Abstract
Background
Carivalan® dosage form containing carvedilol and ivabradine is widely indicated for patients with stable angina. Three precise, eco-friendly and reproducible spectrophotometric techniques were created for resolving the researched drugs in their mixtures using zero and/or ratio spectra. Technique I is a factorized dual wavelength coupled with spectrum subtraction (FDW-SS), technique II is a ratio difference, and technique III is a constant center coupled with spectrum subtraction (CC-SS). Moreover, CAR and IVA were simultaneously determined in the existence of their oxidative degradation products exploiting the newly developed induced tripartite amplitude difference coupled with ratio subtraction (ITAD-RS) technique.
Results
The calibration curves for CAR and IVA showed linearity within 3.0–30.0 µg/ml, each. Techniques' precision, accuracy, and linearity ranges were resolved and validated in harmony with ICH guidelines. Additionally, the specificity was examined by examining created combinations of the proposed drugs with LOD of 0.258 and 0.290 for CAR, while for IVA 0.272 and 0.204.
Conclusion
These techniques were used to determine the presence of the provided drugs in Carivalan® tablets. There is statistical comparison between the found results of the offered spectrophotometric techniques and the previously reported ones with no discernible variance in the acquired results.
Similar content being viewed by others
Background
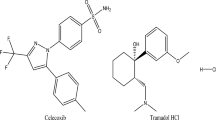
Carvedilol (Fig. 1a) is chemically [1-(9H-carbazol-4-yloxy)-3-[[2-(2methoxyphenoxy) ethyl] amino] propan-2-01]. It is a non-selective beta-blocker which works by reducing heart beats and facilitating smoother blood circulation. Additionally, it widens some blood arteries, acting similarly to an alpha blocker, which in turns helps in lowering the blood pressure [1, 2].
Ivabradine (Fig. 1b) is chemically (3-[3-[[(7S)-3,4-dimethoxy-7-bicyclo[4.2.0]octa-1,3,5-trienyl]methyl-methylamino]propyl]-7,8-dimethoxy-2,5-dihydro-1H-3-benzazepin-4-one). It is a drug that lowers heart rate by precisely and selectively blocking the cardiac pacemaker current; (If) and hence regulates the heart rate via direct sinus node inhibition without affecting myocardial contractility or blood pressure [3, 4].
Carivalan® containing CAR and IVA has received a widespread indication for treating the symptoms of stable angina, where few analytical HPTLC [5] and HPLC [6, 7] techniques for analyzing CAR and IVA have been stated. In the meantime, their simultaneous determination was achieved spectrophotometrically by only one reported research [8], while no spectrophotometric techniques have been previously conveyed for the declared drugs' resolving in the occurrence of their degradation products.
It is well known that the chromatographic analytical technique, which is distinguished by its superior accuracy and reproducibility in the process of samples' separation and analysis in parts per million (PPM) level as well as its speed and automation [9], is one of the most well-liked and frequently used techniques in the field of drug analysis. However, and particularly in light of the current conditions faced by the majority of the world's nations, especially developing nations, many analysts and researchers now turn to the usage of less expensive analytical techniques while keeping accuracy and precision.
The UV spectrophotometric approach is at the forefront of the methodologies that achieve most researchers' objectives because of its ease of use, low implementation cost besides widespread accessibility in laboratories for quality control [10].
This work focuses on the creating and validation of three green UV spectrophotometric techniques for the selective resolution of CAR and IVA in their pure forms and Carivalan® tablets by utilizing zero and/or ratio spectra; FDW-SS, RD [11,12,13] and CC-SS techniques [13,14,15]. Furthermore, induced tripartite amplitude difference (ITAD), a newly developed spectrophotometric technique, was able to solve the issue of resolving and analyzing CAR and IVA in their quaternary mixture with their oxidative degradates and succeeded in determining them without even requiring any preliminary separation steps. Green assessment of the offered, official and the reported techniques was achieved by using Analytical Eco-Scale [16], National Environmental Methods Index (NEMI) [17], Green Analytical Procedure Index (GAPI) [18] and Analytical Greenness Metric (AGREE) [19]. The proposed techniques' suitability for the intended usage has been validated in accordance with ICH criteria [20].
Factorized dual wavelength coupled with spectrum subtraction technique (FDW-SS)
This technique depicts coupling between dual wavelength [21,22,23,24] and spectrum subtraction [24,25,26] for analyzing CAR and IVA in their mixture, where one drug X shows a noteworthy absorbance difference at two selected wavelengths which is directly proportional to its concentrations. While the other interfering drug Y shows equal absorbance reading with an insignificant absorbance difference at the same selected wavelengths (ΔA = zero), thus its effect is negligible. Recovering the parent D0 of X is accomplished by firstly preparing X's factorized ∆A spectrum using computer software via dividing (D0) X at any concentration within Beer's law by the ΔA between the chosen wavelengths; \(\frac{X (D^\circ ) }{\Delta A}\).
