Abstract
The leaves and bark of Syringa oblata Lindl are used as folk medicine which has heat-clearing, detoxifying, dampness-removing and jaundice-relieving effects. There are many studies about leaves of S. oblata because of its abundant resource, however, less reports about the components of S. oblata flowers. The previous studies on S. oblate flowers were mainly focused on the volatile components and its traditional pharmacological activity. Thus, this study aimed to investigate the nonvolatile chemical constituents and the coagulation activity of S. oblate flowers. The chemical constituents of S. oblate flowers were isolated with various column chromatographies and coagulation activity of the major constituents was investigated by assaying the activated partial thromboplastin time (APTT), prothrombin time (PT), thrombin time (TT) and fibrinogen (FIB) on plasma of rabbit in vitro. Fifteen known compounds (namely compound 1-15) were isolated from S. oblata flowers. Compound 6, 10, 11 and 14 were isolated from Syringa genus for the first time. Compound 1, 2, 4, 5, 8 and 9 were isolated from the plant for the first time. The results of coagulation activity showed that water part of S. oblate flowers, lauric acid and kaempferol-rutinose significantly shorten PT (P < 0.001), TT (P < 0.001) and APTT (P < 0.001) compared with blank group, thus revealed that water extract of S. oblate flowers, lauric acid and kaempferol-rutinose possessed the procoagulant activity, but the effects were not better than that of Yunnan Baiyao as positive control.
Similar content being viewed by others
Introduction
Syringa oblata Lindl, a medicinal plant which has the characteristics of trees or shrubs of the Oleaceae family, is native to north China. S. oblate tastes bitter, and has quality of cold. Chinese Materia Medica records that the leaves and bark of S. oblata have been used as folk medicine, which have heat-clearing, detoxifying, dampness -removing and jaundice-relieving effect [1].
Many studies were reported on the chemical constituents of S. oblate in China. Zhang et al. [2,3,4,5,6] isolated more than 50 compounds from the twigs, bark, leaves, alabastrum, seeds, and seed crust of S. oblate. These compounds were identified as oleanolic acid, lupinic acid, lupeol, 4-hydroxyphenethyl alcohol, 3,4-dihydroxyphenylethanol, p-hydroxyphenylethanol acetate, 2-(3,4-dihydroxy) phenyl ethyl acetate, p-hydroxyphenylethyl propyl ester, (8E)-ligstroside, oleuropein, syringopicroside, lariciresinol and esculetin, respectively. Tian et al. [7] isolated 9 compounds, including (+) pinoresinol-4″-O-β-d-glucopyranoside, (+)lariciresinol-4-O-β-d-glucoside, and epipinoresinol-4-O-β-d-glucopyranoside, from the leaves of S. oblate. Zhou [8] reported 2-furancarboxylic, mannitol, cyclohexane-1,2,3,4,5,6- hexaol, succinic acid, p-hydroxyphenylethyl alcohol and formononetin isolated from the leaves of S. oblate. Yang et al. [9] analyzed the compositions in the essential oil from fruits and leaves of S. oblate. Jiao et al. [10] found that the main component of the dried flowers of S. oblate were the same as those in the fruits and leaves.
These references indicate that triterpenes, phenethyl alcohol, phenylpropanoid and iridoid compounds are the main components in S. oblate. However, the flavonoids, organic acids and other constituents have been less reported.
In view of the fact that the S. oblate has a wide range of biological activities, including antibacterial, anti-inflammatory, antiviral, anti-tussive and expectorant effect, liver protection and cholagogue etc. [11]. The previous studies on S. oblate flowers were mainly focused on the volatile components and its traditional pharmacological activity. Thus, this study aimed to investigate the nonvolatile chemical constituents and the coagulation activity of S. oblate flowers.
Methods
Chemicals and material
The chemicals and material were similar to our previous research [12].
Plant material
Syringa oblata flowers were collected in April 2015 from the Kaifeng region of Henan Province, China and identified by Professor Changqin Li. A voucher specimen was deposited in National R & D Center for Edible Fungus Processing Technology, Henan University.
Animal
The male rabbit (approximately 20 months old, weight from 2.0 to 2.5 kg) was provided by Kaifeng Key Laboratory of Functional Components in Health Food (2016-02) to evaluate anticoagulant effect in vitro.
Ethics information
The study obtained ethical clearance from the Ethics Committee of College of Medical, Henan University (NO: 2016-36). The rabbits were treated as per the guidelines on the care and use of animals for scientific purposes.
