Abstract
Background
Babassu (Orbignya phalerata Mart.) is a palm tree well distributed in Latin America, whose fruit has a mesocarp and kernel used for human feeding, and empirically related to the treatment of gastritis, vaginitis, and wound healing.
Main body of the abstract
The activities attributed to babassu can guide new research on health applications and, for this reason, this study aimed to report in vitro and in vivo biological activities of O. phalerata constituents through a systematic review. Searches terms were applied in five world databases and the data from the publications were collected according to PICOS criteria, including the fruit component, concentration/dose, time of exposure, and comparative groups. All outcomes were reported and the most relevant outcomes were described by a narrative synthesis and a risk of bias assessment. A total of 28 in vitro (n = 15) and in vivo (n = 11) studies were included, and two showed both experimental designs. The studies were heterogeneous, with the predominance of metabolic analysis, wound and peptic ulcer healing, besides in vivo toxicity, among others. For in vitro analysis, antioxidant tests, cell viability and antimicrobial activity predominated. All in vivo ones used rodents. Meanwhile, tumor and non-tumor cell lines, bacteria strains, Leishmania amazonensis, Artemia salina, and antioxidant reactions were considered for in vitro protocols.
Short conclusion
The most frequent applications included mesocarp and kernel in a wide range of extracts, emulsions, and concentrations. Their low in vitro lethality and cytotoxicity, and no acute toxicity in vivo open possibilities for the development of long-term toxicity assays with repeated doses in rodents and interventions in clinical trials.
Graphic abstract

Similar content being viewed by others
Background
Orbignya phalerata Mart. (syn. Attalea speciosa), belonging to the Arecaceae family, is a palm tree well distributed in different biomes in Latin America, such as Amazon rainforest, Atlantic forest, and especially in Cerrado and Caatinga, and popularly known as Babassu, uauaçu, and catolé [1]. The genus Orbignya has more than 20 species, but the binomial nomenclature Orbignya phalerata Mart. was adopted by this review because it is the most used and recent in the bibliography consulted (Fig. 1). Its fruit is generally completely used, but only its mesocarp and kernel are edible. The studies related to babassu describe about the mesocarp and/or the kernel, mainly. The first is used in cosmetics industry [2] and the mesocarp flour has been empirically consumed for the treatment of gastritis, vaginitis, and topically as wound healing [3].
Plant species are continuously studied about their potential developing new drugs and products based on the diverse biological activities and pharmaceutical properties, mainly influenced by primary and secondary plant metabolites. Primary constituents, such as fatty acids, carbohydrates, and amino acids, are crucial macromolecules aiding plant survival and structural development. Meanwhile, secondary metabolites, derived from primary metabolism, play key roles in physiological processes and act as a defense mechanism against biological or chemical agents, such as polyphenols, which are one of the most common types of bioactive compounds, including the flavonoids catechin, epicatechin, proanthocyanidin, and others, all capable of scavenging or neutralizing reactive or radical species [4], which explains, at least in part, their antioxidant, anti-inflammatory, and antimicrobial properties, to name a few [5].
Among the edible parts of babassu, the kernel stands out for its elevated concentrations of fatty acids, primarily lauric acid (12:0; 46.89%), myristic acid (C14:0; 16.95%), and oleic acid (C18:1; 13.54%) [6,7,8]. Conversely, the mesocarp flour is a source of energy (1375 kJ/100 g), complex carbohydrates (79.19%), potassium (3.62%), magnesium (0.39%), phosphorus (0.35%), and a small content of protein (1.41%) [9].
In human metabolism, while carbohydrates are an energy source, flavonoids act as scavenger molecules. Simultaneously, fatty acids not only contribute to caloric supply but also play specific roles such as bactericidal [10]. Regarding the total phenolic compounds in the mesocarp (558.87 mg/100 g), the most important among them are the flavonoids extracted by organic solvents [9] (Table 1).
Preclinical trials have elucidated the biological effects of babassu crude components and its extracts. However, there is no evidence of compiled findings in a systematic review. Therefore, this study reported in vivo and in vitro biological activities of O. phalerata constituents in order to contribute to the development of original studies and applications for health care and research.
Main text
Methods
This systematic review was conducted according to PRISMA guidelines [11] (Preferred Reporting Items for Systematic Reviews and Meta-Analyzes) and registered in PROSPERO [12] (International Prospective Register of Systematic Reviews, registration no CRD 42022302309).
Publications eligibility was established based on the guiding question “Do different parts of babassu contain compounds capable of exerting biological effects in vivo and/or in vitro?” and PICOS criteria [P: Population; I: Intervention; C: Comparator; O: Outcome; S: Study] (Table 2). Only original articles from indexed journals were eligible, with no limitation of period or language. All publications from gray literature or not fully published articles were excluded.
For animal model studies, topical, intraperitoneal, parenteral, or rectal interventions were excluded. To be considered, both in vivo and in vitro designs should clearly describe the type of substance, concentration, and intervention period, at least for the main outcome.
Data sources, searches and studies selection
Explorations in the LILACS [13], SciELO [14], Science Direct [15], Web of Science [16], and PubMed [17] databases were performed by search terms registered in MeSH, combined by AND and OR Boolean operators (Table 3) during April 1st to April 7th, 2022 and again on March 24th, 2022 to update indexes. The searches were exported to Rayyan for prior systematization and metrics [18].
Initially, the studies were screened using the title and abstract (by ND and IOC), independently. Those considered eligible were read in full (ND, IOC) and references were kept for possible inclusion if they met the criteria. Disagreements were solved by consensus and, when necessary, a third researcher (JMCS) was consulted.
Data extraction and description of results
Data extraction included authors, publication year, title, locality, and study design. For in vivo publications, characteristics of the population were collected and for in vitro assays, cell lines, strain, and/or chemical reactions were described. Interventions included fruit part and its product, concentration, time of exposure, and comparative groups. Favorable or unfavorable outcomes were reported, as well as the most relevant assessment instruments and results. The compiled data were organized into tables, facilitating a comprehensive narrative synthesis and outcomes comparison, when applicable. Values were expressed as mean and standard deviation, when available, and compared with control and/or parallel groups. Results were considered significant when p < 0.05.
Risk of bias assessment
The risk of bias assessment for in vivo experiments was carried out independently by ND and IOC using the SYRCLE Risk of Bias Tool and the individual results were compared between ten domains distributed in six biases (selection, performance, detection, attrition, reporting, and other bias). Each domain was defined as “yes” (low risk of bias), “no” (high risk of bias), or “unclear” (uncertain risk) [19].
For in vitro analysis, the tool developed by the World Cancer Research Fund/University of Bristol was used with adaptations [20]. Each of the six questions was answered with “yes” (low risk of bias), “no” (high risk of bias), “not clear” if details were not recorded properly, or “not applicable”. Risk of bias analysis was recorded individually by study and by domain. Inter-rater reliability was determined by Cohen’s kappa coefficient [21].
