Abstract
Background
The most commonly occurring mechanism driving ischemic heart disease, ischemic stroke, and myocardial infarction is thrombosis. It is normally characterized by platelet activation and aggregation. Thrombolytics have been used in the treatment of several forms of thrombosis, but their adverse effects have limited their usefulness. Thus, there is a need to develop alternatives from medicinal plants known to possess antithrombotic activity such as Costus afer.
Results
The phytochemical evaluations indicated the presence of flavonoids, alkaloids, cardiac glycosides, tannins, terpenoids, and saponins. The antithrombotic profiling showed that streptokinase had the highest percentage clot lysis, followed by ethylacetate fraction of the extract, which was higher than aspirin and other fractions of the extract.
Conclusion
The present findings show that C. afer stem extract and various fractions possess antithrombotic activities. However, further studies are needed to characterize the antithrombotic bioactive compounds present in the different fractions that are responsible for the activities.
Similar content being viewed by others
Background
Cardiovascular diseases are described as range of conditions that affect the heart. Coronary artery disease, heart rhythm problems, and heart defects are all included in the category of cardiovascular diseases [1]. Heart attacks, coronary artery disease, and stroke could all be caused by restricted or clogged blood vessels in the cardiovascular system. Risk factors associated with cardiovascular diseases include age, sex, ethnicity, diet, physical inactivity, obesity, diabetes, dyslipidemia, smoking, and genetic predisposition [2, 3].
The most common pathogenic cause behind ischemic heart disease and ischemic stroke is thrombosis [4]. Thrombosis occurs when blood vessels become blocked because of excessive blood clotting or defective anti-clotting factor function [5]. Several variables, including significant activation of thrombin proteinases and activated factor X, cause excessive blood clot formation. According to Choi et al. [5] inhibition of proteinases like thrombin and activated factor X, as well as direct suppression of fibrin and blood clot formation, may be used to treat thrombosis. Platelet activation and aggregation are typical features of thrombogenesis. The expression of cell surface receptors is induced by platelet activation. adhesion, and small molecules which affect the leukocytes, endothelial cells, and other vascular cells [6]. Platelet granules contain adhesive proteins, coagulation factors, mitogenic and angiogenic factors, CXC and CC chemokines, and other factors including platelet factor 4, PF4/CXCL4 that may influence neutrophils and macrophages, contributing to atherosclerosis [6].
Several cardioprotective agents play essential role in inhibiting or reducing the development of coronary artery diseases. Some examples of these cardioprotective agents are, heparin, fondaprinux, rivaroxaban, streptokinase, aspirin [7,8,9]. However, the usefulness of some of these agents has been limited due to their adverse effects [8].
Costus afer is a tropical perennial medicinal plant with an unbranched creeping rhizome that grows in wet or shaded forests and along riverbanks. C. afer can be found throughout Africa's forest belt from Senegal to Ethiopia, in the East to Tanzania, Malawi, and Angola, and in the South and West to South Africa and West Africa. In Nigeria, Ghana, Togo, and Cameroon, it is a common plant [10, 11]. Costus is a pantropical genus of around 70 species, 40 of which are found in tropical America, 25 in tropical Africa, and 5 in Southeast Asia [10]. C. afer stem extract is effective in the treatment of cough, inflammation, diabetes, arthritis, and rheumatism. Its decoction has also been used as a laxative, purgative, and diuretic in the past. Due to the presence of bioactive substances such polyphenolics, saponin, alkaloids, and glycosides, it is widely known for its antioxidant, anti-inflammatory, and hepatoprotective properties [10, 12].
Methods
Ethical approval
The Ethical Committee of the Faculty of Biological Sciences, University of Nigeria, Nsukka, approved the study protocol (Approval number: UNN/FBS/EC/1036; date: 27th October 2020). Prior to the collection of blood samples, each study participant filled and signed a written consent form.
Drugs
The drugs used in the study were of proven quality. The aspirin used was a product of Juhel Nigeria limited while streptokinase was a product of Abbott laboratories, United States of America.