Secondly, ∆A is recorded in the laboratory mixtures between the chosen wavelengths then multiplied by X's factorized ∆A spectrum.
While D0 of the interfering drug Y is obtained via spectrum subtraction [25,26,27] technique; D0 (X + Y) − D0 (X).
Induced tripartite amplitude difference coupled with ratio subtraction technique (ITAD-RS)
An advanced technique that can be used on a ratio spectrum of quaternary mixtures made up of \( \left( {\frac{{\left[ {X + Y + Z + W} \right]}}{{W^{\prime } }}} \right) \) at selected wavelengths at which component X has a ΔP noteworthy value and the amplitude readings of Y are not equal (ΔP ≠ 0) at the same chosen wavelengths.
Meanwhile, component Z exhibits an equal amplitude value with a zero difference (ΔP = 0); thus, the effect of Z is canceled. It is a moderation of both induced dual-amplitude difference [27] and dual-amplitude difference [28] techniques.
The effect of Y was canceled via calculating the equality factor [13, 29] of Y by getting average Py1/Py2 at the suggested wavelengths (FY). Component Y will be canceled by calculating the difference. As a result, P (FyPm1–Pm2) is only connected to X.
A factorized induced ratio spectrum of X \(;\frac{X/W^{\prime }}{ \Delta P. Fy}\) is set via division of the ratio spectrum of X by the calculated induced amplitude difference.
For laboratory mixtures, the induced amplitude difference values (\(\Delta\)P. Fy) at the suggested wavelengths are noted and multiplied by X's factorized induced ratio spectrum to acquire X's ratio spectrum.
Multiplying the recovered ratio spectrum of X by the divisor, W', will attain the parent D0 of X; \(\frac{X}{{W}^{\prime }}\times {W}^{\prime }=X ({\text{D}}0)\)
Finally, subtracting the regained D0 of X from the quaternary mixture's D0 spectrum and applying the same previously mentioned steps, the other components in the mixtures will be determined.
Exploiting the factorized induced ratio spectrum and the divisor’s spectrum multiplication in this newly developed method allows the mentioned component's determination at its λmax through minimal signal output analysis of data. In addition, random error is highly minimized since the preparation of factorized spectrum is founded on the response only in contrast to normalized spectrum which is based on concentration.
Methods
Instrumentation
Shimadzu double-beam spectrophotometer (UV, Japan), Sonicator (Model 3510, UK), Analytical Balance, Italy, Digital Stirring Hot Plate, China, Pure laboratory flex to obtain distilled water
Silica gel F254 (20 × 20 cm) pre-coated TLC plates (Sigma-Aldrich, Germany). The degradation tracing and plate visualization were carried out using a UV lamp with a wavelength of 254.0 nm.
Reagents and chemicals
Pure samples of CAR and IVA were kindly obtained from Global Napi, 6th of October, Egypt. The pureness was certified to be 99.88 ± 0.95 and 99.60 ± 1.06, respectively, depending on their official [1] and reported [8] techniques.
The market-available Carivalan® tablet dosage form, made in France by Les Laboratories Servier Industrie, was purchased. According to the product label, Carivalan ® batch number (29,044) contains 12.5 mg of CAR and 5.0 mg of IVA. 30% H2O2 (ADWIC, Egypt).
Standard solutions
To prepare stock solutions of carvedilol and ivabradine (100.0 µg/ml), in volumetric flasks, 100 ml, 10 mg of each of CAR and IVA was dissolved separately in distilled water.
In two rounded flasks, 10 mg of each of CAR and IVA was refluxed with 10 ml of 30% H2O2 for 8 h at 80 °C. After refluxing, hydrogen peroxide was evaporated using hot plate at 50 °C, transfer oxidative degraded product to volumetric flask, 100 ml, which is completed with distilled water to prepare standard solutions of 100.0 µg/ml for each.
Laboratory mixtures
Exact amounts of the stated drugs were conveyed into different volumetric flasks, 10 ml, with varied ratios and topped off with distilled water to produce variable sets as follows:
Set A: Binary mixture containing CAR and IVA.
Set B: Quaternary mixture containing CAR and IVA along with their oxidative degradation products.
Procedures
Stress stability studies
Studied drugs’ stress studies were conducted as per ICH guidelines [30] by applying different stress acidic, basic, photolytic, thermal and oxidative surroundings. Different molar concentrations of HCl and NaOH were tried out where, in each trial, 10 ml of each of the investigated medications was combined individually with either HCl or NaOH. Reflux was applied at 100 °C for 3 h.
To conduct oxidative trials, 10 mg of each medication was refluxed individually in 10 ml of 10, 20 and 30% H2O2 for 8 h at 80 °C.
Drugs’ photostability was tested on their solid powdered form by the exposing them to UV light, 254.0 nm for 10 h.
Finally, drugs’ thermal stress testing was performed in glass ampoules by heating them in a thermostatic oven at 10 °C boosts (50–100 °C) for 10 h.