Results
Search results and study characteristics
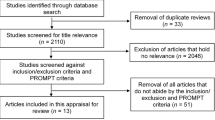
Figure 2 highlights the study selection process. Initially, 424 publications were identified, of which 146 were duplicated ones and removed automatically. A total of 278 articles were eligible by the title and abstract, but only 23 were selected for full-text review and 15 of them met the inclusion criteria. On the other hand, 13 articles not included by unidentifiable reasons in the initial search were included.
Screening flow diagram based on The PRISMA 2020 Statement [11]
According to the included reports, 39.3% (n = 11) refer exclusively to in vivo (Table 4) and 53.6% (n = 15) to in vitro assays (Table 5). Moreover, two articles showed both experimental designs and, for this reason, were described in both tables [22, 23]. Regardless of the taxonomy mentioned in the publications (Orbignya phalerata, O. martiana, O. speciosa, or Attalea speciosa), only the articles of Gaitan et al. [22] and Hovorková et al. [8] are not from Brazil, suggesting great interest about the plant and fruits in the Brazilian scenario. In preclinical interventions with animals, metabolic analysis and toxicity predominated (Fig. 3A), while in vitro assays highlight antioxidant tests (Fig. 3B).
Risk of bias assessment
Individual and for each domain in vivo
SYRCLE RoB Tool identified 130 entries distributed in ten domains for each one of the 13 studies (Table 6). It was found uncertain risk or inappropriate records in 80 entries (81.54%), low risk was present in 45 (34.61%) and high risk in five studies (3.85%). The average agreement by Cohen’s kappa was 0.53 (68%), classified as intermediate to good [21].
Uncertain risk prevailed for the domains related to sequence generation, allocation concealment, random housing, and random outcome assessment (Fig. 4). There was no record of uncertain risk related to selective outcome reporting. In the domain related to other sources of bias, only one entry was registered due to the lack of clearness if the same animals or distinct animals were submitted to the different tests and if there was no contamination by the induction of some substance used in control groups [24].
In vivo risk of bias assessment by domain. (legend) Domains: D1—sequence generation; D2—baseline characteristics; D3—allocation concealment; D4—random housing; D5—blinding (performance bias); D6—random outcome assessment; D7—blinding (detection bias); D8—incomplete outcome data; D9—selective outcome reporting; D10—other sources of bias. Yes: low risk; No: high risk; Unclear: uncertain risk [19]
The analysis of the effective risk of bias revealed a higher frequency of low risk in baseline characteristics (92.3% of entries for the domain), selective outcome reporting, and other sources of bias (84.61% for both). Low risk was evidenced due to the appropriate description regarding the specificities of the animals. As well as for high risk of bias, specific analysis indicates that the circumstances in the intervention route were not similar in all groups [23], and there was animal loss with unknown reason, groups equalization, and randomization of the final sample [25]. Besides that, some primary outcomes were not showed [26, 27].
Individual and for each domain in vitro
The analysis of 17 studies checked 102 entries divided into six questions (Table 7). There was a predominance of low risk of bias by adequate methods reporting (65.69%), high risk in eight (7.84%), and uncertain risk in seven studies (6.86%). The average agreement by Cohen’s kappa was 0.39 (51.96%), classified as low [21].
The most detailed and complete descriptions were seen in antimicrobial evaluation of hydrolyzed kernel lipid emulsion in strains of pathogenic and commensal microorganisms [8], kernel oil in pathogenic strains [10], and mesocarp with epicarp methanolic extract in viability, changes in morphology, and metabolism in different cell lines [28].
The high risk of bias was highlighted by the use of a single organism and the mean values of primary outcomes have not been presented [29]. Nine studies (47.05%) demonstrated the non-application of two questions, most frequently for the impossibility of comparing cell lines/strains or culture conditions between groups, as they were assays with a single microorganism, strain or distinct chemical reactions (Q4, Q6) [23, 29,30,31,32,33,34,35,36].
When analyzing the methodological quality by domain (Fig. 5), the selective result was avoided in 94.11% of the studies and the low risk of bias was in 76.47%, regarding the validation of cells and strains repository and the use of a control group (Q1, Q2). Less frequently, there were some doubts about the cell line origin (Q1, 5.88%) and technical repeats (Q2, 17.65%) [22, 29, 30, 37].
In vitro risk of bias assessment by domain. (legend) Q1—cells/strains from a validated repository or appropriately verified; Q2—technical repeats and controls inclusion; Q3—use of different cell lines/strains/reactions; Q4—comparable conditions between groups/assays; Q5—selective outcome reporting; Q6—comparison of different cell lines/strains/substances. Yes: low risk; Não: high risk; Unclear: uncertain risk. Questions adapted from Lewis et al. [20]
The most frequent classification for 'high risk' was based on the use of a single cell line/strain in 41.17% of the investigations [22, 29, 31,32,33, 38] and, less frequently, for the reason of the impossibility in comparing reactions due to different outcomes [23], and again, due to selective report when the result was presented by a single sample [29].
In vivo interventions and main results
Animals' interventions and related results are described in Table 4. The antithyroid effect was evaluated by offering acutely mesocarp aqueous extract (MAE) and kernel pressed paste with skin (KPPS) after a previous iodine-rich diet. This effect was observed by thyroid uptake reduction of 125I and by the ratio between 125I and monoiodotyrosine (MIT) coupled to diiodotyrosine (DIT). As a result, 125I uptake was suppressed by MAE, but not significantly by KPPS. However, the ratio 125I/MIT + DIT was high for both MAE and KPPS, suggesting a thionamide-like antithyroid effect [22].
Concerning the effect on lipid metabolism, urea and the development of type 1 diabetes, three studies offering MAE evaluated the intake of 50 mg/kg for 30 days in five mouse strains and found a significant increase in total cholesterol (TC) levels only in CBA strain (27 vs. 9 mg/dL) and a decrease only in C3H/HePas (18 vs. 47 mg/dL). There was a significant decrease in urea levels, except for C57BL/6 (49 vs. 67 mg/dL). Despite the glycolipid profile outcome mentioned, levels of lipoproteins, triglycerides (TG) or glucose were not demonstrated [39]. Another intervention offered a dose ten times lower than the previous study for 40 non-consecutive days, associated or not with resistance training (RT). They verified weight of loss after an 8-week supplementation period and, associated with RT, after 4 weeks. In addition, animals supplemented combined or not with RT had retroperitoneal fat reduction up to 73%, a decrease in TC levels (MAE: 79 mg/dL; MAE/RT: 70 vs. 97 mg/dL) and TG (MAE: 90 mg/dL; MAE/RT: 82 mg/dL vs. 166 mg/dL). There was a reduction in glucose only in RT animals (131 vs. 145 mg/dL). Animals trained without supplementation showed greater Delta force, suggesting a possible ergolytic effect of the substance [6].