Plant material
Mr. Alfred Ozioko of the Bioresources Development and Conservation Program, Nsukka, Enugu State, Nigeria, identified the plant sample, fresh C. afer stem obtained from Ozom Mgbagbu-Owa, Ezeagu Local Government of Enugu State.
Preparation of plant material
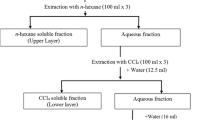
The method described by Njoku et al. [13] was used for the extraction of the plant material. For extraction, the stem was washed with distilled water, air-dried at room temperature, and powdered. The powder (1000 g) was macerated for 48 h at room temperature in 2.5 L of ethanol (80%). The mixture was filtered through Whatman No. 1 filter paper, and the filtrate was concentrated using a rotary evaporator to produce a semi-solid chocolate extract.
Fractionation of the plant extract
The method described by Njoku et al. [14] was used for the fractionation of the plant extract. n-hexane, ethylacetate, and 20% aqueous-ethanol (v/v) were used to fractionate the samples. To make a stock solution, 20 g of crude extract was weighed and diluted in 250 ml of 20% aqueous-ethanol (v/v). The solution was then put onto a separating funnel with 250 ml of n-hexane added to it. The mixture was allowed to stand for 20 min before the upper component was collected in a beaker for appropriate separation. After washing the aqueous-ethanol component with n-hexane many times, the various n-hexane fractions were recovered. Using ethyl acetate, the operation was repeated. Different fractions were collected and concentrated in the end. The different fractions were used for further studies.
Qualitative phytochemical screening of the plant fractions
The qualitative and quantitative phytochemical profiling of the crude plant extract and different fractions were done using the methods of Harbone [15], and Trease and Evans [16], and Soni and Sosa [17].
Blood sample collection
Healthy human volunteers (n = 18) with no history of oral contraceptive or anticoagulant medication had their blood samples taken in an aseptic environment. To create clots, 5 mL of blood was weighed into micro-centrifuge tubes.
Determination of thrombolytic activity
The method described by Ramjan et al. [18]. Each volunteer's venous blood (5 mL) was drawn and placed in one of eighteen pre-weighed sterile micro-centrifuge tubes, which were then incubated at 37 °C for 45 min. After the clot had formed, the fluid was entirely discharged from each micro-centrifuge tube, and the clot weight was calculated by subtracting the weight of the clot-containing tube from the weight of the tube alone. 100 µl of streptokinase (SK) and aspirin were used as a positive control; 100 µl of distilled water was used as a negative non-thrombolytic control; and 100 µl of each sample was separately put into the micro-centrifuge tubes. After that, all the tubes were incubated at 37 °C for 90 min to check for clot lysis. The discharged fluid was discarded after incubation, and the tubes were weighed again to see if there was a difference in weight after the clot was disrupted. The following formula was used to calculate the percentage of clot lysis:
Statistical analysis
Statistical Product and Service Solution (SPSS 15.0) version was used for the statistical analysis. A one-way analysis of variance (ANOVA) was used to assess statistical differences, and Duncan's Multiple Range Test was used to establish significant differences between the mean values of the different samples at P < 0.05.
Results
Phytochemical profiling of C. afer stem crude extract and fractions
The phytochemical profiling of the different fractions of C. afer stem ethanol extract revealed the presence of different phytoconstituents such as flavonoids, alkaloids, terpenoids, glycosides, saponins, and tannins as shown in Table 1.
Antithrombotic profiling C. afer stem crude extract and fractions
The antithrombotic activity profiling of the different samples showed that the standard drug, streptokinase exerted the highest thrombolytic effect, followed by the crude extract and ethylacetate fraction, respectively. However, the percentage clot lysis of aspirin, a known anti-inflammatory drug is lower than those of the crude extract and ethylacetate fraction.
