation and high mortality rate in COVID-19 shares biological and clinical characteristics with immunosuppression associated with sepsis in the presence of lymphopenia. The mechanisms responsible for lymphopenia associated with COVID-19 need to be explored, as they may be responsible for the delay in virus elimination and the increased mortality rate among intensive care unit patients. Interestingly, COVID-19 severity was directly correlated with lymphopenia and increased arginase activity. In vitro studies also showed that the proliferative capacity of T cells was significantly reduced among COVID-19 patients and could be restored through arginine supplementation [118].
However, the hypothesis that the problem could arise from an exacerbated production of arginase is not explored, which consequently depletes arginine and increases ornithine, leading to a potential chain of reactions [89].
Excess NO production during viral infections can have a negative impact on the host’s immune system, leading to the suppression of the type 1 helper T cell-dependent response. The biosynthesis of NO via iNOS expression has been observed in several microbial infections, and it has proved to have an antimicrobial effect on bacteria and protozoa. Nevertheless, this effect is not transferable to pulmonary infections caused by neurotropic viruses, as it exhibits opposite effects. Prolonged activity of iNOS leads to NO overproduction, resulting in the generation of reactive nitrogen oxide species that cause tissue damage via strong oxidative and nitration reactions [122].
The expression of iNOS in cells is strictly restricted to situations in which a cell is stimulated by bacterial lipopolysaccharides or pro-inflammatory cytokines [80]. Once expressed, iNOS is responsible for producing a large amount of NO for an extended period of time [123]. It is widely acknowledged that NO has two opposite effects [42]. When present in low concentrations, NO can be protective against pathogenic intruders; however, when present in high concentrations as a result of iNOS overexpression, NO can be toxic and harmful to the human body and be responsible for a variety of illnesses [80]. The beneficial or detrimental activity of iNOS-derived NO is contingent upon its concentration [124].
NO is a crucial signaling molecule in the development of inflammation in the joints, intestines, and lungs. It exhibits an anti-inflammatory effect under normal physiological conditions, but its excessive production in abnormal situations assumes a pro-inflammatory role. Consequently, selective NO inhibitors represent a significant therapeutic advancement in treating inflammatory diseases [80].
Drugs that inhibit the synthesis or effects of NO, such as arginine analogues that compete with endogenous arginine for NOS and its transporters into cells, have been studied. Because of this competition, NO levels are reduced, as are its pro-inflammatory effects [125].
Arginine depletion, a tumor treatment strategy, may prove effective in treating COVID-19 due to its association with the inflammatory process caused by macrophage-released NO. Depletion of dietary arginine or use of arginases attenuates the severe inflammatory response of COVID-19, as well as other pathophysiological and inflammatory conditions [44]. Grimes and collaborators suggest that this approach has potential as a therapeutic strategy against the virus, including SARS-CoV-2 [4].
It is known that a virus-infected cell absorbs more arginine than other amino acids in the culture medium [90]. This phenomenon could potentially explain the decrease in plasma arginine observed in critically ill patients with COVID-19 [120, 121].
Lower levels of this amino acid may be associated with increased consumption by the multiplying virus during the early stages of infection, when viral proteins are produced in greater quantities. This is supported by the findings of Melano and collaborators [97] and by other pioneering investigation into other viruses [91].
Despite the studies presented, researchers endorse the utilization of arginine for enhancing the prognosis of viral infections, including COVID-19. They discuss the evidence of its functional contribution, its actions on endothelial cells and the immune system, as well as its therapeutic potential [38, 119, 120].
Caution should be used when interpreting these findings, as plasma arginine levels may be reduced during early infection due to high consumption by infected cells [90], thus promoting viral replication and disease severity [91].
However, arginine may be beneficial in later stages of the disease, as it affects other metabolic functions [66]. This supports the assertion that arginine depletion should only occur for a specified period due to its metabolic significance, but it ought to be avoided in the initial stages of viral infections [3].
In this context, it is uncertain whether arginine supplementation therapy is effective in severe cases of COVID-19. The Arg-iONS-ON axis is believed to contribute to the pulmonary inflammatory storm that can result in fatalities [4, 79]. These findings raise concerns as the mechanism in arginine remains unclear. Additional research is necessary to investigate the role of arginine dysregulation in COVID-19 [120].
L-lysine, an amino acid with similar biochemical properties and acting as a competitive antagonist of arginine [3], is assumed to be a viable complementary therapy for COVID-19 cases rather than arginine, but further investigation is required [4].
Toxicology
When considering amino acid supplementation, it is important to analyze the risks associated with both low (deficiency or lack of benefit) and high intake (generating toxicity). This risk–benefit approach should provide a useful method of analysis that evaluates the potential adverse effects of increasing dietary intake of individual amino acids, as well as the adverse effects of excessive supplement intake, especially for amino acids involved in neurotransmitters [126].
Administration of isolated supplemental amino acids in low doses consistent with normal dietary intake would not require a risk assessment if absorption, metabolism and excretion values are like the kinetics of protein diet amounts consumed. Although there is evidence on the negative health effects of supplementing with some amino acids, not all have been subjected to extensive toxicity testing [126].