Extraction and isolation
The extracted method was similar to our previous research [12]. The air-dried flowers of S. oblata (1.4 kg) were extracted with 70% ethanol to yield the crude extract (So. TE 378 g). The extract (378 g) was dissolved in MeOH-H2O (v:v = 3:1, 500 mL), and then mixed with D101 macroporous adsorbent resin. TE was separated by macroporous resin column chromatography, eluted with 20%, 40%, 60%, and 90% ethanol. After evaporation of the solvent, 235 g of water part, 27 g of 20% ethanol part, 61 g of 40% ethanol part, 20 g of 60% ethanol part and 35 g of 90% ethanol part were obtained.
The 60% ethanol part was separated on a silica gel H column by medium pressure liquid chromatography (MPLC), eluted with dichloromethane-methanol (v:v = 1:0–2:1) to obtain 2 fractions (F1–F2) based on TLC analyses. F1 was separated on a silica gel H column by MPLC, eluted with dichloromethane-acetone (v:v = 1:0–0:1) and then separated by Sephadex LH-20 (methanol) to obtain compound 1 (3 mg). F2 was separated with Sephadex LH-20 (methanol) and Sephadex LH-20 (methanol/water, 3:1, v/v) to obtain compound 2 (18 mg).
40% ethanol part was separated on a silica gel H column by MPLC, eluted with dichloromethane-methanol (v:v = 50:1–1:1) to obtain 6 fractions (P1–P7) based on TLC analyses. P1 was separated with Sephadex LH-20 (dichloromethane/methanol, 1:1, v/v) and Sephadex LH-20 (methanol) to obtain P1-a and P1-b. F1-a was subjected to atmospheric pressure chromatographic column of silica gel H with CHCl2-acetone (v:v = 1:0–0:1) to obtain compound 3 (20 mg). Compound 4 (16 mg) was obtained by the same separation method from F1-b. P2 was subjected to ordinary pressure chromatographic columns of silica gel H with dichloromethane-methanol (v:v = 80:1–15:1), and then separated with Sephadex LH-20 (methanol) to obtain compound 5 (4 mg). P3 was separated with Sephadex LH-20 (dichloromethane/methanol, 1:1, v/v) and Sephadex LH-20 (methanol), and then subjected to atmospheric pressure chromatographic column of silica gel H with dichloromethane 2-acetone- methanol (v:v:v = 50:25:1) to obtain compound 6 (30 mg). P4 was separated on a silica gel H column by MPLC, eluted with dichloromethane-methanol (v:v = 100:1–5:1), and then separated with Sephadex LH-20 (methanol) to obtain compound 7 (11 mg). P5 was separated on a silica gel H column by MPLC, eluted with dichloromethane-methanol (v:v = 20:1–1:1), and then separated with Sephadex LH-20 (methanol) to obtain compound 8 (5 mg). P6 was separated with Sephadex LH-20 (dichloromethane/methanol, 1:1, v/v) and Sephadex LH-20 (methanol) to obtain compound 2 (74 mg). P7 was subjected to atmospheric pressure chromatographic column of silica gel H with dichloromethane-acetone-MeOH (v:v:v = 1:1:0.1–1:1:0.2) and then separated with Sephadex LH-20 (MeOH) to obtain compound 9 (39 mg).
The 90% ethanol part was separated by MPLC that was filled with silica gel H, eluted with petroleum ether-ethyl acetate (100:1–2:1, v/v) and dichloromethane- methanol (50:1–5:1, v/v) to obtain S1–S5. S1 was subjected to atmospheric pressure chromatographic column of silica gel H with petroleum ether-ethyl acetate-acetone (v:v:v = 100:1:1–2:1:1) and petroleum ether-ethyl acetate (v:v = 100:1–5:1) to obtain compound 10 (86 mg). S2 was subjected to decompressed chromatographic column of silica gel H with petroleum ether-ethyl acetate (v:v = 50:1–5:1), and then subjected to atmospheric pressure chromatographic column of silica gel H with petroleum ether- dichloromethane (v:v = 1:1–0:1) and petroleum ether-ethyl acetate (v:v = 20:1) to obtain compound 11 (25 mg). S3 was subjected to atmospheric pressure chromatographic column of silica gel H with petroleum ether- dichloromethane (v:v = 2:1–0:1) and dichloromethane-methanol (v:v = 20:3–10:1) to merge the same components based on TLC analysis. This part was then subjected to atmospheric pressure chromatographic column of silica gel H with petroleum ether-ethyl acetate (v:v = 20:3) to obtain compound 12 (45 mg). S4 was recrystallized to obtain white un-dissolved substance and yellow dissolved substance. The white un-dissolved substance was subjected to atmospheric pressure chromatographic column of silica gel H with dichloromethane-methanol (v:v = 50:1–3:1) to obtain compound 13 (39 mg). The yellow substance was separated with Sephadex LH-20 (dichloromethane/methanol, 1:1, v/v) to afford compound 14 (16 mg). S5 was recrystallized to obtain compound 15 (13 mg).