Discussion
Thrombolytic agents such as anticoagulants are essential in the prevention and management of thromboembolic disorders [19]. Thus, plant-derived thrombolytic compounds are attractive and have become a field of research.
Phytochemicals have continued to be a major source of drugs for pharmaceuticals due to their several medicinal effects. The qualitative phytochemical analysis of the C. afer stem extract showed the presence of flavonoids, alkaloids, saponins, flavonoids, tannins, glycosides, and terpenoids as presented in Table 1. This is consistent with the results obtained by Ezejiofor et al. [20], on the ethanolic leaf extract of C. afer. Alkaloid such as ambinine is known to possess antithrombotic and anticoagulant activities, while tannins are known to inhibit platelet activation and thrombus formation [21, 22]. Flavonoids and saponins are known for their antioxidant properties which play a role in preventing oxidative damage of the cell. Therapeutic potentials of antioxidants in controlling degenerative diseases with marked oxidative damage from reactive oxygen species or free radicals could be ameliorated by flavonoids [23].
Thrombolytic activity profiling of C. afer stem crude extract and different fractions showed a significant clot lysis activity when compared to the control as presented in Table 2, thus indicating that C. afer is a potential cardioprotective and thrombolytic agent. Among the fractions of C. afer stem evaluated, ethyl acetate exerted the highest thrombolytic activity followed by n-hexane fraction, while hydro-ethanol fraction (20% v/v) showed the least activity. Also, the percentage clot lysis of C. afer stem ethylacatate fraction was significantly higher than that of aspirin. The different thrombolytic activities could be attributed to the presence of different phytoconstituents in the plant fractions. Some flavonoids are known to prevent the formation of thrombus by inhibiting platelet aggregation [24]. In addition, alkaloids have been described to exert antithrombotic effect by significantly degrading blood clot and delaying the plasma recalcification time [21]. According to Ezejiofor et al. [20], C. afer leaves extract caused a dose dependent increase in blood clotting time in albino mice, with no substantial effect on the hemoglobin, packed cell volume, and red blood cell components while there was a decrease in the platelet count, white blood cell, neutrophils, and lymphocytes. The decrease in platelet component of the blood of albino mice could be responsible for the increase in clotting time observed leading to delayed platelet clot formation.
Normally, blood clot formation results from the cyclooxygenase pathway. Prostaglandins are associated with localized pain, inflammation and thus, promote blood clotting by activating platelets [24]. Cyclooxygenase (COX 1 and 2) act on arachidonate and converts it to prostaglandin H2 (PGH2), which is the precursor of many other prostaglandins and thromboxane [24]. On the other hand, thromboxane synthase converts PGH2 to thromboxane A2, which is the source of other thromboxane which cause the constriction of blood vessels and platelet aggregation [25]. However, flavonoids such as quercetin and myricetin have been reported to inhibit platelet aggregation as well as thromboxane [26]. Thus, the presence of these polyphenolic compounds as well as other phytocompounds could be responsible for the observed antithrombotic effects of C. afer extract and different organic solvent fractions.
Conclusions
The present findings show that C. afer stem extract and various fractions possess antithrombotic activities. These antithrombotic effects could be attributed to the phytoconstituents present in the stem extract and fractions. However, further studies are needed to characterize the antithrombotic bioactive compounds present in the different fractions that are responsible for the activities.
Availability of data and materials
All data and material are available upon request.