Pharmacology established the principles of toxicokinetic, which refers to the link between the toxic dose and the site of action, and toxicodynamic, which concerns the relationship between the site of action of the toxicant and the resulting consequences in the body. However, these principles have not yet been applied to amino acid metabolism. Given the direct correlation between arginine and NO, and the subsequent impact of NO on endothelial function, neurotransmitter signaling, angiogenesis, leukocyte, platelet, and macrophage functions, additional methods for investigating relationships between amino acids and other relevant physiological outcomes should be used, such as involvement in the production of NO. The dose–response relationship of a specific amino acid, such as arginine, can result in positive and negative effects depending on its circulating concentration or intake levels. More research is needed to identify the exact levels of amino acid intake and their corresponding physiological effects, as well as to establish risk–benefit thresholds that establish safe intake ranges for the general population [127].
Arginine is generally considered safe for most individuals when consumed in moderation. However, excessive intake can potentially lead to adverse effects, including nausea, vomiting, diarrhea, and headaches. The recommended dosage may vary depending on the medical condition being treated. The use of this amino acid as a supplement entails a certain degree of risk and potential benefit. Current scientific research indicates that the recommended dosage of arginine can range from 2 to 15 g per day for a period of 2 to 12 weeks. Adherence to these intervals can enhance the therapeutic benefits of L-arginine while reducing any adverse effects [78, 128].
As previously discussed, arginine has been demonstrated to possess a number of beneficial properties, including anticancer, antioxidant, anti-inflammatory, and neuroprotective effects. Additionally, it has been shown to confer protection against a range of cardiovascular and liver diseases. However, further research is required to elucidate the underlying mechanisms of action, with a particular focus on gene expression studies and the establishment of recommended and effective doses. Furthermore, additional research is required to investigate the safety issues associated with arginine, including its potential genotoxicity, maternal toxicity, and teratogenic consequences [78] and especially in the event of a viral infection.
Future perspectives
Arginine is a crucial amino acid that plays a vital role in regulating various cellular processes. Its supplementation is necessary in several pathologies, while its depletion is important in other diseases. Understanding the entire cellular mechanism dependent on this amino acid is necessary in future studies to obtain a greater overview of the pros and cons of supplementation or deprivation of this amino acid.
Conclusion
According to research, caution should be exercised when supplementing or depleting the amino acid arginine. Individuals with some pathology, especially those caused by viruses and/or with latent viruses, such as herpesviruses, and/or who have been exposed to other studied viruses, should avoid arginine supplements and the consumption of foods rich in arginine.
However, as prophylaxis or antiviral therapy, control of arginine intake as well as the use of lysine supplements, its antagonist, is recommended for short periods starting after a possible viral exposure, or when faced with stimuli that can eliminate viruses from their latent state, and/or at the very beginning of the viral manifestation, in order to avoid a large viral multiplication and consequently control the infection. Long-term arginine depletion can significantly affect cellular metabolism and its use as supplemental therapy needs case-by-case evaluation.
Availability of data and materials
Data used in the discussion were found in peer-reviewed journals and previously published case reports. Appropriate citations and references are included in the article.
Code availability
Not applicable.
Abbreviations
- Arg:
-
L-arginine
- NO:
-
Nitric oxide
- NG:
-
Guanidine
- L-hArg:
-
L-homoarginine
- Lys:
-
L-lysine
- Arg1:
-
Arginase 1
- Arg2:
-
Arginase 2
- NOSs:
-
Nitric oxide synthases
- NOS:
-
Nitric oxide synthase
- ADS:
-
Arginine deficiency syndrome
- PDE:
-
Pyridoxine-dependent epilepsy
- GA1:
-
Glutaric aciduria type 1
- T2DM:
-
Type 2 diabetes mellitus
- FRs:
-
Free radicals
- ROS:
-
Reactive oxygen species
- RNS:
-
Reactive nitrogen species