The coagulation activity of Syringa oblata Lindl flowers in vitro
Blood samples were drawn from Rabbit’s Auricular vein without anaesthesia. The method was similar to our previous research [12]. APTT, PT,TT and FIB were determined.
For all the tests mentioned above, blank solvent (dimethyl sulphoxide: Tween 80: normal saline = 2:1:17) was used as negative control, while the drugs of breviscapine (13.3 mg/mL) and Yunnan baiyao (5 mg/mL) used in the clinics were used as positive control. All the samples were dissolved in blank solvent. The concentrations of compounds were 5 mg/mL and all the extract samples were 15 mg/mL. PT, APTT, TT and FIB tests were conducted with Semi-Automated Coagulation Analyzer (CPC Diagnostics Pvt. Ltd, India).
Statistical analysis
The results of coagulation activity were expressed as mean ± standard deviation. The data analysis was performed by SPSS19.0 software with single factor analysis of variance (ANOVA One-Way) to determine the significant difference. The difference between groups with P < 0.05 and P < 0.001 were regarded as significant and highly significant, respectively. Results were shown in Table 1.
Results
Chemical constituents in S. oblate flowers
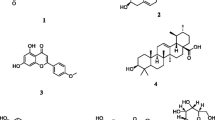
Fifteen known compounds (1–15) were isolated and identified from S. oblata flowers. The structures of compounds were shown in Fig. 1.
Compound 1
Yellow power. The molecular formula was C15H10O7. EI-MS m/z: 302[M]+. 1H-NMR (400 MHz, DMSO-d6) δ: 12.49 (1H, s, 5-OH), 7.67 (1H, s, H-2′), 7. 55 (1H, d, J = 8.0 Hz, H-6′), 6.89 (1H, d, J = 8.0 Hz, H-5′), 6.40 (1H, s, H-8), 6.18 (1H, s, H-6); 13C-NMR (100 MHz, DMSO-d6) δ: 146.74 (C-2), 135.66 (C-3), 175.77 (C-4), 156.09 (C-5), 98.15 (C-6), 163.95 (C-7), 93.29 (C-8), 160.65 (C-9), 102.90 (C-10), 121.90 (C-1′), 115,55 (C-2′), 145.01 (C-3′), 147.66 (C-4′), 115.01 (C-5′), 119.90 (C-6′). The above spectral data were basically consistent with those reported previously [13] and thus, compound 1 was identified as quercetin.
Compound 2
A yellow, needle-shaped crystal. Its molecular formula was C27H30O15. EI-MS m/z: 594 [M]+. 1H-NMR (400 MHz, DMSO-d6) δ: 12.57 (1H, s, 5-OH), 10.86 (1H, s, 7-OH), 10.14 (1H, s, 4′-OH), 8.00 (2H, d, J = 12.0 Hz, H-2′, 6′), 6.89 (2H, d, J = 8.0 Hz, H-3′, 5′), 6.42 (1H, d, J = 4.0 Hz, H-8), 6.21 (1H, d, J = 4.0 Hz, H-6), 5.32 (1H, d, J = 8.0 Hz, H-1′′), 4.44 (1H, brs, H-1′′′); 13C-NMR (100 MHz, DMSO-d6) δ: 156.63 (C-2), 133.26 (C-3), 177.43 (C-4), 161.24 (C-5), 98.76 (C-6), 164.14 (C-7), 93.79 (C-8), 156.90 (C-9), 104.04 (C-10), 120.93 (C-1′), 130.93 (C-2′, 6′), 159.93 (C-4′), 115.14 (C-3′, 5′), 101.36 (C-1′′), 74.21 (C-2′′), 76.39 (C-3′′), 69.96 (C-4′′), 75.78 (C-5′′), 66.92 (C-6′′), 100.81 (C-1′′′), 70.39 (C-2′′′), 70.63 (C-3′′′), 71.85 (C-4′′′), 68.29 (C-5′′′), 17.77 (C-6′′′). The above data were basically consistent with those reported in the Ref. [14]. Thus, compound 2 was identified as kaempferol-3-O-α-l-rhamnosyl-(1 → 6)-β-d-glucoside (kaempferol- rutinose).
Compound 3
A white powder. The molecular formula was C8H10O2. EI-MS m/z: 138 [M]+. 1H-NMR (C5D5N, 400 MHz) δ: 7.33 (2H, d, J = 8.0 Hz, H-2, 6), 7.18 (2H, d, J = 8.0 Hz, H-3, 5), 4.11 (2H, d, J = 12.0 Hz, H-8), 3.05 (2H, t, J = 7.0 Hz, H-7); 13C-NMR (C5D5N, 100 MHz) δ: 130.59 (C-1), 130.54 (C-2, 6), 116.07 (C-3, 5), 157.17 (C-4), 39.58 (C-7), 63.86 (C-8). The above data were basically consistent with those reported in the Ref. [15]. Thus, compound 3 was identified as 4-Hydroxyphenethyl alcohol.