On the other hand, Silva et al. [25] observed weight gain with intake of 3.3 mL/day of a suspension of MAE 20 mg/mL. In this study, the animals were not subjected to any type of physical labor and weight gain occurred between the 20th and 50th days, but with an abrupt drop afterwards. There was blood glucose fluctuation with lower levels on the 30th day and returning to baseline on the 60th. They did not observe significant changes in immunoglobulin IgG levels, possibly due to the low effect of MAE on T lymphocyte activation and cytokine production.
Acute toxicity effects were observed with high doses of mesocarp extracts and measured by organ relative weight and histological and biochemical analysis. No deaths were registered after a single dose up to 5000 mg/kg of mesocarp ethanolic extract (MEE) [27], as well as significant physical and or behavioral changes were not found. The same investigation also evidenced a reduction in urea levels at 5000 mg/kg (26 vs. 40 mg/dL) and increased TG at 1000 mg/kg (104 vs. 54 mg/dL) in a dose-dependent way. Alkaline phosphatase (ALP) increased after 3000 and 5000 mg/kg intake (23 and 21 U/L, respectively, vs. 6 U/L). No toxic effects were observed with gradual doses up to 4000 mg/kg [24]. MAE, mainly associated with RT, increased both aspartate (AST) and alanine aminotransferase (ALT) [6]. Alterations in locomotor activity and motor coordination can also be a sign of toxicity. Thus, a single dose of up to 3 g/kg of MAE did not affect these outcomes [23].
Low toxicity was observed since no changes in the number of medullary cells and a decrease of splenic cells only in BALB/c and C3H/HePas (both 3 × 107 vs. 5 × 107) was noted. Weight loss in organs was reported for HePas after 30 days exposure at 50 mg/kg of MAE [39]. On the other hand, MAE 5 mg/kg was able to increase the number of medullary cells in the absence of RT in Swiss mice (30 × 106 vs. 13 × 106), but in the scenario of splenocyte levels’ preservation (41 × 106). When associated with RT, splenic cells were reduced (30 × 106) [6].
After different doses and periods, Maia and Rao [24] evaluated the effect of mesocarp chloroform extract intake on the inflammatory process. Analyzing the carrageenan-induced inflammation in edema paw, it decreased up to 32% with progressive doses. The edema reduction caused by formaldehyde-induced arthritis was also observed on the 7th and 8th days of treatment. In this same study, seven-day intake at 250 mg/kg inhibited subcutaneous granuloma, measured by the weight of the cotton pellet introduced (112 vs. 192 mg) and it reduce leukocyte migration (24.19 vs. 23.62 cells/mm3) and inflammatory exudate after a subcutaneous sponge implant impregnated with carrageenan.
In a stage before exudate induction, Silva and Parente [40] verified a significant inhibition in vascular permeability progression following a single dose of an isolated polysaccharide from mesocarp composed of alpha-(1 → 4) linked D-glucopyranose residues. Meanwhile, Barbosa et al. [26] observed the same effect in microvessels after volumes greater than 0.06 mL of the crude kernel oil twice a day. This study also reported attenuation in leukocyte adhesion with intake of 0.02 mL.
When analyzing proinflammatory cytokines, MAE intervention decreased interleukin-6 (IL-6) and increased tumoral necrosis factor-alpha (TNF-α) levels [6]. However, kernel oil was not able to cause significant changes in IL-1, IL-6, and TNF-α [26].
Leukocyte-specific activity is closely related to inflammatory stages. In this regard, five days of α-glucan from mesocarp increased zymosan-like phagocytic activity induced by colloidal carbon [40]. However, MAE associated with RT increased total splenic macrophages, but reduced activated macrophages, without changes in monocytes [6]. There was an activated T helper decrease and an increase of B cells, suggesting an immunomodulatory effect well [6].
Other outcomes, similar or antagonistic, were also evaluated. Although the antithrombotic effect of the aqueous mesocarp suspension was indicated by the prothrombin time increase (11.2 vs. 10 s) and activated partial thromboplastin time (33.6 vs. 29.5 s) [41], on the other hand, the chloroform extract of mesocarp given for three days did not extend the hemostasis time (98.0 vs. 90.3 min) [23].
Regarding peptic ulcer induction, prevention, or treatment, Maia and Rao induced ulceration with phenylbutazone and observed an average score of 1.03 for the lesion in five of six animals treated with phenylbutazone, on a 0–4 scale [24]. In opposition, animals treated with mesocarp chloroformic extract for 21 days did not exhibit lesions. In a different study, the prophylactic and therapeutic effect of MAE was compared to omeprazole, before or after ethanol-induced ulcers. In the preventive treatment, the effect was similar to omeprazole in the absence of lesions (60% of the animals), but superior to the drug in the absence of hyperemia, bleeding, and preservation of folds (100% vs. 60% on all outcomes). Despite the accentuated related-inflammatory lesions, such damage reached only the mucosa in MAE-treated animals, while there was deeper damage up to the submucosa in those ones receiving vehicle. Moreover, microscopic analysis showed MAE prevented necrosis in 80% of animals [42].
Scheibe et al. [43] evaluated 21-day MAE intake for healing. After this period, the animals underwent laparotomy with cecum exteriorization. Significantly, they identified a grade II adhesion (two adhesions between organs or between an organ and abdominal wall) in 100% of the animals on the 21st postoperative day compared to the negative control. Morphological evaluations did not identify polymorphonuclear leukocytes (vs. moderate presence), and mild congestion and angiogenesis (vs. severe) and moderate fibroblasts (vs. severe) were detected in MAE-exposed animals. Collagen production was also more intense than in the control group. Silva et al. [44] used the same procedures, but offered a MAE single dose. They found similar results, except for collagen production.
Finally, antipyretic and analgesic properties of mesocarp chloroform extract were analyzed [24]. After pyrexia induced by Saccharomyces cerevisiae, no time-related decrease in body temperature was observed (1 h: 39.18 vs. 39.18 °C; 2 h: 39.1 vs. 39.3 °C; 3 h: 39.18 vs. 39.31 °C). The analgesic effect was compared to morphine after exposure to a hot-plate at 55 °C. After 30 min of 250 mg/kg intake, the extract was not able to promote analgesia. Reaction time was similar to negative control and shorter than morphine (extract: 3.5 s; control: 2.83 s; morphine: 14.0 s). In contrast, the same dose was able to reduce acetic acid-induced writhing around 62% [24].