- iNOS:
-
Inducible NO synthase
- AP:
-
Acute pancreatitis
- AST:
-
Serum aspartame transaminase
- ALT:
-
Serum alamine transaminase
- HSV:
-
Herpes simplex virus
- HHV:
-
Human herpes virus
- PFU:
-
Viral plaque-forming units
- CMV:
-
Cytomegalovirus
- MDHV:
-
Marrek’s disease
- HCV:
-
Hepatitis C virus
- Peg-rhArg1:
-
Pegylated recombinant human arginase 1
- SARS:
-
Severe acute respiratory syndrome
- ARDS:
-
Acute respiratory distress syndrome
References
Stasyuk N, Gayda G, Yepremyan H, Stepien A, Gonchar M (2017) Fluorometric enzymatic assay of l-arginine. Spectrochim Acta A Mol Biomol Spectrosc 170:184–190. https://doi.org/10.1016/j.saa.2016.07.019
Li H, Liu Q, Zou Z, Chen Q, Wang W, Baccarelli AA, Deng F, Guo X, Wu S (2021) L-arginine supplementation to mitigate cardiovascular effects of walking outside in the context of traffic-related air pollution in participants with elevated blood pressure: a randomized, double-blind, placebo-controlled trial. Environ Int 156:106631. https://doi.org/10.1016/j.envint.2021.106631
Pedrazini MC, Da Silva MH, Groppo FC (2022) L-lysine: Its antagonism with L-arginine in controlling viral infection. narrative literature review. Br J Clin Pharmacol 88:4708–4723. https://doi.org/10.1111/bcp.15444
Grimes JM, Khan S, Badeaux M, Rao RM, Rowlinson SW, Carvajal RD (2021) Arginine depletion as a therapeutic approach for patients with COVID-19. Int J Infect Dis 102:566–570. https://doi.org/10.1016/j.ijid.2020.10.100
Wu G (2009) Amino acids: metabolism, functions, and nutrition. Amino Acids 37:1–17. https://doi.org/10.1007/s00726-009-0269-0
Nelson DL, Cox MM (2017) Lehninger principles of biochemistry, 7th edn. WH Freeman and company, New York City
Morris SM (2006) Arginine: beyond protein. Am J Clin Nutr 83:508S-512S. https://doi.org/10.1093/ajcn/83.2.508S
Rath M, Müller I, Kropf P, Closs EI, Munder M (2014) Metabolism via arginase or nitric oxide synthase: two competing arginine pathways in macrophages. Front Immunol. 5:532. https://doi.org/10.3389/fimmu.2014.00532
Brosnan ME, Brosnan JT (2004) Renal arginine metabolism. J Nutr 134:2791S-2795S. https://doi.org/10.1093/jn/134.10.2791S
Wu G, Morris SM (1998) Arginine metabolism: nitric oxide and beyond. Biochem J 336:1–17. https://doi.org/10.1042/bj3360001
Newsholme P, Rebelato E, Abdulkader F, Krause M, Carpinelli A, Curi R (2012) Reactive oxygen and nitrogen species generation, antioxidant defenses, and β-cell function: a critical role for amino acids. J Endocrinol 214:11–20. https://doi.org/10.1530/JOE-12-0072
Windmueller HG, Spaeth AE (1981) Source and fate of circulating citrulline. Am J Physiol 241:E473-480. https://doi.org/10.1152/ajpendo.1981.241.6.E473
Dhanakoti SN, Brosnan JT, Herzberg GR, Brosnan ME (1990) Renal arginine synthesis: studies in vitro and in vivo. Am J Physiol-Endocrinol Metab 259:E437. https://doi.org/10.1152/ajpendo.1990.259.3.E437
Watford M (1991) The urea cycle: a two-compartment system. Essays Biochem 26:49–58
Morris SM (2016) Arginine metabolism revisited. J Nutr 146:2579S-2586S. https://doi.org/10.3945/jn.115.226621
Ball RO, Urschel KL, Pencharz PB (2007) Nutritional consequences of interspecies differences in arginine and lysine metabolism. J Nutr 137:1626S-1641S. https://doi.org/10.1093/jn/137.6.1626S
Ansermet C, Centeno G, Lagarrigue S, Nikolaeva S, Yoshihara HA, Pradervand S, Barras J, Dattner N, Rotman S, Amati F, Firsov D (2020) Renal tubular arginase-2 participates in the formation of the corticomedullary urea gradient and attenuates kidney damage in ischemia-reperfusion injury in mice. Acta Physiol 229:e13457. https://doi.org/10.1111/apha.13457
Bollenbach A, Cordts K, Hanff E, Atzler D, Choe C, Schwedhelm E, Tsikas D (2019) Evidence by GC-MS that lysine is an arginase-catalyzed metabolite of homoarginine in vitro and in vivo in humans. Anal Biochem 577:59–66. https://doi.org/10.1016/j.ab.2019.04.019
Schmidt Z, Murthy G, Ennis M, Stockler-Ipsiroglu S, Elango R (2020) Impact of enteral arginine supplementation on lysine metabolism in humans: a proof-of-concept for lysine-related inborn errors of metabolism. J Inherit Metab Dis 43:952–959. https://doi.org/10.1002/jimd.12233
Bol S, Bunnik EM (2015) Lysine supplementation is not effective for the prevention or treatment of feline herpesvirus 1 infection in cats: a systematic review. BMC Vet Res 11:284. https://doi.org/10.1186/s12917-015-0594-3
Griffith RS, DeLong DC, Nelson JD (1981) Relation of arginine-lysine antagonism to herpes simplex growth in tissue culture. Chemotherapy 27:209–213. https://doi.org/10.1159/000237979