Spectral characteristics
D0 spectra of each examined drugs were measured at 200.0–400.0 nm.
Construction of calibration graphs
To prepare calibration standards across concentrations of 3.0–30.0 µg/ml for CAR and IVA, from each standard stock solution (100.0 µg/ml), an accurately transferred amounts were conveyed to separate series of volumetric flasks, 10 ml, and finalized with distilled water. The D0 spectra acquired after scanning solutions between 200.0 and 400.0 nm were recorded. The calibration graph for each suggested technique was performed via the mean of three experiments, as shown below:
Factorized dual wavelength coupled with spectrum subtraction technique (FDW-SS)
The regression equations were created by plotting the stored D0 maximum absorbance of CAR and IVA at 285.6 and 287.0 nm, respectively, against the associated concentrations. A factorized ∆A spectrum for IVA was constructed using spectrophotometric software by division of D0 of a certain concentration of IVA by (∆A) at 285.2 nm and 255.0 nm.
Ratio difference technique
The ratio spectra were produced via division of the scanning spectra of the CAR and IVA solutions by the absorption spectra of the IVA (15.0 µg/ml) and CAR (15.0 µg/ml) standard solutions, respectively. CAR's and IVA's concentrations were plotted against their respective amplitude differences at 252.6 and 275.0 nm and 294.0 and 320.0 nm, respectively.
Constant center coupled with spectrum subtraction technique (CC-SS)
The regression equations have been calculated by setting two calibration curves. The first one is between the maximum absorbance of D0 of CAR and IVA at 285.6 nm and 287.0 nm and their related concentrations, respectively. The second one is between the ∆A at 294.0 nm and 320.0 nm versus amplitudes at 294.0 nm for IVA after generating a ratio spectrum by dividing of the stored absorption spectra of IVA by the absorption spectrum of CAR; 15.0 µg/ml.
Induced tripartite amplitude difference coupled with ratio subtraction technique (ITAD-RS)
The regression equations were computed by plotting the maximum absorbance of the stored D0 of CAR and IVA at 285.6 nm and 287.0 nm versus their corresponding concentrations, respectively.
The equality factor of IVA ratio spectra was calculated by using amplitude ratios' average at 296.0 nm (P296.0) and 242.7 nm (P242.7) using CAR's oxidative degradate spectrum as a divisor.
Via dividing the ratio spectrum of a certain concentration of CAR using CAR's oxidative degradate's spectrum as a divisor by ∆P at 242.7 nm and 296.0 nm after multiplying the former by F, (FP242.7–P296.0), factorized induced ratio spectrum for CAR was created.
Through the division of the ratio spectrum of IVA using CAR's oxidative degradate's spectrum as a divisor by the value of the ∆P at 242.7 nm and 296.0 nm, factorized ratio spectrum for IVA was created.
Laboratory mixtures' analysis
Different mixtures in different sets, set A (binary mixture of CAR and IVA) and set B (quaternary mixture of CAR and IVA with their oxidative degradates), were analyzed by carrying out the following manipulation actions for each method:
Analysis of set A containing CAR and IVA in their binary mixtures
Factorized dual wavelength coupled with spectrum subtraction technique (FDW-SS)
The D0 of IVA was obtained by noting ΔA at 285.2 nm and 255.0 nm and multiplying by IVA's factorized ΔA spectrum in the prepared laboratory mixtures of CAR and IVA. CAR's D0 spectrum was resulted from the subtraction of IVA's D0 spectrum from the spectra of laboratory mixture.
Ratio difference technique
The D0 of binary laboratory mixture in set A was divided separately by the absorption spectrum of standard IVA' and CAR' (15.0 µg/ml, each). The amplitudes of the obtained ratio spectra were noted at 252.6 and 275.0 nm for CAR and 294.0 and 320.0 nm for IVA. The concentrations of CAR and IVA were resolved from their related regression equations.
Constant center coupled with spectrum subtraction technique (CC-SS):
Via employing the formerly manipulated ratio spectra in RD method, the following steps were employed: Firstly, IVA's ratio spectrum in each mixture was recorded at 294.0 nm and 320.0 nm using CAR's D0 as a divisor. Secondly, the postulated amplitude at 294.0 was acquired from the correspondent related regression equation followed by subtracting the postulated amplitude from the recorded one to obtain a constant value; CAR/CAR'. Finally, via multiplying the constant value by the spectra of 15.0 µg/ml standard CAR', the parent D0 of CAR was obtained in which its concentration might be determined using the matching regression equation built at its maxima.
As a result, the D0 of IVA could be calculated via subtraction of the acquired spectra of CAR from the spectra of its related mixtures' spectra. The concentration of IVA was estimated using the relevant regression equation.