Abbreviations
- COX:
-
Cyclooxygenase
- PGH2:
-
Prostaglandin H2
- cAMP:
-
Cyclic adenosine monophosphate
- SK:
-
Streptokinase
- SD:
-
Standard deviation
References
World Health Organization (2021) Cardiovascular diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds). Accessed on 10th May 2021
Mina TK, Mahrous A (2018) Drug induced cardiotoxicity: mechanism, prevention, and management. https://doi.org/10.5772/intechopen.79611. Accessed on 10th May 2021
Lipshultz SE, Cochran TR, Franco VI, Miller TL (2013) Treatment related cardiotoxicity in survivors of childhood cancer. Nat Rev Clin Oncol 10(12):697–710. https://doi.org/10.1038/nrclinonc.2013.195
Raskob GE, Angchaisuksiri P, Blanco AN, Buller H, Gallus A, Hunt BJ, Hylek EM, Kakkar A, Konstantinides SV, McCumber M, Ozaki Y, Wendelboe A, Weitz JI (2014) Thrombosis: a major contributor to global disease burden. Arterioscler Thromb Vasc Biol 34(11):2363–2371. https://doi.org/10.1161/ATVBAHA.114.304488
Choi JH, Park SE, Kim SJ, Kim S (2015) Kaempferol inhibits thrombosis and platelet activation. Biochimie 115:177–186. https://doi.org/10.1016/j.biochi.2015.06.001
Nagareddy P, Smyth SS (2013) Inflammation and thrombosis in cardiovascular disease. Curr Opin Hematol 20(5):457–463. https://doi.org/10.1097/MOH.0b013e328364219d
Tomaru T, Kawano H, Tsujiuchi Y, Suzuki J, Nakajima T, Uchida Y (2005) Mechanism of antithrombotic effect of heparin and antithrombin in balloon-injured arteries. Life Sci 77(21):2611–2625
Papathanasopoulos A, Kouroupis D, Henshaw K, McGonagle D, Jones EA, Giannoudis PV (2011) Effects of antithrombotic drugs fondaparinux and tinzaparin on in vitro proliferation and osteogenic and chondrogenic differentiation of bone-derived mesenchymal stem cells. J Orthop Res 29(9):1327–1335
Sugihara H, Idemoto Y, Kuwano T, Nagata Y, Morii J, Sugihara M, Ogawa M, Miura SI, Saku K (2016) Evaluation of the antithrombotic effects of Rivaroxaban and Apixaban using the total thrombus-formation analysis system®: In vitro and ex vivo studies. J Clin Med Res 8(12):899–907
Njoku UO, Nwodo OFC, Ogugofor MO (2017) Cardioprotective potential of methanol extract of Costus afer leaf on carbon tetrachloride-induced cardiotoxicity in albino rats. Asian J Pharm Res Health Care 9(2):51–58. https://doi.org/10.18311/ajprhc/2017/8363
Aweke G (2007) Costus afer (Ker Grawl) In: Schmelzer GH, Guribraukin A (eds) Plant resources of TROPICAL Africa (PROTA) Wageningen, Netherlands
Omokhua GE (2011) Medicinal and socio-cultural importance of Costus Afer (Ker Grawl) in Nigeria. Afr Res Rev 5(5):282–287. https://doi.org/10.4314/afrrev.v5i5.22
Njoku UO, Ogugofor MO (2021) Antidiarrheal effect of methanol leaf fraction of Cola hispida Brenan and Keay in rats. Trop J Pharm Res 20(7):1455–1462
Njoku UO, Ogugofor MO (2021) Glyphaea brevis (Spreng) Monachino (Tiliaceae) leaf fractions protect against pentylenetetrazole (PTZ)-induced convulsion in mice. Trop J Pharm Res 20(6):1199–1204
Harborne JB (1973) Phytochemical methods: a guide to modern techniques of plant analysis. Chapman and Hall Ltd, London, p 279
Trease GE, Evans WC (2002) Pharmacognosy. 15th Edn. Saunder Publishers, London, pp 42 - 44, 221 -229, 246 – 249, 404 -306, 331–332, 391–393.