In vitro assays and main results
In vitro studies are useful for preliminary testing of substances in a controlled environment. The studies included are described in Table 5. Six studies evaluating antimicrobial and phagocytic activities on pathogenic or commensal strains [8, 10, 27, 31, 37, 45] and two investigations against protozoa [29, 38] were identified. Cellular assays were carried out with tumor or non-tumor cell lines to study viability or cytotoxicity, morphological or metabolic changes [22, 28, 31,32,33, 36]. Lethality assays were carried out in microcrustaceans (Artemia salina Leach.) [45].
Assays using chemical reactions or yeasts can also be developed to measure preliminary effective concentrations (EC50) for achieving half desirable effects. This review included six studies which evaluated the antioxidant activity of babassu [23, 30, 32, 34, 35, 45].
The most common component of O. phalerata was the kernel (52.9% of the studies) [8, 10, 22, 30,31,32,33, 36, 45]. MAE and mesocarp alcoholic extracts were tested in 47% and one publication associated mesocarp with epicarp [22, 23, 28, 29, 35, 37, 38, 46]. Only Silva et al. [34] used endocarp, flowers, and leaf extracts. The concentrations ranged a lot from 500 mg/mL of MEE [46] to assess the antimicrobial effect to 1 μg/mL of MAE for the antioxidant activity [23].
The disk diffusion assay was used to evaluate the effect of mesocarp on pathogenic bacteria strains. At 250 and 500 mg/mL, MEE promoted concentration-dependent inhibition zones on Staphylococcus aureus (18.5 mm), methicillin-resistant S. aureus (17.4 mm), and Enterococcus faecalis (14.4 mm). The most relevant minimum inhibitory concentration was observed for E. faecalis, while 500 mg/mL inhibited the growth of them completely. In contrast, there was no inhibition of Escherichia coli and Pseudomonas aeruginosa [46]. Similar levels of inhibition on methicillin-resistant or sensitive S. aureus were observed with lower concentrations of the hydroalcoholic extract [37].
Kernel oil also did not demonstrate efficacy on Gram-positive and negative strains [45], but after hydrolysis, it inhibited 80% of Clostridium perfringens, S. aureus, and Enterococcus cecorum [8]. Furthermore, the fixed oil increased the effectiveness of antibiotics over S. aureus, P. aeruginosa, and E. coli [10].
After evaluating mononuclear phagocyte activity and bactericidal potential, Pessoa et al. [31] found a higher rate of phagocytosis by lipid microemulsion (69.1 vs. 47%), and the bactericidal activity was higher by isolated oil (47.9%). When testing the leishmanicidal effect of MAE, it was observed a low activity when compared to Glucantime® (LC50 > 500 vs. 440.3 μg/mL) [38]. On the other hand, microparticles loaded with the same extract demonstrated upper effectivess, but lower than pentamidine (IC50 12 vs. 0.8 pg/mL) [29].
In a study developed by Rennó et al. [28] using the trypan blue method, leukemic promyelocytes (HL-60) were more sensitive to the crude ethanolic extract from mesocarp combined with epicarp when compared to negative control (ID50 9.3 vs. 125 μg/mL), whose activity was time dependent (150 μg/mL: 8.6 h; 2 mg/mL: 0.4 h). The concentration of 1.2 mg/mL promoted changes in HL-60 morphology, with decrease in size and cytoplasmic/nuclear condensation. Interestingly, there was an increase in levels of 6-phosphofructo-1-kinase (PFK) enzyme at 300 μg/mL were tested, 6.6 times greater than the negative control. In this study, non-tumor cells showed greater resistance, such as L929 (127 vs. 88.7 μg/mL) and human lymphocytes (141.2 vs. 84.4 μg/mL). It is important to highlight that both erythroleukaemic sensitive to chemotherapy line and its multidrug-resistant counterpart were equally affected.
Regarding cytotoxicity, Santos et al. [32] tested a lipid nanoemulsion from kernel oil droplets in L929 cells and it was found a time and concentration-dependent IC50 after 24 h of exposure (396.1 μg mL−1), 48 h (363.3 μg mL−1) and 72 h (333.1 μg mL−1). Santos et al. did not find kernel oil toxic effects on L929 after MTT (3–4,5-dimethyl-thiazol-2-yl-2,5-diphenyltetrazolium bromide) assay. Conversely, the same effect was not verified on peritoneal macrophages. Furthermore, they reported an increased migration of L929 cells in the scratch assay, concentration-dependent nitric oxide attenuation (0.31 to 0.29 vs. 1.15 μM), and higher levels of INF-γ (interferon-γ) production with 6.25 μg/mL of the nanoemulsion (2214.2 vs. 980.4 pg/mL), TNF-α (107.7 vs. < 0.9 pg/mL) and IL-6 with 3.12 μg/mL (1286.1 vs. 584.4 pg/mL) by macrophages, suggesting a modulation of inflammatory response in wound healing situations.
Concerning phagocyte viability, a higher viability index similar to culture medium was found for lipid microemulsion when compared to isolated oil (98 vs. 94.3%) [31]. Another study using benign prostatic hyperplasic cells observed viability inhibition up to 75% after 24–72 h of exposure to a 300 μg/mL of nanocomposite with lipophilic extract. Even after the addition of fetal bovine serum, the inhibition was sustained. There was also induction of disorganization (disassembly) or disruption in the structure of actin microfilaments in a time-progressive manner, and a progressive lactate dehydrogenase (LDH) release. For this enzyme, there was a release of up to 75% after 48 h. Immunoreactivity for proliferation cell nuclear antigen (PCNA) was also tested and proved to be 50% lower when compared to the negative control in association of apoptosis induction [36]. The single report about multicellular organisms observed no toxicity on A. salina (LC50 > 1000 μg/mL) after testing concentrations up to 50 mg/mL of kernel oil [45].
The antithyroid effect in porcine thyroid slices was tested by Gaitan et al. [22] with different babassu components. The study verified that, although 125I absorption by thyroid tissue was similar between the substances, the extracts showed a higher incorporation of 125I/MIT + DIT, mostly after MME (mesocarp methanolic extract) exposure indicating iodine organification inhibition. Concerning thyroperoxidase activity, the MME and kernel peel evidenced an EC50 of 140 and 160 μg/mL, respectively, for thyroperoxidase-catalyzed iodination. Tyrosinase inhibition was also tested and a higher IC50 was found for the mesocarp ethyl acetate in comparison with extract or hydroalcoholic fraction for both monophenolase and diphenolase activity [35].