Analysis of set B containing CAR and IVA with their oxidative degradates in their quaternary mixtures
Induced tripartite amplitude difference coupled with ratio subtraction technique (ITAD-RS)
CAR/CAR's degradate ratio spectrum in mixtures of set B was acquired after multiplication of CAR's factorized induced ratio spectrum by (FP242.7 − P296.0) of each mixture’s ratio spectrum. After multiplication of the former ratio spectrum, CAR/CAR's degradate by the D0 spectrum of the CAR oxidative degradates, CAR's D0 was calculated.
The ratio spectrum comprising, IVA + IVA's degradate + CAR's degradate/CAR's degradate was acquired by subtraction of the obtained ratio spectrum; CAR/CAR's degradate from that of quaternary mixture.
For IVA, its ratio spectrum in the combination can be acquired by multiplication of ΔP at 242.7 nm and 296.0 nm by the formerly set IVA's factorized ratio spectrum. IVA's parent D0 was acquired after multiplication of the acquired ratio spectrum by the spectrum of the CAR oxidative degradate.
CAR and IVA concentrations were figured using their respective regression equations generated at their maxima.
Application to pharmaceutical formulation
Five Carivalan® tablets were crushed and blended together. Amounts equivalent to 25.0 mg CAR and 10.0 mg IVA were precisely moved to beaker, 100 ml, dissolved in 30 ml distilled water, sonicated and filtered into a volumetric flask, 100 ml, then accomplished with distilled water.
Further dilutions were proceeded to produce concentrations of 10.0 µg/ml for CAR and 4.0 µg/ml for IVA. The proposed techniques were used for the analysis of the examined Carivalan® following the described measures under laboratory-prepared mixtures analysis to compute their concentrations utilizing the related regression equation for each proposed drug.
Results
As a result of the current global conditions that most countries are going through either economically or practically, the main movement has become toward using analytical techniques at the lowest costs and in the easiest and most accurate ways at all once.
This was the main reason behind the resort to the use of spectrophotometric technique in drugs' analysis, as it proved accuracy, reliability and simplicity among the rest of the other analytical techniques as well as its availability in most quality control laboratories.
Unfortunately, the presence of extremely overlapped spectra made several challenges to use direct absorbance measurements in zero-order spectra, which led to numerous obstacles during spectrophotometric analysis, particularly for multicomponent mixtures.
Therefore, the primary objective is to concentrate on creating highly advanced eco-friendly spectrophotometric techniques that enable both the researcher and the analyst to explore complex mixtures and to overcome any challenges that may arise.
Carvedilol and ivabradine were chosen as a model for demonstrating the successful application of new spectrophotometric techniques for their analysis, either in their binary or in their quaternary mixture with their oxidative degradation products without the need for pre-separation procedures.
Complete CAR’s and IVA’s oxidative degradation was observed. Quite the reverse, they conveyed relative acidic, basic, photolytic and thermal stability. Samples were frequently taken out to track the drugs' degradation; for the drugs in question, total oxidation was verified by the disappearance of the TLC spot using TLC aluminum plates.
CAR and IVA along with their oxidative degradation products were UV scanned and found to be highly overlapped in the UV region 200.0–340.0 nm, Fig. 2. Direct measurement of the studied drugs was not possible. Thus, two sets were constructed, one for determining CAR and IVA in their binary mixture (set A) exploiting zero or ratio spectra through the application of FDW-SS, RD and CC-SS methods [15], while the other set (set B) was created for the proposed drugs' determination in the presence of their oxidative degradates exploiting the innovative induced tripartite amplitude difference together with ratio subtraction (ITAD-RS) spectrophotometric technique.
Set A: CAR and IVA in their binary mixture:
An overlapped (D0) spectrum was observed for CAR and IVA. Using suitable recommended techniques, simultaneous determination of the considered drugs was performed.
Factorized dual wavelength coupled with spectrum subtraction technique (FDW-SS)
This suggested technique was used to analyze CAR and IVA in their binary mixture via picking two wavelengths (285.2 nm and 255.0 nm) where the ΔA is proportional to IVA concentration versus a zero ΔA for CAR at the selected wavelengths, as shown in Fig. 3a.
Figure 3b shows how the original (D0) of IVA was acquired from each combination after noting (ΔA) value at 285.2 nm and 255.0 nm and multiplying this value by IVA's factorized ΔA spectrum.
Subtraction of IVA's (D0) spectrum from (D0) spectrum of the mixture yielded CAR's parent (D0) spectrum, Fig. 3.c.
Via relieving the recorded absorbance values at the maxima of each of CAR and IVA in their corresponding regression equations, each drug's concentration was calculated, Table 1.
Ratio difference technique
The paramount principle of this technique is that D0 of the mixture is divided by D0 of the interfering element obtaining a ratio spectrum. At this moment, the studied drug has a marked (ΔP) at two wavelengths opposing a canceled contribution of the interfering component for being a constant all over the curve. Thus, the interfering component’s determination is possible.
This proposed technique was utilized to determine CAR and IVA simultaneously in binary set A mixtures. Dividing the mixture’s absorption spectra by the absorption spectrum of IVA' (15.0 µg/ml) was performed to achieve a ratio spectrum. CAR in the mixture was determined using the amplitudes at 252.6 and 275.0 nm, Fig. 4.