Compound 4
A white powder. The molecular formula was C8H8O4. EI-MS m/z: 168 [M]+. 1H-NMR (C5D5N, 400 MHz) δ: 8.19 (1H, dd, J = 8.0 Hz, 4.0 Hz, H-6), 8.09 (1H, d, J = 4.0 Hz, H-2), 7.32 (1H, d, J = 8.0 Hz, H-5), 3.74 (3H, s, 3-OCH3); 13C-NMR (C5D5N, 100 MHz) δ: 123.34 (C-1), 116.03 (C-2), 148.15 (C-3), 152.58 (C-4), 113.61 (C-5), 124.75 (C-6), 168.98 (C-7), 5.58 (C-8). The above data were basically consistent with those reported in the Ref. [16]. Thus, compound 4 was identified as vanillic acid.
Compound 5
This compound was a yellow-brown, needle-shaped crystal. The molecular formula was determined to be C9H8O4. EI-MS m/z: 180 [M]+. 1H-NMR (DMSO-d6, 400 MHz) δ: 7.42 (1H, d, J = 16.0 Hz, H-7), 7.01 (1H, s, H-2), 6.96 (1H, dd, J = 4.0, 8.0 Hz H-6), 6.76 (1H, d, J = 8.0 Hz, H-5), 6.18 (1H, d, J = 16.0 Hz, H-8); 13C-NMR (DMSO-d6, 100 MHz) δ: 125.72 (C-1), 114.60 (C-2), 144.34 (C-3), 148.09 (C-4), 115.36 (C-5), 115.74 (C-6), 145.55 (C-7), 121.04 (C-8), 168.00 (9-COOH). The above data were basically consistent with those reported in the Ref. [17]. Thus, the compound 5 was identified as caffeic acid.
Compound 6
A white powder. The molecular formula was C17H24O8. EI-MS m/z: 566 [M]+. 1H-NMR (400 MHz, C5D5N) δ: 6.60 (2H, s, H-3, 5), 6.03 (1H, m, H-β), 5.11 (2H, m, H-γ), 3.74 (6H, s, 2 × OCH3), 3.92–4.38 (6H, m, H-2′–6′), 3.34 (2H, d, J = 8.0 Hz, H-α); 13C-NMR (100 MHz, C5D5N) δ: 153.67 (C-2, 6), 137.82 (C-β), 136.26 (C-1), 134.45 (C-4), 115.87 (C-γ), 107.13 (C-3, 5), 105.02 (C-1′), 75.98 (C-2′), 78.58 (C-3′), 71.51 (C-4′), 78.28 (C-5′), 62.53 (C-6′), 56.46 (2 × OCH3), 40.44 (C-α). The above data were basically consistent with those reported in the Ref. [18]. Thus, the compound 6 was identified as dictamnoside A.
Compound 7
A yellow powder. The molecular formula was C24H30O13. EI-MS m/z: 526 [M]+. 1H-NMR (400 MHz, C5D5N) δ: 7.52 (1H, s, H-3), 6.65 (1H, s, H-5′′), 6.63 (1H, d, J = 1.5 Hz, H-2′′), 6.49 (1H, dd, J = 1.5 Hz, 8.5 Hz, H-6′′), 4.18 (2H, m, 2 × H-α), 3.02 (1H, m, H-5), 2.72 (2H, t, J = 6.5 Hz 2 × H-β), 2.07 (1H, q, H-9), 4.56 (1H, d, J = 8.0 Hz, H-1′), 1.41 (3H, d, J = 4.0 Hz, 10-Me); 13C-NMR (100 MHz, C5D5N) δ: 94.84 (C-1), 152.92 (C-3), 107.77 (C-4), 26.82 (C-5), 33.53 (C-6), 171.67 (C-7), 73.49 (C-8), 21.26 (C-10), 166.63 (C-11), 99.23 (C-1′), 73.20 (C-2′), 77.34 (C-3′), 70.16 (C-4′), 76.57 (C-5′), 61.38(C-6′), 128.75 (C-1′′), 116.21 (C-2′′), 145.12 (C-3′′), 143.76 (C-4′′), 115.55 (C-5′′), 119.62 (C-6′′), 64.93 (C-α), 33.88 (C-β). The above data were basically consistent with those reported in the Ref. [19]. Thus, the compound 7 was identified as Lilacoside.
