Abstract
Background
In March 2020, the World Health Organization declared the coronavirus disease 2019 as a global pandemic. Though antiviral drugs and antimalarial drugs are considered treatment options for treating coronavirus disease 2019 (COVID-19), no specific antivirals are currently available for its treatment. Efficient use of drug discovery approaches including repurposing or repositioning of drugs used in the treatment of severe acute respiratory syndrome coronavirus (SARS-CoV) and the Middle East respiratory syndrome coronavirus (MERS-CoV) is considered recently. The widespread application of corticosteroid therapy in COVID-19 should be backed with careful documented pragmatic research of its use in this context.
Main body
This article aims to analyze various trials registered across the globe providing an overall picture of the use of corticosteroids in the treatment of COVID-19. An extensive search was conducted on the clinical trial registries around the world to identify all the trials reporting information regarding the use of corticosteroids in COVID-19. Our initial search returned 231 trials, out of which 60 trials were finally included in the analysis. Fifty-six studies were interventional trials, and all the trials had clearly defined primary and secondary outcomes of interest, of which only 11 trials had evaluation of respiratory rate as one of their outcomes.
Conclusion
Few preliminary trial findings show promising results and recommend the use of methylprednisolone and dexamethasone in the severe form of the disease; however, there is insufficient data to prove its benefits over its risks. Routine use of corticosteroids should be favored only after a better insight is obtained, with the completion of these trials.
Similar content being viewed by others
Background
An outbreak of pneumonia caused by a new coronavirus spread in Wuhan province of China in December 2019. Sequencing of the sampling from patients with pneumonia revealed the viral genome phylogenetically closer to severe acute respiratory syndrome coronavirus (SARS-CoV) and the Middle East respiratory syndrome coronavirus (MERS-CoV) [1]. The Coronavirus Study Group named the causative agent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and the disease caused by this virus was named coronavirus disease 2019 (COVID-19 or 2019-nCoV) by the World Health Organization (WHO) [2, 3]. These viruses are enveloped, positive, single-stranded RNA viruses belonging to the family Coronaviridae, which can cause an array of symptoms including fever, dry cough, myalgia, fatigue, and dyspnea [4]. SARS-CoV-2 transmits from human-to-human by respiratory droplets caused by coughing or sneezing [5, 6]. The WHO declared COVID-19 as a Public Health Emergency of International Concern in January 2020 [7]. The infection has spread over to 216 countries (15,745,102 confirmed cases and 639,317 confirmed deaths) since its outbreak in November 2019 (as of 30 January 2021; Fig. 1).
Global COVID-19 spread showing number of confirmed cases as of 30 January 2021 (Source: https://covid19.who.int/)
Detection and diagnosis of this novel coronavirus mostly relied on molecular-based approaches such as nucleic acid testing, virus antigen, or serological antibody testing (against the N-protein of SARS-CoV) [8]. Treatment option includes antiviral drugs such as favipiravir, remdesivir, lopinavir, and ritonavir and antimalarial drugs such as chloroquine or hydroxychloroquine. Nevertheless, no vaccine or specific antiviral treatment recommended for COVID-19 is currently available [9]. The uncontrolled scenario of COVID-19 demands the use of effective drug discovery approaches for effective control of the disease [10,11,12,13,14,15,16]. Among these approaches, drug repurposing or drug repositioning is a time-effective way of treating a disease. One of the examples of successful application of drug discovery approach is drug repositioning of antivirals, and it has triggered a number of in vitro studies as well as clinical trials for a number of chemical molecules to evaluate their efficacy against COVID-19 [17,18,19]. Drug repurposing of corticosteroids has also been implemented recently as a part of a drug discovery approach. There are several studies reporting the use of corticosteroids in the treatment of severe coronavirus infections including COVID-19. The effectiveness of corticosteroids in some patients with SARS-CoV has resulted in a widespread application of this therapy in COVID-19, especially in patients in the ICU with severe infections, as these drugs prevent lung injury caused by severe community-acquired pneumonia (sCAP) due to their potential pharmacological effects on the suppression of exuberant and dysfunctional systematic inflammation [20].