Similarly, for the estimation of IVA in the prepared mixture utilizing CAR' (15.0 µg/ml) as a divisor, the amplitudes at 294.0 and 320.0 nm were used, Fig. 5.
The estimated ΔP of the ratio spectra at 252.6 and 275.0 nm and 294.0 and 320.0 nm, respectively, was shown to have a linear relationship with the corresponding concentrations of CAR and IVA, respectively, Table 1.
RD is advantageous over FDW method which requires the specific choice of two wavelengths for the studied drug opposing an equal absorbance value for the other interfering component, while in the RD approach, the wavelength selection is considerably more flexible because the interfering element is a straight line for being a constant.
Constant center coupled with spectrum subtraction technique (CC-SS)
This proposed technique is regarded as a complement to the previously applied RD method, where a ratio spectrum for the binary mixture in set A was obtained with a marked amplitude difference at 294.0 and 320.0 for IVA using CAR' as a divisor. Meanwhile, the practical ratio amplitude of each mixture was noted at 294.0 nm {(IVA/CAR') + (CAR/CAR')}. The supposed ratio amplitude value of (IVA/CAR') was obtained by putting the recorded ratio amplitude in the equation expressing the linear relationship between the ΔP at 294.0 and 320.0 nm with the associated ratio amplitudes at 294.0 nm.
, where P1, P2 are the ratio amplitudes of the ratio spectra of different concentrations of IVA (3.0–30.0 µg/ml) by utilizing CAR; 15.0 µg/ml as a divisor at 294.0 and 320.0 nm.
The constant value (C.V.);CAR/CAR' was figured as the deviation among the measured amplitudes of the mixture's ratio spectra at 294.0 nm and the postulated amplitudes obtained from the established regression equation.; [Precorded] − [Ppostulated].
The recovered CAR's D0 in the mixture, Fig. 3c, was obtained by multiplication of the laboratory mixture's obtained constant (CAR/CAR') by the CAR's divisor.
For IVA, its recovered D0, Fig. 3b, can be calculated by subtraction of the acquired spectra of CAR from the corresponding spectra of their laboratory-generated mixtures.
By substituting the recorded absorbance values at the maxima of each of CAR and IVA in their corresponding regression equations, each drug's concentration was calculated, Table 1
Set B: CAR and IVA in their quaternary mixture with their oxidative degradates:
The determination of CAR and IVA existed with their degradates was not that possible by the commonly used spectrophotometric techniques. Thus, a novel technique, namely, induced tripartite amplitude difference was devised to assign the targeted drugs by overcoming their completely overlapped spectra.
Induced tripartite amplitude difference coupled with ratio subtraction technique (ITAD-RS)
On the ratio spectrum of CAR, two wavelengths, 242.7 nm and 296.0 nm, were chosen using its oxidative degradate as a divisor.
The ratio spectrum of IVA degradate demonstrates equal amplitude values at these specified wavelengths. The ratio spectrum of IVA, on the other hand, revealed un-equalized values. As a result, an equality factor (F) of 2.497 [P296.0/P242.7] was calculated by obtaining the average of ΔP of diverse concentrations of IVA's ratio spectra using CAR degradate's spectrum as a divisor at (296.0 nm and 242.7 nm). This factor prospered to match the amplitudes of IVA at the proposed wavelengths, Fig. 6a.
After calculating the ΔP at 242.7 nm and 296.0 nm along with the equality factor for each set B quaternary mixture, multiplication of the previous value with the previously prepared CAR's factorized induced ratio spectrum resulted in an additional spectrum reflecting the CAR/CAR's degradate ratio spectrum in the mixture, Fig. 6b. By multiplication of the generated ratio spectrum by CAR oxidative degradate's D0 spectrum, CAR’s D0 was successfully obtained, Fig. 6c.
Subtracting the acquired CAR's ratio spectrum from that of the quaternary mixture resulted in a ratio spectrum comprising IVA + IVA's degradate + CAR's degradate/CAR's degradate. The ratio spectrum of IVA/CAR's degradate in the combination was obtained by recording ΔP at 242.7 nm and 296.0 nm and then multiplying by IVA's factorized ratio spectrum, Fig. 6d. The parent D0 of IVA was revealed afterward by multiplication of the acquired ratio spectrum by the spectrum of CAR oxidative degradate, Figure 6e
CAR and IVA concentrations were obtained using the respective regression equations generated at their maxima, Table 1.