Pedrazini MC, Groppo FC (2021) L-lysine therapy to control the clinical evolution of pityriasis rosea: clinical case report and literature review. Dermatol Ther. https://doi.org/10.1111/dth.14679
Roberts JJ, Solanki NS, Kurmis R, Lammerink S, Wong KL, Greenwood JE (2013) Prophylaxis against Herpes Simplex Virus reactivation in patients with facial burns: a potential role for L-lysine. J Burn Care Res Off Publ Am Burn Assoc 34:e368-369. https://doi.org/10.1097/BCR.0b013e3182685b59
Miller CS, Foulke CN (1984) Use of lysine in treating recurrent oral herpes simplex infections. Gen Dent 32:490–493
Gaby AR (2006) Natural remedies for Herpes simplex. Altern Med Rev J Clin Ther 11:93–101
Jones JD, Petersburg SJ, Burnett PC (1967) The mechanism of the lysine-arginine antagonism in the chick: effect of lysine on digestion, kidney arginase, and liver transamidinase. J Nutr 93:103–116. https://doi.org/10.1093/jn/93.1.103
Stutz MW, Savage JE, O’Dell BL (1971) Relation of dietary cations to arginine-lysine antagonism and free amino acid patterns in chicks. J Nutr 101:377–384. https://doi.org/10.1093/jn/101.3.377
Austic RE, Nesheim MC (1970) Role of kidney arginase in variations of the arginine requirement of chicks. J Nutr 100:855–867. https://doi.org/10.1093/jn/100.7.855
Ding X, Li M, Peng C, Wang Z, Qian S, Ma Y, Fang T, Feng S, Li Y, Wang X, Li J, Wu J (2019) Uric acid transporters BCRP and MRP4 involved in chickens uric acid excretion. BMC Vet Res 15:180. https://doi.org/10.1186/s12917-019-1886-9
Brugaletta G, Zampiga M, Laghi L, Indio V, Oliveri C, De Cesare A, Sirri F (2023) Feeding broiler chickens with arginine above recommended levels: effects on growth performance, metabolism, and intestinal microbiota. J Anim Sci Biotechnol 14:33. https://doi.org/10.1186/s40104-023-00839-y
Bouchereau J, Schiff M (2020) Inherited disorders of lysine metabolism: a review. J Nutr 150:2556S-2560S. https://doi.org/10.1093/jn/nxaa112
Böger RH (2014) The pharmacodynamics of L-arginine. Altern Ther Health Med 20:48–54
Rashid J, Kumar SS, Job KM, Liu X, Fike CD, Sherwin CMT (2020) Therapeutic potential of citrulline as an arginine supplement: a clinical pharmacology review. Pediatr Drugs 22:279–293. https://doi.org/10.1007/s40272-020-00384-5
Grantham R, Gautier C, Gouy M, Mercier R, Pavé A (1980) Codon catalog usage and the genome hypothesis. Nucleic Acids Res 8:r49–r62
Griffith RS, Norins AL, Kagan C (1978) A multicentered study of lysine therapy in herpes simplex infection. Dermatology 156:257–267. https://doi.org/10.1159/000250926
LoBue SA, Tailor P, Carlson SM, Mano F, Giovane RA, Schaefer E, LoBue TD (2019) Recurrent herpes zoster ophthalmicus in a young, healthy individual taking high doses of l-Arginine. Am J Ophthalmol Case Rep 16:100547. https://doi.org/10.1016/j.ajoc.2019.100547
LoBue SA, Goldman A, Giovane RA, Carlson SM, Bivona M, Albear S, LoBue TD (2020) Recurrent herpes zoster ophthalmicus preceded by anabolic steroids and high-dose L-arginine. Case Rep Ophthalmol Med 2020:1–4. https://doi.org/10.1155/2020/8861892
Adebayo A, Varzideh F, Wilson S, Gambardella J, Eacobacci M, Jankauskas SS, Donkor K, Kansakar U, Trimarco V, Mone P, Lombardi A, Santulli G (2021) l-Arginine and COVID-19: an update. Nutrients 13:3951. https://doi.org/10.3390/nu13113951
Gambardella J, Khondkar W, Morelli MB, Wang X, Santulli G, Trimarco V (2020) Arginine and endothelial function. Biomedicines 8:277. https://doi.org/10.3390/biomedicines8080277
Murray PJ, Wynn TA (2011) Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol 11:723–737. https://doi.org/10.1038/nri3073
Mills C (2012) M1 and M2 macrophages: oracles of health and disease. Crit Rev Immunol 32:463–488. https://doi.org/10.1615/CritRevImmunol.v32.i6.10
Flora Filho R, Zilberstein B (2000) Óxido nítrico: o simples mensageiro percorrendo a complexidade. Metabolismo, síntese e funções. Rev Assoc Médica Bras 46:265–271. https://doi.org/10.1590/S0104-42302000000300012
Starikova EA, Rubinstein AA, Mammedova JT, Isakov DV, Kudryavtsev IV (2023) Regulated arginine metabolism in immunopathogenesis of a wide range of diseases: is there a way to pass between Scylla and Charybdis? Curr Issues Mol Biol 45:3525–3551. https://doi.org/10.3390/cimb45040231
Anakha J, Kawathe PS, Datta S, Jawalekar SS, Banerjee UC, Pande AH (2022) Human arginase 1, a Jack of all trades? 3 Biotech 12:264. https://doi.org/10.1007/s13205-022-03326-9
Field GC, Pavlyk I, Szlosarek PW (2023) Bench-to-bedside studies of arginine deprivation in cancer. Mol Basel Switz 28:2150. https://doi.org/10.3390/molecules28052150