Soni A, Sosa S (2013) Phytochemical analysis and free radical scavenging potential of herbal and medicinal plant extracts. J Pharmacogn Phytochem 2(4):22–24. Corpus ID: 41059142
Ramjan A, Hossain M, Runa JF, Md H, Mahmodul I (2014) Evaluation of thrombolytic potential of three medicinal plants available in Bangladesh, as a potent source of thrombolytic compounds. Avicenna J Phytomed 4(6):430–436
Vedantham S, Piazza G, Sista AK, Goldenberg NA (2016) Guidance for the use of thrombolytic therapy for the treatment of venous thromboembolism. J Thromb Thrombol 41(1):68–80. https://doi.org/10.1007/s11239-015-1318-z
Ezejiofor AN, Igweze Z, Amadi CN (2017) Evaluations of some biological properties of ethanolic leave extract of Costus afer (ker gawl)”. IOSR J Pharm Biol Sci 12(1):62–68. https://doi.org/10.9790/3008-1201026268
Chang S, Yang Z, Han N, Liu Z, Yin J (2018) The antithrombotic, anticoagulant activity and toxicity research of ambinine, an alkaloid from the tuber of Corydalis ambigua var amurensis. Regul Toxicol Pharmacol. https://doi.org/10.1016/j.yrtph.2018.03.004
Zhu L (2018) Tannic acid inhibits protein disulfide isomerase, platelet activation and thrombus formation. Blood 132(supplement 1):2418. https://doi.org/10.1182/blood-2018-99-114418
Anyasor GN, Ogunwenmo KO, Olatunji AO, Blessing EA (2010) Phytochemical, proximate and mineral element composition of stem of Costus afer (Bush cane). Asian J. Plant Sci. 2(5):607–612
Bojić M, Maleš Z, Antolić A, Babić I, Tomičić N (2019) Antithrombotic activity of flavonoids and polyphenols rich plant species. Acta Pharm 69:483–495. https://doi.org/10.2478/acph-2019-0050
Ricciotti E, FitzGerald GA (2011) Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol 31(5):986–1000. https://doi.org/10.1161/ATVBAHA.110.207449
Rucker D, Dhamoon AS (2020) Physiology, Thromboxane A2. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK539817/ Accessed on 20th May 2021
Lescano CH, de Lima FF, Mendes-Silvério CB, Justo AFO, Baldivia DS, Vieira CP, Sanjinez-Argandoña EJ, Cardoso CAL, Mónica FZ, de Oliveira IP (2018) Effect of polyphenols from Campomanesia adamantium on platelet aggregation and inhibition of cyclooxygenases: Molecular docking and in vitro analysis. Front Pharmacol 9:617. https://doi.org/10.3389/fphar.2018.00617
Acknowledgements
The authors appreciate Mr. Alfred Ozioko of Bioresource Development and Conservation Program, Nsukka Enugu state Nigeria for authenticating the plant sample.
Plant authentication
Mr. Alfred Ozioko of the Bioresources Development and Conservation Program, Nsukka, Enugu State, Nigeria, identified the plant sample, fresh Costus afer stem obtained from Ozom Mgbagbu-Owa, Ezeagu Local Government of Enugu State.
Funding
No funding received.
Author information
Authors and Affiliations
Contributions
Conceptualization, MOO, UON, and OUN.; Methodology, MOO, UON, OUN, and GE-SB; Formal Analysis, MOO, UON; Investigation, MOO, UON, OUN; Resources, UON, OUN, and GE-SB; Data Curation, MOO and UON; Writing—Original Draft Preparation, MOO and UON; Writing—Review & Editing, OUN and GE-SB; Supervision, OUN and UON. All authors have read and approved that the manuscript should be published.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted according to the guidelines of the Declaration of HELSINKI and approved by the Ethics and Biosafety Committee of Faculty of Biological Sciences, University of Nigeria Nsukka, Enugu Nigeria (Approval number: UNN/FBS/EC/1036; date: 27th October 2020).” Informed consent was obtained from all the study participants. They filled and signed a consent form before the collection of the blood sample.
Consent for publication
Not applicable.
Competing interests
The authors have declared no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ogugofor, M.O., Njoku, U.O., Njoku, O.U. et al. Phytochemical analysis and thrombolytic profiling of Costus afer stem fractions. Futur J Pharm Sci 8, 4 (2022). https://doi.org/10.1186/s43094-021-00392-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43094-021-00392-3