Main body
Corticosteroids and their therapeutic role
The Infectious Diseases Society of America (IDSA) guidelines strongly recommends the use of dexamethasone in critically ill patients to treat acute respiratory distress syndrome (ARDS) and systemic inflammation, backed by moderate evidence. Dexamethasone at a total daily dose of 6 mg IV or PO for 10 days (or until discharge) or alternative glucocorticoids like methylprednisolone 32 mg and prednisone 40 mg are suggested. The level of recommendation decreases with decreasing severity of the disease. In non-severe COVID-19, the use of glucocorticoids is not recommended as there is a dearth of solid evidence. Additionally, experiences from SARS and MERS show risk of worsening clinical status, delayed viral clearance, and other adverse events [21]. Currently, available data on safety and effectiveness of corticosteroids in this setting is very few and inconclusive [20, 22, 23]. The value of corticosteroids as a treatment option in patients with severe COVID-19 infection needs careful documented pragmatic research in this context. In order to obtain strong clinical evidence, several studies have been launched that were registered on various clinical trial registries across the globe. The detailed analysis of these trials will give an overall picture of the use of corticosteroids in the treatment of COVID-19 around the world. This will help to identify the lacunae to be filled with definitive clinical evidence in order to reposition corticosteroid for COVID-19 treatment. Therefore, this study aims to analyze various trials registered across the globe providing an overall picture of the use of corticosteroids in the treatment of COVID-19.
Search strategy
An extensive search was conducted to identify all the trials reporting information regarding the use of corticosteroids in COVID-19. We searched the following clinical trial registries: Clinicaltrials.gov, Chinese Clinical Trial Registry (ChiCTR), Clinical Research Information Service (CRiS)–Republic of Korea, EU Clinical Trials Register, ISRCTN Registry, Iranian Registry of Clinical Trials (IRCT), German Clinical Trials Register (DRKS), Japan Primary Registries Network (JPRN), and Clinical Trial Registry–India. The search was run until 23 June 2020. In Clinicaltrials.gov, the following keywords were used for search: “(COVID-19 OR SARS-CoV-2 OR 2019-nCoV OR severe acute respiratory syndrome coronavirus 2 OR Wuhan coronavirus OR 2019 novel coronavirus OR novel coronavirus–infected Pneumonia) AND (“glucocorticoids” OR “steroids” OR “corticosteroids” OR “hydrocortisone” OR “prednisone” OR “methylprednisolone” OR “dexamethasone” OR “prednisolone”). A similar strategy was adapted for the other registries. We included the English language and interventional and non-interventional studies. No restrictions were placed on the dose or formulation of the intervention. All trials must have studied the safety and efficacy of steroids in COVID-19 care.
Recovery of trials
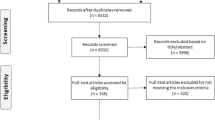
Our initial search returned 231 trials, out of which 62 potentially relevant trials were identified. Potentially eligible trials were identified by three authors by screening titles and study description. All eligible trials were then assessed independently by three authors, and potentially relevant trials were selected in accordance with the predefined inclusion criteria. Any disagreement was reviewed and resolved by a fourth independent reviewer. Authors of individual trials were contacted if necessary. After a careful review of the study description, out of 62 articles, 2 trials did not satisfy the inclusion criteria and were excluded from the analysis. Finally, data from 60 trials were included in the final review and synthesis of results. This is shown in Fig. 2.
Data abstraction and study appraisal
We extracted the following general data from each study: trial number, title, origin (country) of study, intervention, treatment arms, doses, mean age of participants, stage of COVID–19, expected start and end date of trial, primary outcomes of the study, blinding, randomization, and study design.
Scrutiny of trials
Our initial search of the clinical trial registries resulted in 231 trials, of which 167 trials did not satisfy the inclusion criteria and three trials did not have complete data, and after removing the duplicates, 60 trials were included in the final analysis. Thus, 60 trials with 31,732 patients were included in this systematic review. The included trials were classified into trials that included only steroid therapy and those that included steroids in addition to other standard treatment as shown in Table 1.
Type of trials
Among the included trials, 57 trials were quantitative studies and the remaining three trials were qualitative studies, i.e., non-interventional studies, as shown in the Table 2.
Heterogeneity of trials
All 60 trials included were heterogenous in that they had various inclusion and exclusion criteria and different treatment protocols for the treatment of various stages of COVID-19. The most common stage of COVID-19 among these trials is pneumonia, which is shown in Table 1.
Methodological quality of the trials
Among the 60 trials, 54 were randomized. It was unclear how randomization was carried out in three of the trials. Among 54 randomized trials, only 21 trials were blinded, of which 8 were single blinded, 8 were double blinded, 2 were triple blinded, and 3 were quadruple blinded, as shown in Table 2.