Sindhu R, Supreeth M, Prasad SK, Thanmaya M (2023) Shuttle between arginine and lysine: influence on cancer immunonutrition. Amino Acids 55:1461–1473. https://doi.org/10.1007/s00726-023-03327-9
Chen D, Ma H, Hong H, Koh SS, Huang SM, Schurter BT, Aswad DW, Stallcup MR (1999) Regulation of transcription by a protein methyltransferase. Science 284:2174–2177. https://doi.org/10.1126/science.284.5423.2174
Bannister AJ, Kouzarides T (2011) Regulation of chromatin by histone modifications. Cell Res 21:381–395. https://doi.org/10.1038/cr.2011.22
Zhang F, Rakhimbekova A, Lashley T, Madl T (2023) Brain regions show different metabolic and protein arginine methylation phenotypes in frontotemporal dementias and Alzheimer’s disease. Prog Neurobiol 221:102400. https://doi.org/10.1016/j.pneurobio.2022.102400
Fuhrmann J, Thompson PR (2016) Protein arginine methylation and citrullination in epigenetic regulation. ACS Chem Biol 11:654–668. https://doi.org/10.1021/acschembio.5b00942
Fuhrmann J, Clancy KW, Thompson PR (2015) Chemical biology of protein arginine modifications in epigenetic regulation. Chem Rev 115:5413–5461. https://doi.org/10.1021/acs.chemrev.5b00003
Chen C-L, Hsu S-C, Chung T-Y, Chu C-Y, Wang H-J, Hsiao P-W, Yeh S-D, Ann DK, Yen Y, Kung H-J (2021) Arginine is an epigenetic regulator targeting TEAD4 to modulate OXPHOS in prostate cancer cells. Nat Commun 12:2398. https://doi.org/10.1038/s41467-021-22652-9
Rajapakse NW, Mattson DL (2009) Role of L-arginine in nitric oxide production in health and hypertension. Clin Exp Pharmacol Physiol 36:249–255. https://doi.org/10.1111/j.1440-1681.2008.05123.x
Ströhle A, von Bibra H, Hahn A (2016) L-Arginine and vascular health. Med Monatsschr Pharm 39:515–520
Popovic PJ, Zeh HJ, Ochoa JB (2007) Arginine and immunity. J Nutr 137:1681S-1686S. https://doi.org/10.1093/jn/137.6.1681S
Wilmore D (2004) Enteral and parenteral arginine supplementation to improve medical outcomes in hospitalized patients. J Nutr 134:2863S-2867S. https://doi.org/10.1093/jn/134.10.2863S
Längle F, Steininger R, Waldmann E, Grünberger T, Benditte H, Mittlböck M, Soliman T, Schindl M, Windberger U, Mühlbacher F, Roth E (1997) Improvement of cardiac output and liver blood flow and reduction of pulmonary vascular resistance by intravenous infusion of L-arginine during the early reperfusion period in pig liver transplantation. Transplantation 63:1225–1233. https://doi.org/10.1097/00007890-199705150-00007
Morris CR, Kato GJ, Poljakovic M, Wang X, Blackwelder WC, Sanchdev V, Hazen SL, Vichinsky EP, Morris SM, Gladwin MT (2005) Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension and mortality in sickle cell disease. JAMA J Am Med Assoc 294:81–90. https://doi.org/10.1001/jama.294.1.81
Rodriguez PC, Hernandez CP, Quiceno D, Dubinett SM, Zabaleta J, Ochoa JB, Gilbert J, Ochoa AC (2005) Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. J Exp Med 202:931–939. https://doi.org/10.1084/jem.20050715
Palencia JYP, Saraiva A, Abreu MLT, Zangeronimo MG, Schinckel AP, Pospissil Garbossa CA (2018) Effectiveness of citrulline and N-carbamoyl glutamate as arginine precursors on reproductive performance in mammals: a systematic review. PLoS ONE 13:e0209569. https://doi.org/10.1371/journal.pone.0209569
Dickerson BL, Sowinski R, Kreider RB, Wu G (2023) Impacts of microgravity on amino acid metabolism during spaceflight. Exp Biol Med 248:380–393. https://doi.org/10.1177/15353702221139189
Viribay A, Burgos J, Fernández-Landa J, Seco-Calvo J, Mielgo-Ayuso J (2020) Effects of arginine supplementation on athletic performance based on energy metabolism: a systematic review and meta-analysis. Nutrients 12:1300. https://doi.org/10.3390/nu12051300
Angeli G, Barros TLD, Barros DFLD, Lima M (2007) Investigação dos efeitos da suplementação oral de arginina no aumento de força e massa muscular. Rev Bras Med Esporte 13:129–132. https://doi.org/10.1590/S1517-86922007000200012
McKnight JR, Satterfield MC, Jobgen WS, Smith SB, Spencer TE, Meininger CJ, McNeal CJ, Wu G (2010) Beneficial effects of l-arginine on reducing obesity: potential mechanisms and important implications for human health. Amino Acids 39:349–357. https://doi.org/10.1007/s00726-010-0598-z
Hu S, Han M, Rezaei A, Li D, Wu G, Ma X (2017) L-arginine modulates glucose and lipid metabolism in obesity and diabetes. Curr Protein Pept Sci 18:599–608. https://doi.org/10.2174/1389203717666160627074017
Szlas A, Kurek JM, Krejpcio Z (2022) The potential of L-arginine in prevention and treatment of disturbed carbohydrate and lipid metabolism—a review. Nutrients 14:961. https://doi.org/10.3390/nu14050961