Compound 8
A yellow powder. The molecular formula was C21H20O12. EI-MS m/z: 465 [M]+. 1H-NMR (400 MHz, DMSO-d6) δ: 12.65 (1H, s,5-OH), 7.59 (1H, d, J = 4.0 Hz, H-6′), 7.57 (1H, d, J = 4.0 Hz, H-2′), 6.85 (1H, d, J = 8.0 Hz, H-5′), 6.39 (1H, s, H-8), 6.20 (1H, d, J = 4.0 Hz, H-6), 5.46 (1H, d, J = 8.0 Hz, Glc-H-1′′), 3.17–3.24 (5H, m, Rha-H-2″–6″); 13C-NMR (100 MHz, DMSO-d6) δ: 156.11 (C-2), 133.30 (C-3), 177.36 (C-4), 161.18 (C-5), 98.63(C-6), 164.21(C-7), 93.45(C-8), 156.28 (C-9), 103.78 (C-10), 121.52 (C-1′), 115.15 (C-2′), 144.75(C-3′), 148.41(C-4′), 116.16(C-5′), 121.11(C-6′), Glc: 100.92(C-1″), 74.06(C-2″), 77.46(C-3″), 69.92(C-4″), 76.48 (C-5″), 60.95 (C-6″). The above data were basically consistent with those reported in the Ref. [20]. Thus, the compound 8 was identified as quercetin-3-O-β-d-glucoside.
Compound 9
Was a yellow powder. The molecular formula was C27H30O16. EI-MS m/z: 610 [M]+. 1H-NMR (400 MHz, DMSO-d6) δ: 12.60 (1H, s, 5-OH), 10.84 (1H, s, 7-OH), 9.68 (1H, s, 4′-OH), 9.19 (1H, s, 3′-OH), 7.55 (2H, d, J = 8.0 Hz, H-2′, 6′), 6.85 (1H, d, J = 8.0 Hz, H-5′), 6.39 (1H, d, J = 4.0 Hz, H-8), 6.20 (1H, d, J = 4.0 Hz, H-6), 5.35 (1H, d, J = 4.0 Hz, H-1′′), 4.38 (1H, brs, H-1′′′); 13C-NMR (100 MHz, DMSO-d6) δ: 156.42 (C-2), 133.30 (C-3), 177.37 (C-4), 161.23 (C-5), 98.68 (C-6), 164.07 (C-7), 93.59 (C-8), 156.61 (C-9), 103.98 (C-10), 121.18 (C-1′), 115.23 (C-2′), 144.76 (C-3′), 148.42 (C-4′), 116.26 (C-5′), 121.59 (C-6′), 101.17 (C-1′′), 74.08 (C-2′′), 76.44 (C-3′′), 70.00 (C-4′′), 75.91 (C-5′′), 67.01 (C-6′′), 100.76 (C-1′′′), 70.38 (C-2′′′), 70.56 (C-3′′′), 71.84 (C-4′′′), 68.26 (C-5′′′), 17.77 (C-6′′′). The above data were basically consistent with those reported in the Ref. [21]. Thus, the compound 9 was identified as rutin.
Compound 10
A white solid. The molecular formula was C16H32O2. EI-MS m/z: 256 [M]+. 1H-NMR (CDCl3, 400 MHz) δ: 0.88 (3H, t, J = 9.0 Hz, H-16), 1.23–1.29 (24H, m, H-4–15), 1.61 (2H, m, H-3), 2.34 (2H, t, J = 7.5 Hz, H-2); 13C-NMR(CDCl3, 100 MHz) δ: 179.3 (–COOH), 34.23 (C-2), 24.83 (C-3), 29.21–29.85 (C-4–13), 32.08 (C-14), 22.85 (C-15), 14.27 (C-16). The above data were basically consistent with those reported in the Ref. [22]. Thus, the compound 10 was identified as palmitic acid.
Compound 11
A white solid. The molecular formula was C12H24O2. EI-MS m/z: 200 [M]+. 1H-NMR (CDCl3, 400 MHz) δ: 0.87 (3H, t, J = 7.0 Hz, H-12), 1.26 (16H, m, H-4–11), 1.80 (2H, m, H-3), 2.52 (2H, t, J = 8.0 Hz, H-2); 13C-NMR (CDCl3, 100 MHz) δ: 175.9(-COOH), 34.89 (C-2), 25.67 (C-3), 29.63–29.99 (C-4–9), 32.14 (C-10), 22.96 (C-11), 14.29 (C-12). The above data were basically consistent with those reported in the Ref. [23]. Thus, the compound 11 was identified as lauric acid.