The antioxidant potentiality of babassu is detailed in Table 5. EC50 required for DPPH• (2,2-diphenyl-1-picryl-hydrazyl) scavenging was described for kernel oil [37, 45] and for a nanoemulsion [32]. Nobre et al. [30] did not find thiobarbituric acid reactive substances (TBARS) inhibition and evidenced a low effect on deoxyribose degradation, DPPH•, and iron chelation by kernel methanolic extract. In this study, the expected effect occurred only for FRAP (ferric-reducing antioxidant power), but required higher concentrations than quercetin (1560 vs. 155.2 μmol L−1Fe2+). Likewise, no antioxidant effect of MAE up to 1000 μg/mL was detected. Endocarp, flower, and leaf alcoholic extracts also did not demonstrate antioxidant potential based on reaction with DPPH•. Furthermore, there was no yeast survival when the same extracts were associated with tert-butylhydroperoxide [23]. A positive result was verified only by Silva et al. [35] for mesocarp ethyl acetate fraction on DPPH• (IC50 of 3.38 μg/mL), ABTS•+ (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) scavenging (2.04 μg/mL), and FRAP (15.41 mmol Fe2+).
Discussion
In vivo studies
The fruit of babassu has shown favorable biological activities. However, the absence of results or undesirable outcomes were also observed, such as the antithyroid effects.
In animal models, the sex, age, weight, and metabolic condition are essential to optimize the relevance of the results [47]. Despite the analysis of beneficial effects, the in vivo trials seem to be useful for acute, subacute, or chronic toxicity tests, since even natural substances cannot be considered completely safe. Notably, widely studied nutrients also exhibit upper limits and restrictions [48]. In this context, investigations subjected animals to varied experimental conditions, including a single dose [23, 27], short or moderate durations throughout their lifetime [6, 39], or progressive dose regiments [24], but not always waiting for long latency periods [23], which limits the outcomes.
One of the alterations found that could indicate possible toxicity suggests that even after 14 days of latency, ALP increase was not followed by changes in liver and kidney functions. The hypothesis suggests that young animals are susceptible to alterations in this enzyme according to their diet [27]. In turn, increasing levels in aminotransferases should be evidence of oxidative stress due to lipid peroxidation, notably when there is a greater oxygen demand, as the rodents submitted to RT [6]. In terms of immunotoxicity, the decrease of splenic cells and spleen weight were both selective. The authors suggest sparse effects due to the divergence of results in strains with distinct haplotypes [39]. In short, despite the cited changes, all publications suggest low toxicity of the kernel and the mesocarp, not only with a single dose intervention up to 5 g/kg [23, 24, 27], but also with low doses up to 40 days [6, 39].
Results on metabolic effects show discrepant changes in serum TG. This marker increase could suggest an increment in lipogenesis due to the high carbohydrate content in the mesocarp (79.2%) [6], but it did not worse with doses above 1000 g/kg [27]. The presence of fibers in the mesocarp (17.9%) could also be associated with TC and LDL (low-density lipoprotein) decrease by reducing lipids absorption when they interact with lipase and/or colipase and limit the enzymatic activity [6, 49].
The reason for urea level reduction lies in the fact that foods with a high carbohydrate content, mostly resistant types, are not absorbed and remain available for gut microbiota fermentation. In this condition, endogenous proteins and plasma nitrogen would be recruited to ensure microorganism growth, reducing plasma urea levels, especially when dietary protein intake is deficient [50].
Investigations have also displayed weight gain and glycemic fluctuation after intervention with MAE, which would contraindicate mesocarp intake, mainly for type II diabetes patients, in which weight loss is the most common aim, or when the risk of developing diabetes is increased [25]. However, the glycemic load of foods with a high content of digestible carbohydrates can be attenuated in association with proteins, lipids, and dietary fiber, constituting an essential strategy for maintaining adequate glycemic levels [51]. In opposition, the absence of weight gain and retroperitoneal adiposity reduction was demonstrated by Soares et al. [6] after MAE intake associated or not with RT, indicating a possible adjuvant effect of mesocarp in fat loss and weight control.
The anti-inflammatory activity of mesocarp chloroform extract suggests its application in subacute situations due to the inhibition of granuloma, a proliferative phase of inflammation. However, as the chloroform extract had no effect on pyrexia or leukocyte migration, arachidonate metabolism is not possibly involved in the mechanism [24].
Despite the high content of saturated fatty acids in crude kernel oil, the hypothesis is that vascular permeability decrease and leukocyte adhesion observed by Barbosa et al. [26] may have attributed to the anti-inflammatory action of oleic acid and the antioxidant effect of α-tocopherol.
Other positive effects on inflammation processes demonstrated by α-glucan propose that the residual chains of 1 → 3 bonds not hydrolyzed by amylase may be long enough to contribute to phagocytic amplification and vascular protection [40].
In vitro studies
The selective antibacterial effect of MEE, mainly on E. faecalis and S. aureus, has likely clinical effects because these strains are associated with nosocomial infections and usually resistant to antibiotics. Its mechanism of action probably involves the generation of complexes between phenolic acids, proteins, and polysaccharides capable of breaking cell wall and inhibiting microorganisms' enzymes [37, 46]. In turn, the synergistic effect between mesocarp and antibiotics may occur by different pathways, including changes in drug receptors [10, 52]. The microparticle encapsulation system can delay compound cytoplasm release, which improves the effect [29]. Applications with crude kernel oil on gram-positive or negative strains were not effective [45]. After its hydrolysis, it showed a selective effect against pathogenic bacteria, but not commensal strains [8]. This effectiveness is related to the free lauric acid, which can cross cell membranes, acidify the intracellular medium, and block bacterial growth, besides the oxidative effect after phagocyte activation [31, 53].
The results observed against tumor cells only demonstrated relative selectivity of mesocarp with epicarp ethanolic extract, including over chemoresistant cell lines, a phenomenon that represents the main failure of antineoplastic treatments. However, the absence of PFK inhibition on HL-60 suggests that the remaining tumor cells may have increased metabolic activity in face of extract toxicity [28, 36].
Fibroblasts have been employed because they are abundant cells in the human body and one of the first to come into contact with substances during absorptive processes. Thus, nanoemulsions based on kernel oil extracts showing low toxic effects against fibroblasts in vitro can predict in vivo studies, as well as the low toxicity against A. salina by a wide number of plant species [32, 45, 54].
Experimental evidence demonstrated the antithyroid effect of mesocarp flours, imputing its intake for the persistence of endemic goiter in Maranhão (Brazil) in the mid-1990s [22]. Nonetheless, since the sodium chloride iodination strategy was implemented in the 1950s, the prevalence of iodine deficiency disorders in Brazil has reduced from 20.7% in 1955 to 1.4% in 2000 [55].
The inhibition of tyrosinase activity promoted by the mesocarp ethyl acetate fraction was justified by the high concentration of proanthocyanidins (453.7 mg CE/g) in the inhibitory steps of monophenolase and diphenolase activity [35]. This enzymatic inhibition is one of the key mechanisms for alterations in melanogenesis, responsible for some characteristics of malignant melanoma [56]. The mechanism of this phytochemical consists of blocking L-tyrosine oxidation, depressing L-dopa oxidation products, and preventing pigment synthesis [57].