Kurhaluk N (2023) The effectiveness of L-arginine in clinical conditions associated with hypoxia. Int J Mol Sci 24:8205. https://doi.org/10.3390/ijms24098205
Asrani KH, Cheng L, Cheng CJ, Subramanian RR (2018) Arginase I mRNA therapy—a novel approach to rescue arginase 1 enzyme deficiency. RNA Biol 15:914–922. https://doi.org/10.1080/15476286.2018.1475178
Helman G, Pacheco-Colón I, Gropman A (2014) The urea cycle disorders. Semin Neurol 34:341–349. https://doi.org/10.1055/s-0034-1386771
Kang S-S, Wong PWK, Melyn MA (1983) Hyperargininemia: Effect of ornithine and lysine supplementation. J Pediatr 103:763–765. https://doi.org/10.1016/S0022-3476(83)80481-8
Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39:44–84. https://doi.org/10.1016/j.biocel.2006.07.001
Ghafourifar P, Cadenas E (2005) Mitochondrial nitric oxide synthase. Trends Pharmacol Sci 26:190–195. https://doi.org/10.1016/j.tips.2005.02.005
Kanter M (1998) Free radicals, exercise and antioxidant supplementation. Proc Nutr Soc 57:9–13. https://doi.org/10.1079/pns19980004
Skerget M, Kotnik P, Hadolin M, Hras AR, Simonic M, Knez Z (2005) Phenols, proanthocyanidins, flavones and flavonols in some plant materials and their antioxidant activities. Food Chem 89:191–198. https://doi.org/10.1016/j.foodchem.2004.02.025
Yang L, Zhang X, Wang Z, Lin X, Zhang Y, Lu J, Wu L, Yao S, Jing W, Huang X, Wang P (2024) Decoction regulating phytochemicals’ micromorphology changes and anti-inflammation activity enhancements originated from herb medicine supermolecules. Chin Med 19:19. https://doi.org/10.1186/s13020-023-00864-z
Bondonno CP, Croft KD, Ward N, Considine MJ, Hodgson JM (2015) Dietary flavonoids and nitrate: effects on nitric oxide and vascular function. Nutr Rev 73:216–235. https://doi.org/10.1093/nutrit/nuu014
Caldwell RW, Rodriguez PC, Toque HA, Narayanan SP, Caldwell RB (2018) Arginase: a multifaceted enzyme important in health and disease. Physiol Rev 98:641–665. https://doi.org/10.1152/physrev.00037.2016
Oyovwi MO, Atere AD (2024) Exploring the medicinal significance of l-Arginine mediated nitric oxide in preventing health disorders. Eur J Med Chem Rep 12:100175. https://doi.org/10.1016/j.ejmcr.2024.100175
Cinelli MA, Do HT, Miley GP, Silverman RB (2020) Inducible nitric oxide synthase: Regulation, structure, and inhibition. Med Res Rev 40:158–189. https://doi.org/10.1002/med.21599
Sharma JN, Al-Omran A, Parvathy SS (2007) Role of nitric oxide in inflammatory diseases. Inflammopharmacology 15:252–259. https://doi.org/10.1007/s10787-007-0013-x
McClave SA, Chang W, Dhaliwal R, Heyland DK (2006) Nutrition support in acute pancreatitis: a systematic review of the literature. J Parenter Enter Nutr 30:143–156. https://doi.org/10.1177/0148607106030002143
Poropat G, Giljaca V, Hauser G, Štimac D (2015) Enteral nutrition formulations for acute pancreatitis. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD010605.pub2
Saka M, Tüzün A, Ateş Y, Bağci S, Karaeren N, Dağalp K (2004) Acute pancreatitis possibly due to arginine use: a case report. Turk J Gastroenterol Off J Turk Soc Gastroenterol 15:56–58
Al-Malki AL (2015) Suppression of acute pancreatitis by L-lysine in mice. BMC Complement Altern Med 15:193. https://doi.org/10.1186/s12906-015-0729-x
Siriviriyakul P, Sriko J, Somanawat K, Chayanupatkul M, Klaikeaw N, Werawatganon D (2022) Genistein attenuated oxidative stress, inflammation, and apoptosis in L-arginine induced acute pancreatitis in mice. BMC Complement Med Ther 22:208. https://doi.org/10.1186/s12906-022-03689-9
Yildiz BD, Hamaloglu E (2010) Basic experimental pancreatitis models for beginners. Surg Sci 1:31–39. https://doi.org/10.4236/ss.2010.12007
Buchwalow I, Schnekenburger J, Tiemann K, Samoilova V, Bankfalvi A, Poremba C, Schleicher C, Neumann J, Boecker W (2013) L-arginine-NO-cGMP signalling pathway in pancreatitis. Sci Rep 3:1899. https://doi.org/10.1038/srep01899
Irwin KK, Renzette N, Kowalik TF, Jensen JD (2016) Antiviral drug resistance as an adaptive process. Virus Evol 2:vew014. https://doi.org/10.1093/ve/vew014
Sanchez MD, Ochoa AC, Foster TP (2016) Development and evaluation of a host-targeted antiviral that abrogates herpes simplex virus replication through modulation of arginine-associated metabolic pathways. Antiviral Res 132:13–25. https://doi.org/10.1016/j.antiviral.2016.05.009
Kaplan AS, Shimono H, Ben-Porat T (1970) Synthesis of proteins in cells infected with herpesvirus. Virology 40:90–101. https://doi.org/10.1016/0042-6822(70)90382-X
Tankersley RW (1964) Amino acid requirements of herpes simplex virus in human cells. J Bacteriol 87:609–613. https://doi.org/10.1128/jb.87.3.609-613.1964