Compound 12
A white powder. The molecular formula was C30H48O3. EI-MS m/z: 456 [M]+. 1H-NMR (C5D5N, 400 MHz) δ: 5.50 (1H, brs, H-12), 3.45 (1H, dd, J = 8.0 Hz, 4.0 Hz, H-3), 3.32 (1H, dd, J = 4.0 Hz, 4.0 Hz, H-18), 1.28 (3H, s, H-27), 1.24 (3H, s, H-25), 1.02 (3H, s, H-30), 1.01 (3H, s, H-29), 0.95 (3H, s, H-23), 0.89 (3H, s, H-26); 13C-NMR (C5D5N, 100 MHz) δ: 38.93 (C-1), 28.09 (C-2), 78.06 (C-3), 39.38 (C-4), 55.80 (C-5), 18.79 (C-6), 39.74 (C-8), 48.11 (C-9), 37.37 (C-10), 23.82 (C-11), 122.54 (C-12), 144.81 (C-13), 42.16 (C-14), 28.31 (C-15), 23.69 (C-16), 46.47 (C-17), 42.00 (C-18), 346.66 (C-19), 30.96 (C-20), 4.21 (C-21), 33.18 (C-22), 28.78 (C-23), 16.55 (C-24), 15.55 (C-25), 17.43 (C-26), 26.17 (C-27), 180.16 (C-28), 233.27 (C-7, 29), 3.76 (C-30). The above data were basically consistent with those reported in the Ref. [16]. Thus, the compound 12 was identified as oleanolic acid.
Compound 13
Was a white powder. The molecular formula was C30H48O3. EI-MS m/z: 456 [M]+. 1H-NMR (C5D5N, 400 MHz) δ: 5.49 (1H, s, H-12), 3.46 (1H, dd, J = 8.0 Hz, 8.0 Hz, H-3), 2.65 (1H, d, J = 8.0 Hz, H-18), 1.25 (3H, s, H-27), 1.23 (3H, s, H-26), 1.05 (3H, s, H-23), 0.96 (3H, d, J = 8.0 Hz, H-29), 0.88 (3H, s, H-24), 1.01 (3H, d, J = 8.0 Hz, H-30), 1.02 (3H, s, H-25); 13C-NMR (C5D5N, 100 MHz) δ: 37.43 (C-1), 28.11 (C-2), 78.09 (C-3), 39.06 (C-4), 55.80 (C-5), 18.77 (C-6), 33.56 (C-7), 39.94 (C-8), 48.02 (C-9), 39.47 (C-10), 23.90 (C-11), 125.63 (C-12), 139.24 (C-13), 42.48 (C-14), 28.80 (C-15), 24.89 (C-16), 48.02 (C-17), 53.52 (C-18), 39.39 (C-19), 39.37 (C-20), 31.06 (C-21), 37.26 (C-22), 28.67 (C-23), 15.67 (C-24), 16.58 (C-25), 17.52 (C-26), 23.61 (C-27), 179.88 (C-28), 17.43 (C-29), 21.42 (C-30). The above data were basically consistent with those reported in the Ref. [16]. Thus, the compound 13 was identified as ursolic acid.
Compound 14
A yellow powder. The molecular formula was C15H12O5 EI-MS m/z: 272 [M]+. 1H-NMR (C5D5N, 400 MHz) δ: 12.83 (1H, s, 5-OH), 7.55 (2H, d, J = 8.0 Hz, H-2′, 6′), 7.22 (2H, d, J = 8.0 Hz H-3′, 5′), 6.49 (1H, d, J = 4.0 Hz, H-8), 6.18 (1H, s,H-6), 5.51 (1H, dd, J = 4.0 Hz, 4.0 Hz, H-2), 5.32 (2H, s, H-6, 8), 3.33 (1H, dd, J = 12.0 Hz, 12.0 Hz, H-3a), 2.90 (1H, dd, J = 0 Hz, 4.0 Hz, H-3b); 13C-NMR (C5D5N, 100 MHz) δ: 79.65 (C-2), 43.29 (C-3), 196.53 (C-4), 165.16 (C-5), 97.22 (C-6), 168.56 (C-7), 96.11 (C-8), 164.03 (C-9), 102.87 (C-10), 129.76 (C-1′), 128.86 (C-2′, 6′), 116.42 (C-3′, 5′), 159.53 (C-4′). The above data were basically consistent with those reported in the Ref. [24]. Thus, the compound 14 was identified as naringenin.
Compound 15
A white powder. The molecular formula was C35H60O6. EI-MS m/z: 578[M]+. It was compared with reference substance of β-daucosterol, no difference was seen between them in term of the TLC detection. Thus compound 15 was identified as β-daucosterol.
Coagulation time test in vitro
In Fig. 2, water part, lauric acid and kaempferol-rutinose could significantly shorten PT (P < 0.001) compared with the blank group. The 40% ethanol part, 90% ethanol part and So.TE had significant anticoagulant activity (P < 0.001 and 0.001 < P<0.01) compared with the blank group. The effects of water part, lauric acid and kaempferol-rutinose were not different with that of Yunnan Baiyao.