Given the importance of controlling oxidative stress and its pathological consequences, some tests identified the antioxidant potential of babassu to neutralize free radicals and prevent lipid peroxidation. After finding a total phenolic content of 288 mg/g in kernel oil, the antioxidant activity did not overcome the ascorbic acid, there may not be a direct correlation between high levels of phytochemicals and better effects though [45]. It is also suggested that specific flavonoids may act in the intermediate process of lipid peroxidation, but they do not neutralize specific radicals [30]. Some concerns about the interference of kernel oil pigments in antioxidant assays have some grade of plausibility, such as DPPH•, because they can affect the optical density, which can lead to misinterpretations [58]. Therefore, the use of electronic spin resonance may be useful to bypass this interference [32].
The low affinity between MAE and lipids in TBARS can be answered by the lack of interaction with specific lipids, or hydrophilic portions of amphipathic lipids, as they are more sensitive to radical activity [23], even using polar solvents which can extract flavonoids that provide hydroxyl radicals to neutralize reactive species. However, different solvents can optimize the extraction of polyphenols [59, 60]. With the use of ethyl acetate to prepare samples from mesocarp, it was possible to identify monomers and tetramers of catechins. While the monomer is capable of reacting with a single free radical, its polymer can neutralize three radicals simultaneously [61]. On the other hand, the lack of effect by the endocarp, leaf, and flower ethanolic extracts on radicals and protection against tert-butylhydroperoxide, can be explained by low flavonoid content [34].
Conclusion
This review highlights the prevalent use of mesocarp and kernel in alcoholic extracts and emulsions, emphasizing their low lethality and weak cytotoxicity in vitro, along with the absence of acute toxicity in vivo. This opens perspectives for advancing to in vivo toxicity assays with repeated doses. The empirical intake of both mesocarp flour and kernel oil provides a basis for considering an extension to clinical trials to comprehensively understand the potential applications and effects of these substances.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ABTS:
-
2,2′-Azino-bis(3- ethylbenzothiazoline-6-sulfonic acid)
- ALP:
-
Alkaline phosphatase
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- DIT:
-
Diiodotyrosine
- DPPH:
-
2,2-Diphenyl-1-picryl-hydrazyl
- FRAP:
-
Ferric-reducing antioxidant power
- EC50 :
-
Effective concentration
- IC50 :
-
Inhibitory concentration
- ID50 :
-
Inhibitory dose
- IFN-γ:
-
Interferon-γ
- Ig:
-
Immunoglobulin
- IL:
-
Interleukin
- KPPS:
-
Kernel pressed paste with skin
- LC50 :
-
Lethal concentration
- LDH:
-
Lactate dehydrogenase
- LDL:
-
Low-density lipoprotein
- MAE:
-
Mesocarp aqueous extract
- MEE:
-
Mesocarp ethanolic extract
- MIT:
-
Monoiodotyrosine
- MME:
-
Mesocarp methanolic extract
- MTT:
-
3-4,5-Dimethyl-thiazol-2-yl-2,5-diphenyltetrazolium bromide
- PCNA:
-
Proliferation cell nuclear antigen
- PFK:
-
6-Phosphofructo-1-kinase
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyzes
- PROSPERO:
-
International Prospective Register of Systematic Reviews
- RT:
-
Resistance training
- SYRCLE:
-
SYstematic Review Center for Laboratory Animal Experimentation
- TBARS:
-
Thiobarbituric acid reactive substances
- TC:
-
Total cholesterol
- TG:
-
Triglycerides
- TNF-α:
-
Tumoral necrosis factor-alpha
References
Lorenzi H (2002) Árvores brasileiras: Manual de identificação e cultivo de plantas arbóreas do Brasil. Instituto Plantarum, Nova Odessa
Carrazza LR, Ávila JCC, Silva ML (2012) Manual tecnológico de aproveitamento integral do fruto do babaçu. Instituto Sociedade, População e Natureza (ISPN), Brasília. https://sgp.undp.org. Accessed 9 May 2022
Souza MHSL, Monteiro CA, Figueiredo PMS et al (2011) Ethnopharmacological use of babassu (Orbignya phalerata Mart.) in communities of babassu nut breakers in Maranhão, Brazil. J Ethnopharmacol 133:1–5. https://doi.org/10.1016/j.jep.2010.08.056
Pott DM, Osorio S, Vallarino JG (2019) From central to specialized metabolism: an overview of some secondary compounds derived from the primary metabolism for their role in conferring nutritional and organoleptic characteristics to fruit. Front Plant Sci 10:835. https://doi.org/10.3389/fpls.2019.00835
Mushtaq S, Abbasi BH, Uzair B et al (2018) Natural products as reservoirs of novel therapeutic agents. EXCLI J 17:420–451. https://doi.org/10.17179/excli2018-1174
Soares MCR, Silva MCP, Almeida-Junior FAZ et al (2021) Effect of babassu mesocarp as a food supplement during resistance training. J Med Food 24:411–421. https://doi.org/10.1089/jmf.2020.0071
Santos DS, Silva IG, Araújo BQ et al (2013) Extraction and evaluation of fatty acid compositon of Orbignya phalerata Martius oils (Arecaceae) from Maranhão state, Brazil. J Braz Chem Soc 2:355–362. https://doi.org/10.5935/0103-5053.20130045
Hovorková P, Laloučková K, Skřivanová E, (2018) Determination of in vitro antibacterial activity of plant oils containing medium-chain fatty acids against gram-positive pathogenic and gut commensal bacteria. Czech J Anim Sci 63:119–125. https://doi.org/10.17221/70/2017-CJAS
Costa CM, Silvino VO, Freitas VH et al (2023) Babassu mesocarp (Orbignya pharelata Mart.) supplementation decreased markers of muscle damage, pain, and perceived exertion in trained young futsal athletes. RBNE 17:51–58
Nobre CB, Sousa EO, Silva JMFL et al (2018) Chemical composition and antibacterial activity of fixed oils of Mauritia flexuosa and Orbignia speciosa associated with aminoglycosides. Eur J Integr Med 23:84–89. https://doi.org/10.1016/j.eujim.2018.09.009
Page MJ, McKenzie JE, Bossuyt PM et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:71. https://doi.org/10.1136/bmj.n71