Butorov EV (2015) Influence of L-lysine amino acid on the HIV-1 RNA replication in vitro. Antivir Chem Chemother 24:39–46. https://doi.org/10.1177/2040206614566582
Becker Y, Olshevsky U, Levitt J (1967) The role of arginine in the replication of herpes simplex virus. J Gen Virol 1:471–478. https://doi.org/10.1099/0022-1317-1-4-471
Pedrazini MC, da Silva MH (2021) Pityriasis rosea-like cutaneous eruption as a possible dermatological manifestation after Oxford-AstraZeneca vaccine: case report and brief literature review. Dermatol Ther 34:e15129. https://doi.org/10.1111/dth.15129
Pedrazini MC, da Silva MH, Groppo FC (2022) L-lysine in herpesvirus reactivation after ChAdOx1 nCoV-19 vaccine (AZD1222): Minor literature review and case report. Dermatol Ther. https://doi.org/10.1111/dth.15291
Garnett HM (1975) The effect of arginine deprivation on the cytopathogenic effect and replication of human cytomegalovirus. Arch Virol 48:131–145. https://doi.org/10.1007/BF01318146
Melano I, Kuo L-L, Lo Y-C, Sung P-W, Tien N, Su W-C (2021) Effects of basic amino acids and their derivatives on SARS-CoV-2 and influenza-A virus infection. Viruses 13:1301. https://doi.org/10.3390/v13071301
Archard LC, Williamson JD (1971) The effect of arginine deprivation on the replication of vaccinia virus. J Gen Virol 12:249–258. https://doi.org/10.1099/0022-1317-12-3-249
Everitt E, Sundquist B, Philipson L (1971) Mechanism of the arginine requirement for adenovirus synthesis: I. Synthesis of structural proteins. J Virol 8:742–753. https://doi.org/10.1128/jvi.8.5.742-753.1971
Plaat D, Weber J (1979) On the mechanism of arginine requirement for adenovirus synthesis. Arch Virol 60:187–196. https://doi.org/10.1007/BF01317490
Raška K, Prage L, Schlesinger RW (1972) Effects of arginine starvation on macromolecular synthesis in infection with type 2 adenovirus. Virology 48:472–484. https://doi.org/10.1016/0042-6822(72)90058-X
Tan KB (1977) The effect of arginine deprivation on DNA, thymidine kinase and DNA polymerase synthesis in simian virus 40-infected monkey kidney cells. Arch Virol 53:133–138. https://doi.org/10.1007/BF01314854
Romano N, Scarlata G (1973) Amino acids requirements of measles virus in hela cells. Arch Für Gesamte Virusforsch 43:359–366. https://doi.org/10.1007/BF01556153
Loh PC, Oie HK (1969) Role of lysine in the replication of reovirus: I. Synthesis of complete and empty virions. J Virol 4:890–895. https://doi.org/10.1128/jvi.4.6.890-895.19690
Schierhorn KL, Jolmes F, Bespalowa J, Saenger S, Peteranderl C, Dzieciolowski J, Mielke M, Budt M, Pleschka S, Herrmann A, Herold S, Wolff T (2017) Influenza a virus virulence depends on two amino acids in the N-terminal domain of Its NS1 protein to facilitate inhibition of the RNA-dependent protein kinase PKR. J Virol 91:e00198-e217. https://doi.org/10.1128/JVI.00198-17
de Almeida JO, de Oliveira VRT, Avelar JLD, Moita BS, Lima LM (2020) COVID-19: physiopathology and targets for therapeutic intervention. Rev Virtual Quimica 12:1464–1497
Hoffmann M, Kleine-Weber H, Pöhlmann S (2020) A multibasic cleavage site in the spike protein of SARS-CoV-2 is essential for infection of human lung cells. Mol Cell 78:779-784.e5. https://doi.org/10.1016/j.molcel.2020.04.022
Romeu AR, Ollé E (2021) SARS-CoV-2 and the secret of the furin site. https://doi.org/10.20944/preprints202102.0264.v1
Izzo F, Montella M, Orlando AP, Nasti G, Beneduce G, Castello G, Cremona F, Ensor CM, Holtzberg FW, Bomalaski JS, Clark MA, Curley SA, Orlando R, Scordino F, Korba BE (2007) Pegylated arginine deiminase lowers hepatitis C viral titers and inhibits nitric oxide synthesis. J Gastroenterol Hepatol 22:86–91. https://doi.org/10.1111/j.1440-1746.2006.04463.x
Kahán IL, Hajas K, Halász A (1979) The significance of the arginine and arginase of tears in experimentally-induced herpes simplex corneae. Albrecht von Graefes Arch Klin Ophthalmol 209:219–224. https://doi.org/10.1007/BF00414614
Wildy P, Gell PG, Rhodes J, Newton A (1982) Inhibition of herpes simplex virus multiplication by activated macrophages: a role for arginase? Infect Immun 37:40–45. https://doi.org/10.1128/iai.37.1.40-45.1982
dos Santos VAB, Pedrazini MC (2024) L-lysine as an alternative treatment for pityriasis rosea (PR) [Letter]. Clin Cosmet Investig Dermatol 17:433–434. https://doi.org/10.2147/CCID.S461722
Thein DJ, Hurt WC (1984) Lysine as a prophylactic agent in the treatment of recurrent herpes simplex labialis. Oral Surg Oral Med Oral Pathol 58:659–666. https://doi.org/10.1016/0030-4220(84)90030-6