In the Fig. 3, all the samples except 60% ethanol part and dictamnoside A could significantly shorten TT (P < 0.001 and 0.001 < P < 0.01) compared with the blank group. The procoagulant activity of 20% ethanol part was the best one (P < 0.001) compared with the Yunnan Baiyao.
In Fig. 4, water part, 20% ethanol part, 40% ethanol part, 90% ethanol part, So, TE, lauric acid and kaempferol-rutinose could significantly shorten APTT (P < 0.001) compared with the blank group. Water part, 20% ethanol part, 40% ethanol part, 90% ethanol part, So.TE, lauric acid and kaempferol-rutinose had procoagulant activity compared with the Yunnan Baiyao, and 20% ethanol part had a higher activity than that of Yunnan Baiyao, while the others were not better than that of Yunnan Baiyao.
In Fig. 5, water part, 20% ethanol part, 40% ethanol part, 90% ethanol part, So.TE and lauric acid all could significantly increase the FIB content (P < 0.001) compared with the blank group. The procoagulant activity of the positive control was the best one (P < 0.001) compared with the Yunnan Baiyao.
Discussion
Sun et al. [25] found that the volatile compounds in fresh flowers of S. oblate during different flowering periods were different. Dong et al. [6] isolated 8 compounds from the alabastrum of S. oblate, and they were identified as syringopicrogenin-B, oleandic acid, ursolic acid, lupanic acid, luprol, p-hydroxy phenylpropanol, p-hydroxy phenylethanol and β-sitosterol. Triterpenic acids were the main components. In this study, fifteen known compounds were isolated from S. oblata flowers. They were identified as quercetin (1), kaempferol-3-O-α-l-rhamnosyl-(1 → 6)-β-d-glucoside (2, kaempferol-rutinose), 4-Hydroxyphenethyl alcohol (3), vanillic acid (4), caffeic acid (5), dictamnoside A (6), Lilacoside (7), quercetin-3-O-β-D-glucoside (8), rutin (9), palmitic acid (10), lauric acid (11), oleanolic acid (12), ursolic acid (13), naringenin (14), and β-daucosterol (15). Flavonoids, organic acids and Triterpenic acids were the main components.
The previous studies on S. oblate flowers were mainly focused on the volatile components and its traditional pharmacological activity. In the present study we found that the S. oblate flowers had a significant procoagulant activity for the first time. Our researches showed that water part, lauric acid and kaempferol-rutinose all displayed a significant procoagulant activity, and that the procoagulant activity of water part, lauric acid, and kaempferol-rutinose were not better than that of Yunnan Baiyao, which was used as the positive control.
Conclusions
In the present study, fifteen compounds were isolated and identified from S. oblate flowers, including triterpenic acids, fatty acids and flavonone glycosides etc. Water extract of S. oblate flowers, lauric acid and kaempferol-rutinose possessed the procoagulant activity.
Availability of data and materials
The datasets supporting the conclusions of this article are presented in this main paper. Plant materials used in this study have been identified by Professor Changqin Li. A voucher specimen was deposited in National Center for Research and Development of Edible Fungus Processing Technology.
Abbreviations
- APTT:
-
activated partial thromboplastin time
- PT:
-
prothrombin time
- TT:
-
thrombin time
- FIB:
-
fibrinogen
- So.TE:
-
total extract of S. oblata flowers
- Water part:
-
water extract of S. oblata flowers
- 20% ethanol part:
-
20% ethanol extract of S. oblata flowers
- 40% ethanol part:
-
40% ethanol extract of S. oblata flowers
- 60% ethanol part:
-
60% ethanol extract of S. oblata flowers
- 90% ethanol part:
-
90% ethanol extract of S. oblata flowers
References
Editorial board of Chinese Materia Medica, State Administration of traditional Chinese medicine (1999) Chinese materia medica (volume eleventh). Shanghai science and Technology Press, Shanghai, p 415
Zhao M, Han J, Lv SY, Zhang SJ (2012) Study on chemical constituents in twigs of Syringa oblate. Chin Trad Herb Drugs. 43(2):251–254
Zhang SJ, Guo HQ, Han J, Zhao M, Wang JL (2011) Chemical constituents from seeds of Syringa oblate. Chin Trad Herb Drugs. 42(10):1894–1899