Privileges proposed by the developed methods
From the achieved results, it was found that determination of the cited drugs through grabbing the beneficial impact of using spectral factorization gave the proposed methods, FDW-SS and the newly developed ITAD-RS a huge privilege. This was attributed to the great ability of factorized spectrum to recover the parent profile of each drug and their determination at each corresponding maxima. Additionally, manipulation using factorized spectra allows the resolution of the analytes via minimal arithmetic operation and spectrophotometer software's manipulation steps. Moreover, CC-SS method occupied a high capability in analyzing the cited drugs through obtaining their D0 spectra in addition to highest accuracy in their quantification at their λmax. Finally, and through using a spectral divisor, RD method was able to cancel the interfering component in the form of constant, which is a straight line across the curve; as a result, there would not be any crucial measurements at any particular wavelength, and the difference between any two wavelengths will always equal to zero.
Discussion
Method validation
The suggested spectrophotometric techniques were validated in accordance with the ICH guidelines [20]. Accurate linear results were obtained by analyzing 6 concentrations ranging from 3.0 to 30.0 µg/ml of each cited drug conferring to obedience to Beer’s law and their concentration in the pharmaceutical dosage form, Table 1. Accuracy of the results was also checked through analyzing different CAR's and IVA's blind samples where their concentrations were obtained from the corresponding regression equations for the percentage recoveries to be calculated, Table 1. Moreover, three concentration levels were prepared and analyzed in triplicates within and between days for each of CAR and IVA to assess the precision of the employed methods obtaining percentage relative standard deviation (RSD %) not exceeding 2%, Table 1.
Table 2 displays the outcomes of laboratory-prepared mixtures: sets A and B having various ratios of the drugs with or without their oxidative degradates, verifying the specificity of the specified techniques when results were within the calibration range and acceptable. The suggested techniques were additionally successful in distinguishing the drugs contained in Carivalan® tablets. The standard addition approach was used to further evaluate the suggested methods' validity, Table 3.
Statistical analysis
Tables 4 shows a statistical comparison of the found results acquired for the identified drugs using the proposed techniques, official [1] and the reported spectrophotometric technique [8] in pure forms. The computed t and F values were fewer than the theoretical ones, indicating no statistically significant variance in obtained results between the suggested and reported approaches.
Greenness profile assessment
The goal of the green analytical chemistry is to boost the environmental friendliness without compromising the performance of analytical methods by recommending the reduction of hazardous substances' usage. Applying various assessment techniques to compare the proposed methods' levels of greenness allowed for the method's greenness to be evaluated.
Analytical Eco-Scale [31] is a semi-quantitative approach that operates on the method's penalty points (PP) minus 100. National Environmental Methods Index (NEMI) [32] is a circular NEMI pictogram, consisting of four quadrants coded with green if they meet the requirements. Green Analytical Procedure Index (GAPI) [32] is a color-coding representation. The output representation will provide insight into the method's greenness and the list of chemicals that must be verified in this GAPI in accordance with the National Fire Protection Act (NFPA) by applying the necessary data in the 11 quadrants of the GAPI. Analytical Greenness Metric (AGREE) [32, 33] is a software-based quantitative method. The necessary information was entered into the software to obtain the pictogram.
As a result, greenness assessment of the proposed techniques, official [1], and the reported spectrophotometric technique [8] was performed for obtaining a more fruitful and purposeful comparison. The obtained results showed that the proposed spectrophotometric technique is greener than the official and the reported methods as shown in Table 5. Regarding Analytical Eco-Scale, the developed method shows a 97 green Eco-score in comparison with both official and the reported methods where they possess an Eco-score of 79 and 93 for CAR and IVA, respectively.
Additionally, the developed method's greenness assessment profile by applying national environmental method index tool (NEMI) obtained four full green quadrants as the used solvent; water is completely safe regarding persistence, bioaccumulation and corrosiveness, while only 1 and 2 green quadrants for both the official and reported methods of CAR and IVA, respectively, were obtained.
Moreover, method’s complete framework greenness profile was acquired using green analytical procedure index tool (GAPI) where ten green and five yellow zones with a complete absence of any red zones were possessed in comparison with official and reported methods.
Finally, a score value of 0.84 was obtained which is greener than that obtained by official, 0.73, and reported method, 0.76, after applying the analytical greenness metric tool.
Briefly, the usage of distilled water and minimizing instrumental's energy consumption in the proposed methods was reflected by gaining high analytical greenness scores besides having full green NEMI pictogram and a complete absence of any red zones in GAPI pictogram.