Debia N, Castro IO, Sousa JMC (2022) Investigation of the biological effects of compounds present in babassu (Orbignya phalerata Mart.) in preclinical studies. In: PROSPERO: international prospective register of systematic reviews. https://doi.org/10.15124/CRD42022302309. https://www.crd.york.ac.uk/prospero/
Latin American and Caribbean Health Sciences Literature (LILACS) (2022) Latin American and Caribbean center on health sciences information (BIREME/PAHO/WHO). https://lilacs.bvsalud.org/en/. Accessed 24 Oct 2022
Scientific Electronic Library Online (SciELO) (2022). https://www.scielo.org. Accessed 24 Oct 2022
Science Direct (2022) Elsevier. https://www.sciencedirect.com. Accessed 24 Mar 2022
Web of Science (2022) Clarivate. https://www.webofscience.com/wos/author/search. Accessed 24 Mar 2022
PubMed Central (PMC). National Center for Biotechnology Information (NCBI). National Library of Medicine (US), National Center for Biotechnology Information (2022) https://pubmed.ncbi.nlm.nih.gov/. Accessed 24 Mar 2022
Ouzzani M, Hammady H, Fedorowicz Z et al (2016) Rayyan—a web and mobile app for systematic reviews. Syst Rev 5:210. https://doi.org/10.1186/s13643-016-0384-4
Hooijmans CR, Rovers MM, de Vries RBM et al (2014) SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol 14:43
Lewis SJ, Gardner M, Higgins J et al (2017) Developing the WCRF International/University of Bristol methodology for identifying and carrying out systematic reviews of mechanisms of exposure-cancer associations. Cancer Epidemiol Biomark Prev 26:1667–1675. https://doi.org/10.1158/1055-9965.EPI-17-0232
Cohen J (1960) A coefficient of agreement for nominal scales. Educ Psychol Measur 20:37–46. https://doi.org/10.1177/001316446002000104
Gaitan E, Cooksey RC, Legan J et al (1994) Antithyroid effects in vivo and in vitro of babassu and mandioca: a staple food in goiter areas of Brazil. Eur J Endocrinol 131:138–144. https://doi.org/10.1530/eje.0.1310138
Silva APS, Cerqueira GS, Nunes LCC et al (2012) Effects of an aqueous extract of Orbignya phalerata Mart. on locomotor activity and motor coordination in mice and as antioxidant in vitro. Pharmazie 67:260–263. https://doi.org/10.1691/ph.2012.1105
Maia MBS, Rao VS (1989) Anti-inflammatory activity of Orbignia phalerata in rats. Phytother Res 3:170–174. https://doi.org/10.1002/ptr.2650030503
Silva TA, Carvalho-Filho CJ, Barroqueiro ES et al (2016) The effect of high carbohydrate consumption on glucose levels and antibody production in nonobese diabetic mice. Food Nutr Sci 7:866–873. https://doi.org/10.4236/fns.2016.710086
Barbosa MCL, Bouskela E, Cyrino FZGA et al (2012) Effects of babassu nut oil on ischemia/reperfusion-induced leukocyte adhesion and macromolecular leakage in the microcirculation: observation in the hamster cheek pouch. Lipids Health Dis 11:158. https://doi.org/10.1186/1476-511X-11-158
Barroqueiro ESB, Barroqueiro FB, Pinheiro MT et al (2011) Evaluation of acute toxicity of babassu mesocarp in mice. Braz J Pharmacogn 21:710–714. https://doi.org/10.1590/S0102-695X2011005000121
Rennó MN, Barbosa GM, Zancan P et al (2008) Crude ethanol extract from babassu (Orbignya speciosa): cytotoxicity on tumoral and non-tumoral cell lines. An Acad Bras Sci 80:467–476. https://doi.org/10.1590/S0001-37652008000300008
Silva MCP, Brito JM, Ferreira AS et al (2018) Antileishmanial and immunomodulatory effect of babassu-loaded PLGA microparticles: a useful drug target to Leishmania amazonensis Infection. Evid Based Complement Altern Med. https://doi.org/10.1155/2018/3161045
Nobre CB, Sousa EO, Camilo CJ et al (2018) Antioxidative effect and phytochemical profile of natural products from the fruits of “babaçu” (Orbignia speciose) and “buriti” (Mauritia flexuosa). Food Chem Toxicol 121:423–429. https://doi.org/10.1016/j.eujim.2018.09.009
Pessoa RS, França EL, Ribeiro EB et al (2015) Microemulsion of babassu oil as a natural product to improve human immune system function. Drug Des Dev Ther 9:21–31. https://doi.org/10.2147/DDDT.S73756
Santos DS, Moura LD, Radicchi MA et al (2021) Nanoemulsion improves babassu palm oil (Orbignya phalerata) antioxidant properties. Braz Arch Biol Technol 64:e21190387. https://doi.org/10.1590/1678-4324-2021190387
Santos JAA, Silva JW, Santos SM et al (2020) In vitro and in vivo wound healing and anti-inflammatory activities of babassu oil (Attalea speciosa Mart. Ex Spreng., Arecaceae). Evid Based Complement Altern Med. https://doi.org/10.1155/2020/8858291
Silva CG, Herdeiro RS, Mathias SJ et al (2005) Evaluation of antioxidant activity of Brazilian plants. Pharmacol Res 52:229–233. https://doi.org/10.1016/j.phrs.2005.03.008
Silva VC, Barboza JL, Dutra RP et al (2017) Identification of phenolic compounds by LC/MS-MS and antioxidant and anti-tyrosinase activities of the Attalea speciosa Mart. ex Spreng mesocarp. J Chem Pharm Res 19:267–275
Souza PAVRS, Palumbo A Jr, Alves LM et al (2011) Effects of a nanocomposite containing Orbignya speciosa lipophilic extract on benign prostatic hyperplasia. J Ethnopharmacol 135:135–146. https://doi.org/10.1016/j.jep.2011.03.003
Caetano NA, Saraiva A, Pereira R et al (2002) Determinação de atividade antimicrobiana de extratos de plantas de uso popular como anti-inflamatório. Rev Bras Farmacogn 12:132–135. https://doi.org/10.1590/S0102-695X2002000300062
Bezerra JL, Costa GC, Lopes TC et al (2006) Avaliação da atividade leishmanicida in vitro de plantas medicinais. Rev Bras Farmacogn 16:631–637. https://doi.org/10.1590/S0102-695X2006000500008
Pinheiro MT, Guedelha NND, Matos AG et al (2010) Efeito do mesocarpo de babaçu no metabolismo de carboidratos em camundongos de diferentes linhagens. Rev Ciênc Saúde 12:11–17
Silva BP, Parente JP (2001) An anti-inflammatory and immunomodulatory polysaccharide from Orbignya phalerata. Fitoterapia 72:887–893. https://doi.org/10.1016/s0367-326x(01)00338-0
Azevedo APS, Farias JC, Costa GC et al (2007) Anti-thrombotic effect of chronic oral treatment with Orbignya phalerata Mart. J Ethnopharmacol 111:155–159. https://doi.org/10.1016/j.jep.2006.11.005