Zhang SJ, Zhang JF, Wang JL (2006) Chemical constituents in stem bark of Syringa oblate. Chin Trad Herb Drugs. 37(11):1624–1626
Wang JL, Zhang GF, Dong LW, Zhao M, Zhang SJ (2010) Chemical constituents in seed crust of Syringa oblate. Chin Trad Herb Drugs. 41(10):1589–1601
Dong LW, Wang JL, Zhao M, Zhang SJ (2011) Chemical constituents of the alabastrum of Syringa oblata Lindl. Nat Prod Res Dev. 23:658–660
Tian L, Li YJ, Lv SW, Zhang L, Liu T (2013) Chemical constituents of Syringa oblate leaves. Chin J Exp Trad Med Form 19(1):144–147
Zhou YM (2005) Studies on the active constituents from leaves of Syringa oblata Lindl. Shenyang Pharmaceutical University, Shenyang
Yang H, Zhao CX, Fang HZ, Wang DS, Zeng YY, Liang YZ (2007) Chemical components in essential oils from Syringa oblate. Chin Trad Herb Drugs 38(11):1613–1619
Jiao SQ, Zong MX, Zhang NN, Tao Y (2012) Analysis of the chemical constituents by Supercritical CO2 extraction from dried flower of Syringa oblata Lindl. Chem Indus For Prod 32(1):85–88
Yu TJ, Wang LB, Wu LJ (2016) Research progress of the chemical constituents and pharmacological action of Syringa Linn. J Anhui Agric Sci 44(2):168–170
Cao PR, Xie PY, Wang XB et al (2017) Chemical constituents and coagulation activity of Agastache rugosa. BMC Complem Altern Med. 17(1):93
Sang YL, Hao YJ, Yang SS (2008) Studies on chemical constituents of Lamiophlomis rotat. Chin Trad Herb Drugs. 39(11):1622–1624
Yang M (2013) Studies on chemical constituents of persistent calyx of Physalis pubescens L. Soochow University, Soochow
Zhang YL, Feng ZY, Zheng ZK, Cao YG, Li M, Dong CM et al (2014) Chemical constituents from the leaves of Rhemannia glutinosa Libosch. Chin Pharm J. 49(1):15–21
Feng WS, Wang JC, He YH, Zheng XK, Song K, Zhang YL et al (2015) Chemical constituents from the flower buds of Magnolia biondii Pamp. Chin Phar J. 50(24):2103–2106
Li HB, Yu Y, Wang ZZ, Xiao W, Yao XS (2014) Research on antiviral constituents in Re-Du-Ning Injection (I). Chin Trad Herb Drugs. 45(12):1682–1688
Peng W, Han T, Liu QC, Qin LP (2011) Chemical constituents from aerial part of Atractylodes macrocephala. China J Chin Materia Med 36(5):578–581
Damtoft S, Franzyk H, Jensen SR (1995) Biosynthesis of Iridoids in Syringa and Fraxinus: carbocyclic iridoid precursors. Phytochemistry 40(3):785–792
Ni FY, Chen Z, Xu QM, Yang SL, Chen DF (2013) Chemical constituents from Rhodiola sachalinensis. Chin Trad Herb Drugs 44(7):798–802
Wen J, Yuan XH, Liu Z (2015) Study on the chemical constituents of Nerium indicum Mill. J Anhui Agric Sci 43(9):65–66
Liu QR, Li J, Zhao XF, Xu B, Peng WD, Li SX (2016) Studies on constituents from rhizome of Arundo donax. Chin Trad Herb Drugs. 47(7):1084–1089
Qi J, Shi RF, Yu JM et al (2016) Chemical constituents from leaves of Camellia nitidissima and their potential cytotoxicity on SGC7901 cells. Chin Herb Med 8(1):80–84
Zhou XY, Sun XB, Xu XD, Li YD, Zhang HY, Wang YQ et al (2016) Chemical constituents from seeds of Caesalpinia sappan. Chin Trad Herb Drugs 47(10):1653–1656
Sun JW, Yang KY, Li YM, Liu YP (2015) Analysis of aroma components in Syringa oblate at different flowering periods by SPME-GC-MS. China Brewing. 34(7):151–155
Acknowledgements
The authors would like to extend their sincere appreciation to Henan Province University Science and Technology Innovation Team (16IRTSTHN019) and National Science Fund Henan Province (162300410038) for funding this research group.
Funding
This work was funded by Key Project in Science and Technology Agency of Henan Provence (192102110112).
Author information
Authors and Affiliations
Contributions
WYK and ZHL conceived the research idea. LLC, MYH and PRC conducted the experiment, collected the plant specimens, analyzed and interpreted the data as well as prepared the first draft. WYK, YN and CQL critically read and revised the paper. All the authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Cui, L., Hu, M., Cao, P. et al. Chemical constituents and coagulation activity of Syringa oblata Lindl flowers. BMC Chemistry 13, 108 (2019). https://doi.org/10.1186/s13065-019-0621-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13065-019-0621-8