Conclusion
UV spectrophotometric techniques have several advantages over other analytical techniques, including their simplicity, low solvent usage and low cost. The suggested spectrophotometric techniques demonstrated great sensitivity and accuracy for determining CAR and IVA in dosage form, laboratory-generated combinations, and in presence of their oxidative degradates with no interference and excellent outcomes. Additionally, the greenness profile was argued green by applying various green assessment tools as analytical eco-scale, national environmental method index, green analytical procedure index and analytical greenness metrics. Therefore, the proposed method is capable of routine analysis of the cited drugs in quality control laboratories.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CAR:
-
Carvedilol
- IVA:
-
Ivabradine
- FDW-SS:
-
Factorized dual wavelength coupled with spectrum subtraction
- RD:
-
Ratio difference technique
- CC-SS:
-
Constant center coupled with spectrum subtraction technique
- ITAD-RS:
-
Induced tripartite amplitude difference coupled with ratio subtraction technique
References
Pharmacopoeia B (2022) British pharmacopoeia commission. London, TSO, UK, pp. 309–310. for Carvedilol, A-290
National Center for Biotechnology Information, (2022). PubChem compound summary for CID 2585, Carvedilol. https://pubchem.ncbi.nlm.nih.gov/compound/Carvedilol
National Center for Biotechnology Information, (2022). PubChem compound summary for CID 132999, Ivabradine. https://pubchem.ncbi.nlm.nih.gov/compound/Ivabradine
Sulfi S, Timmis AD (2006) Ivabradine–the first selective sinus node If channel inhibitor in the treatment of stable angina. Int J Clin Pract 60(2):222–228. https://doi.org/10.1111/j.1742-1241.2006.00817.x
Prajapati P, Naik K, Tailor P, Shah S (2022) Screening design and response surface methodology for the simultaneous estimation of carvedilol and ivabradine HCl by HPTLC method. J Chromatogr Sci 60(9):859–870. https://doi.org/10.1093/chromsci/bmab130
Nadella NP, Ratnakaram VN, Srinivasu N (2018) Development and validation of UPLC method for simultaneous quantification of carvedilol and ivabradine in the presence of degradation products using DoE concept. J Liq Chromatogr Relat 41(3):143–153. https://doi.org/10.1080/10826076.2018.1427595
Abbas SS, Al-Ani IH, Dayyih WAA, Oriquat G, Hassan SF (2018) Investigation of possible pharmacokinetic interaction between ivabradine and carvedilol in rats using high performance liquid chromatography/mass spectroscopy. Biomed Pharmacol J. 11(1):311–324. https://doi.org/10.13005/bpj/1375
Chanduluru H, Sugumaran A (2022) Three spectrophotometric approaches for measuring ratio spectra of ivabradine and carvedilol in a binary mixture using green analytical principles. Curr Chem Lett 11(3):321–330. https://doi.org/10.5267/j.ccl.2022.3.002
Fares NV, El Fiky HA, Badawey AM, Abd El Ghany MF (2022) Ultra-performance liquid chromatographic and densitometric methods for sensitive determination of xipamide and triamterene in pure and pharmaceutical dosage forms. J AOAC Int 105(1):19–25. https://doi.org/10.1093/jaoacint/qsab110
Victor N, Elfiky H, Badawey A, Abdelghany M (2021) Simultaneous determination of xipamide and triamterene by first derivative, ratio difference, and derivative ratio spectrophotometric methods. APS ASU. 5(1):52–62. https://doi.org/10.21608/APS.2021.58496.1053
Lotfy HM, Hegazy MAM (2013) Simultaneous determination of some cholesterol-lowering drugs in their binary mixture by novel spectrophotometric methods. Spectrochim Acta A Mol 113:107–114. https://doi.org/10.1016/j.saa.2013.04.064
Lotfy HM, Hagazy MAM (2012) Comparative study of novel spectrophotometric methods manipulating ratio spectra: an application on pharmaceutical ternary mixture of omeprazole, tinidazole and clarithromycin. Spectrochim Acta A Mol 96:259–270. https://doi.org/10.1016/j.saa.2012.04.095
Lotfy HM, Ahmed DA, Rahman MKA, Weshahy SAF (2019) Study of efficiency and spectral resolution for mathematical filtration technique using novel unlimited derivative ratio and classical univariate spectrophotometric methods for the multicomponent determination-stability analysis. Heliyon. https://doi.org/10.1016/j.heliyon.2019.e01669
Lotfy HM (2012) Determination of simvastatin and ezatimibe in combined tablet dosage forms by constant center spectrophotometric method. Int J Pharm Pharm Sci 4(4):673–679
Lotfy HM, Ahmed DA, Abd El-Rahman MK, Weshahy SA (2019) Smart spectral processing of data for the estimation of commonly used over-the-counter (OTC) Co-formulated drug; pseudoephedrine hydrochloride and ibuprofen. Spectrochim Acta A Mol 223:117322. https://doi.org/10.1016/j.saa.2019.117322
Gałuszka A, Migaszewski ZM, Konieczka P, Namieśnik J (2012) Analytical Eco-Scale for assessing the greenness of analytical procedures. TrAC Trends Anal Chem 37:61–72. https://doi.org/10.1016/j.trac.2012.03.013
Keith LH, Gron LU, Young JL (2007) Green analytical methodologies. Chem Rev 107(6):2695–2708. https://doi.org/10.1021/cr068359e