Pedrazini MC, da Silva MH, Odone LFG, Groppo FC (2024) L-lysine as a possible supplement for treatment of herpetic epithelial keratitis: a case report and literature review. J Explor Res Pharmacol 9:127–134. https://doi.org/10.14218/JERP.2023.00036
Pedrazini MC, Cury PR, Araújo VC, Wassall T (2007) Efeito da lisina na incidência e duração das lesões de herpes labial recorrente. RGO Rev Gaúcha Odontol 55:7–10
Pedrazini MC, Araújo VC, Montalli VAM (2018) The effect of L-Lysine in recurrent herpes labialis: pilot study with a 8-year follow up. RGO Rev Gaúcha Odontol 66:245–249. https://doi.org/10.1590/1981-863720180003000083517
Fiorentino G, Coppola A, Izzo R, Annunziata A, Bernardo M, Lombardi A, Trimarco V, Santulli G, Trimarco B (2021) Effects of adding L-arginine orally to standard therapy in patients with COVID-19: a randomized, double-blind, placebo-controlled, parallel-group trial. Results of the first interim analysis. EClinicalMedicine 40:101125. https://doi.org/10.1016/j.eclinm.2021.101125
Reizine F, Lesouhaitier M, Gregoire M, Pinceaux K, Gacouin A, Maamar A, Painvin B, Camus C, Le Tulzo Y, Tattevin P, Revest M, Le Bot A, Ballerie A, Cador-Rousseau B, Lederlin M, Lebouvier T, Launey Y, Latour M, Verdy C, Rossille D, Le Gallou S, Dulong J, Moreau C, Bendavid C, Roussel M, Cogne M, Tarte K, Tadié J-M (2021) SARS-CoV-2-induced ARDS associates with MDSC expansion, lymphocyte dysfunction, and arginine shortage. J Clin Immunol 41:515–525. https://doi.org/10.1007/s10875-020-00920-5
Trimarco V, Izzo R, Lombardi A, Coppola A, Fiorentino G, Santulli G (2023) Beneficial effects of L-Arginine in patients hospitalized for COVID-19: new insights from a randomized clinical trial. Pharmacol Res 191:106702. https://doi.org/10.1016/j.phrs.2023.106702
Rees CA, Rostad CA, Mantus G, Anderson EJ, Chahroudi A, Jaggi P, Wrammert J, Ochoa JB, Ochoa A, Basu RK, Heilman S, Harris F, Lapp SA, Hussaini L, Vos MB, Brown LA, Morris CR (2021) Altered amino acid profile in patients with SARS-CoV-2 infection. Proc Natl Acad Sci 118:e2101708118. https://doi.org/10.1073/pnas.2101708118
Sacchi A, Grassi G, Notari S, Gili S, Bordoni V, Tartaglia E, Casetti R, Cimini E, Mariotti D, Garotto G, Beccacece A, Marchioni L, Bibas M, Nicastri E, Ippolito G, Agrati C (2021) Expansion of myeloid derived suppressor cells contributes to platelet activation by l-arginine deprivation during SARS-CoV-2 infection. Cells 10:2111. https://doi.org/10.3390/cells10082111
Akaike T, Maeda H (2000) Nitric oxide and virus infection. Immunology 101:300–308. https://doi.org/10.1046/j.1365-2567.2000.00142.x
MacMicking JD, North RJ, LaCourse R, Mudgett JS, Shah SK, Nathan CF (1997) Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc Natl Acad Sci U S A 94:5243–5248. https://doi.org/10.1073/pnas.94.10.5243
Yang Y, Yu T, Lian Y-J, Ma R, Yang S, Cho JY (2015) Nitric oxide synthase inhibitors: a review of patents from 2011 to the present. Expert Opin Ther Pat 25:49–68. https://doi.org/10.1517/13543776.2014.979154
Stichtenoth DO, Frölich JC (1998) Nitric oxide and inflammatory joint diseases. Br J Rheumatol 37:246–257. https://doi.org/10.1093/rheumatology/37.3.246
Renwick AG (2004) Establishing the upper end of the range of adequate and safe intakes for amino acids: a toxicologist’s viewpoint. J Nutr 134:1617S-1624S. https://doi.org/10.1093/jn/134.6.1617S
Bier DM (2003) Amino acid pharmacokinetics and safety assessment. J Nutr 133:2034S-2039S. https://doi.org/10.1093/jn/133.6.2034S
Kim DR, Martin S, Desai K (2023) The effects of a comparatively higher dose of 1000 mg/kg/d of oral L- or D-arginine on the L-arginine metabolic pathways in male Sprague-Dawley rats. PLoS One. 18(8):e0289476. https://doi.org/10.1371/journal.pone.0289476
Acknowledgements
The authors would like to thank Ms. Thereza C. P. de Castilho for promptly volunteering to review this manuscript for its English language content.
Funding
This study did not receive funding.
Author information
Authors and Affiliations
Contributions
MCP was involved in conceptualization, bibliographical survey, and reviewing and writing of the initial version. EFM and FCG were responsible for the final article review. VABS contributed to writing, figures, and the final article review. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pedrazini, M.C., Martinez, E.F., dos Santos, V.A.B. et al. L-arginine: its role in human physiology, in some diseases and mainly in viral multiplication as a narrative literature review. Futur J Pharm Sci 10, 99 (2024). https://doi.org/10.1186/s43094-024-00673-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43094-024-00673-7