Torres OJM, Santos OJ, Moura RS et al (2018) Activity of Orbignya phalerata and Euterpe edules in the prevention and treatment of peptic ulcer in rats. ABCD Arq Bras Cir Dig 31:e1390. https://doi.org/10.13140/RG.2.2.10672.87041
Scheibe CL, Ribas-Filho JM, Czeczko NG et al (2016) Schinus terebinthifolius raddi (Aroeira) and Orbignya phalerata mart. (Babassu) effect in cecorrahphy healing in rats. Acta Cir Bras 31:402–410. https://doi.org/10.1590/S0102-865020160060000007
Silva CES, Santos OJ, Ribas-Filho JM et al (2015) Effect of Carapa guianensis Aublet (Andiroba) and Orbignya phalerata (Babassu) in colonic healing in rats. Rev Col Bras Cir 42:399–406. https://doi.org/10.1590/0100-69912015006009
Ferreira BS, Almeida CG, Faza LP et al (2011) Comparative properties of Amazonian oils obtained by different extraction methods. Molecules 16:5875–5885. https://doi.org/10.3390/molecules16075875
Barroqueiro ESB, Prado DS, Barcellos PS et al (2016) Immunomodulatory and antimicrobial activity of babassu mesocarp improves the survival in lethal sepsis. Evid Based Complement Altern Med. https://doi.org/10.1155/2016/2859652
Honek J (2017) Preclinical research in drug development. Med Writ 26:5–8
Institute of Medicine (2000) Dietary Reference intakes: applications in dietary assessment. https://doi.org/10.17226/9956. Accessed 1 Nov 2022
Viuda-Martos M, López-Marcos MC, Fernández-López J et al (2010) Role of fiber in cardiovascular diseases: a review. Compr Rev Food Sci Food Saf 9:240–258. https://doi.org/10.1111/j.1541-4337.2009.00102.x
Younes H, Egret N, Hadj-Abdelkader M et al (2006) Fermentable carbohydrate supplementation alters nitrogen excretion in chronic renal failure. J Ren Nutr 16:67–74. https://doi.org/10.1053/j.jrn.2005.10.007
Riccardi G, Rivellese AA, Giacco R (2008) Role of glycemic index and glycemic load in the healthy state, in prediabetes, and in diabetes. Am J Clin Nutr 87:269S-274S. https://doi.org/10.1093/ajcn/87.1.269S
Hemaiswarya S, Kruthiventi AK, Doble M (2008) Synergism between natural products and antibiotics against infectious diseases. Phytomedicine 15:639–652. https://doi.org/10.1016/j.phymed.2008.06.008
Anzaku AA, Akyala AI, Adeola J et al (2017) Antibacterial activity of lauric acid on some selected clinical isolates. Ann Clin Lab Res 5:170. https://doi.org/10.21767/2386-5180.1000170
Arcanjo DDR, Albuquerque ACM, Melo-Neto B et al (2012) Bioactivity evaluation against Artemia salina Leach of medicinal plants used in Brazilian Northeastern folk medicine. Braz J Biol 72:505–509. https://doi.org/10.1590/S1519-69842012000300013
Brasil, Ministério da Saúde. Secretaria de Atenção Primária à Saúde. Prevenção e Controle de Agravos Nutricionais (2022) Deficiência de Iodo. https://aps.saude.gov.br/ape/pcan/iodo. Accessed 24 Oct 2022
Seo SY, Sharma VK, Sharma N (2003) Mushroom tyrosinase: recent prospects. J Agric Food Chem 51:2837–2853. https://doi.org/10.1021/jf020826f
Yang H, Xu P, Song W et al (2021) Anti-tyrosinase and antioxidant activity of proanthocyanidins from Cinnamomum camphora. Int J Food Prop 24:1265–1278. https://doi.org/10.1080/10942912.2021.1958841
Oliveira GLS (2015) Determination in vitro of the antioxidant capacity of natural products by the DPPH• method: review study. Rev Bras Plantas Med 17:36–44. https://doi.org/10.1590/1983-084X/12_165
Moura Filho JM, Nagai LY, Nascimento LCS et al (2017) Determinação do solvente ótimo para extração dos compostos fenólicos do fruto de buriti. Braz J Food Res 8:22–28
Machado AR, Assis LM Silva PP, et al (2010) Influência do solvente na extração de fenóis totais em microalga Spirulina platensis. https://propesp.furg.br/anaismpu/cd2010/pos/574.pdf. Accessed 27 Oct 2022
Zuanazzi JAS, Montanha JA, Zucolotto SM (2017) Flavonoides. In: Simões CMO, Schenkel EP, Mello JCPD. Farmacognosia. Grupo A, Porto Alegre
Godinho JWLS (2017) Estudo de validação de Attalea speciosa Mart. ex. Spreng: aspectos da etnofarmacologia e química. Dissertação (mestrado). Programa de Pós-graduação em Ciências da Saúde—Universidade Federal do Maranhão. https://tedebc.ufma.br/jspui/handle/tede/1766. Accessed 21 Oct 2023
Holanda AC, Freire LS, Alencar GRR et al (2020) Bioacessibilidade dos polifenóis presentes no mesocarpo e na amêndoa do babaçu (Orbignya phalerata Mart.). Braz J Dev 6:19237–19247. https://doi.org/10.34117/bjdv6n4-188
Acknowledgements
João Marcelo de Castro e Sousa (#309109/2022-1) and Paulo Michel Pinheiro Ferreira (#304803/2022-7) are grateful to the public Brazilian agency “Conselho Nacional de Desenvolvimento Científico e Tecnológico” (CNPq) for their personal scholarships.
Funding
This study was partially financed by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brazil (CAPES)—Finance Code 001.
Author information
Authors and Affiliations
Contributions
ND conceived the review idea, conducted the literature review, database searches, data extraction, risk of bias assessment and edited drafts as well as the final version. IOC performed the database searches, data extraction, risk of bias assessment and provided intellectual input into draft versions. ABSS contributed to the review, edited drafts, provided intellectual input into draft versions. VAO edited drafts, provided intellectual input into draft versions. HAN developed the graphic abstract and provided intellectual input into draft versions. PMPF provided intellectual input into draft and final versions. JMCS contributed to the review idea, conducted the risk of bias conflicts, edited drafts and provided intellectual input into draft versions as well as the final version. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. All the co-authors approved this submission.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Debia, N., Castro, I.O., Sousa, A.B.S. et al. Comprehensive preclinical studies on the bioactivity of Orbignya phalerata Mart. (Babassu) and its derived products: a systematic review. Futur J Pharm Sci 10, 11 (2024). https://doi.org/10.1186/s43094-024-00585-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43094-024-00585-6