Płotka-Wasylka J (2018) A new tool for the evaluation of the analytical procedure: green analytical procedure index. Talanta 181:204–209. https://doi.org/10.1016/j.talanta.2018.01.013
Pena-Pereira F, Wojnowski W, Tobiszewski M (2020) AGREE—Analytical GREEnness metric approach and software. Anal Chem 92(14):10076–10082. https://doi.org/10.1021/acs.analchem.0c01887
Validation of analytical procedures Q28, (2003). International conference on harmonization, I., Geneva
Lotfy HM, Hegazy MA, Rezk MR, Omran YR (2015) Comparative study of novel versus conventional two-wavelength spectrophotometric methods for analysis of spectrally overlapping binary mixture. Spectrochim Acta A Mol 148:328–337. https://doi.org/10.1016/j.saa.2015.04.004
Lotfy HM, Saleh SS, Hassan NY, Salem H (2015) Novel two wavelength spectrophotometric methods for simultaneous determination of binary mixtures with severely overlapping spectra. Spectrochim Acta A Mol 136:1786–1796. https://doi.org/10.1016/j.saa.2014.10.084
Mohamed EH, Lotfy HM, Hegazy MA, Mowaka S (2017) Different applications of isosbestic points, normalized spectra and dual wavelength as powerful tools for resolution of multicomponent mixtures with severely overlapping spectra. Chem Cent J 11(1):1–15
Lotfy HM, Hegazy MA, Mowaka S, Mohamed EH (2016) Validated spectrophotometric methods for simultaneous determination of omeprazole, tinidazole and doxycycline in their ternary mixture. Spectrochim Acta A Mol 153:321–332. https://doi.org/10.1016/j.saa.2015.07.106
Rostom Y, Wadie M, Rezk MR, Marzouk HM, Abdel-Moety EM (2022) Fingerprinting and iso-absorptive resolution techniques for the spectrally overlapping dutasteride and silodosin mixture: content uniformity testing along with greenness profile assessment. Spectrochim Acta A Mol 273:121063. https://doi.org/10.1016/j.saa.2022.121063
Lotfy HM, Omran YR (2018) Novel absorptivity centering method utilizing normalized and factorized spectra for analysis of mixtures with overlapping spectra in different matrices using built-in spectrophotometer software. Spectrochim Acta A Mol 200:167–178. https://doi.org/10.1016/j.saa.2018.04.004
Ahmed DA, Lotfy HM (2022) Sticking-pulling strategy for assessment of combined medicine for management of tough symptoms in COVID-19 pandemic using different windows of spectrophotometric platform-counterfeit products’ detection. Spectrochim Acta A Mol 277:121256. https://doi.org/10.1016/j.saa.2022.121256
Ahmed DA, Lotfy HM (2021) Evaluation of in silico and in lab sample enrichment techniques for the assessment of challengeable quaternary combination in critical ratio. Spectrochim Acta A Mol 260:119943. https://doi.org/10.1016/j.saa.2021.119943
Lotfy HM, Mohamed EH (2020) Induced mathematical filtration as an innovative strategy for discrimination and estimation of glycemic control drugs in fixed dose combination. Heliyon. https://doi.org/10.1016/j.heliyon.2020.e05289
Guideline I (2003) Stability testing of new drug substances and products. Q1A (R2), current step, 4(1–24)
Soliman RM, Rostom Y, Mahmoud AM, Fayez YM, Mostafa NM, Monir HH (2023) Novel fabricated potentiometric sensors for selective determination of carbinoxamine with different greenness evaluation perspectives. Microchem J 187:108381. https://doi.org/10.1016/j.microc.2022.108381
Hussein OG, Ahmed DA, Rezk MR, Abdelkawy M, Rostom Y (2023) Exquisite integration of quality-by-design and green analytical approaches for simultaneous determination of xylometazoline and antazoline in eye drops and rabbit aqueous humor, application to stability study. J Pharm Biomed Anal 235:115598. https://doi.org/10.1016/j.jpba.2023.115598
Hussein OG, Rostom Y, Abdelkawy M, Rezk MR, Ahmed DA (2023) Spectrophotometric platform windows’ exploitation for the green determination of alcaftadine in presence of its oxidative degradation product. Spectrochim Acta A Mol 297:122737. https://doi.org/10.1016/j.saa.2023.122737
Acknowledgements
The authors are grateful for Global Napi, 6th of October, Egypt, for providing the reference standards.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
HAEF helped in conceptualization, methodology, software data curation, software, validation, writing—original draft preparation, writing—reviewing and editing. DAA was involved in conceptualization, methodology, software data curation, software, validation, writing—reviewing and editing. NVF, AMB and MFAEG contributed to validation, supervision, writing—reviewing and editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not concerned.
Consent for publication
We certify this manuscript has not been published elsewhere and is not submitted to another journal. All authors have approved the manuscript and agreed to submit it to Future Journal of Pharmaceutical Sciences.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El Fiky, H.A., Abd El Ghany, M.F., Badawey, A.M. et al. A unique revolutionary eco-friendly spectrophotometric technique for solving the spectral overlap in the determination of carvedilol and ivabradine in their binary combination: stability study. Futur J Pharm Sci 10, 8 (2024). https://doi.org/10.1186/s43094-024-00582-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43094-024-00582-9