Steroid treatment
Regarding the steroid treatment, the most common steroid used is methylprednisolone (used in 28 trials) at various dosages depending on the age of the patients. Maximum loading dose of methylprednisolone used is 500 mg IV infusion over 1h in a trial (IRCT20080901001165N52). Steroids were given from a minimum of 3 days to a maximum of 21 days. Other steroids used are budesonide, ciclesonide, dexamethasone, formoterol, prednisolone, prednisone, and hydrocortisone. In 10 trials, the dose of the steroids used was unclear, and in one trial (ChiCTR2000030481), the treatment regimen was not mentioned. This is shown in Table 3.
Figure 3 depicts the number of trials studying different types of steroids, showing majority of the trials (N = 28) have decided to study the effectiveness of methylprednisolone in the treatment of COVID-19.
Primary and secondary outcomes
Table 1 summarizes results from all 60 studies. All the trials had clearly defined primary and secondary outcomes of interest, in which only 11 trials had evaluation of respiratory rate as one of their outcomes. Common outcomes measured are respiratory rate, mortality rate, ventilation free days, days in ICU, patient Sequential Organ Failure Assessment (SOFA) score, Murray lung injury score, National Early Warning Score 2 (NEWS2) score, number of patients with treatment failure, rate of remission and progression, blood oxygen saturation, chest x-ray, steroid-related adverse effect, and toxicity monitoring. Table 4 summarizes the consolidation of completed trials with results. All the completed trials have used methylprednisone, dexamethasone, and hydrocortisone as drug of choices.
The data obtained from this review shows that steroids of different doses and types were included in numerous ongoing clinical trials. Their safety and efficacy in managing symptoms of COVID-19, especially in the pneumonia stage, were tested. The trials also included patients of different age groups at different stages of COVID-19. The COVID-19 infection goes through three stages from asymptomatic phase to ARDS (acute respiratory distress syndrome) phase. The 2019-nCoV, after entering the nasal cavity, adheres to the epithelial cells and binds to ACE2 receptor [24]. Owing to this reason, it may be evident that different corticosteroids act through different mechanisms to minimize the symptoms of COVID-19 infection. Table 3 represents the total number of population recruited in each trial, from which we estimate the total ARDS population recruited to be 3880 patients with disease stages ranging from moderate to severe respiratory distress of which methylprednisolone was the most commonly used corticosteroids. A study by H.P. Wiedemann et al. showed that methylprednisolone increased mortality rates by at least 14 days after the onset of ARDS, which gives an impression that the routine use of methylprednisolone is not effective in ARDS [25]. Another study by Nelson Lee et al. shows that SARS-CoV RNA concentrations in the second and third week of illness were significantly higher in patients who received early hydrocortisone treatment compared to placebo; thus, it is recommended to be avoided, but can be cautiously used in SARS [26]. The potential risks associated with high-dose corticosteroids in treating 2019-nCoV pneumonia include secondary infections, long-term complications, and prolonged virus shedding and escalating towards advanced stages [27]. Another study conducted by G.C. Khilnani and H. Vijay registered increased mortality rate (35.7%) with the use of corticosteroids [28,29,30,31,32,33]. Positively, the RECOVERY trial (Randomised Evaluation of COVID-19 therapy) concluded that in hospitalized patients with COVID-19, corticosteroid reduced 28-day mortality among those receiving invasive mechanical ventilation or oxygen at randomization, but not among patients not receiving respiratory support [34]. Moreover, excessive levels of glucocorticoids have shown to precipitate heart failure by aggravating fluid retention, triggering risk factors like glucose intolerance and dyslipidemia, and by worsening atheromatous vascular disease. Additionally, increased risk of mortality with high serum levels of cortisol have been reported, further establishing a link between use of corticosteroids and increased heart failure risk [35]. Thus, the usage of corticosteroids at various stages of COVID-19 is still questionable with higher mortality rates than the comparator. More information can be gained from results from the completed trials. Though four trials have completed its recruitment, results were not available in the registry. The completed four trials were registered in the Iranian clinical trial registry. The outcomes measured in these trials were mortality rate, need for ICU services, duration of stay in the hospital, assessment of side effects, readmission rate, need for oxygen therapy, blood O2, levels, chest x-ray, PAO2/fio2, and need for invasive mechanical ventilation and intubation.
Conclusion
Numerous interventional and non-interventional studies are being conducted to study the efficacy of corticosteroids in COVID-19. Corticosteroids can regulate immune-mediated lung injury and decrease the development to respiratory failure and death. Dexamethasone has been reported to reduce the duration of mechanical ventilation. Long-term glucocorticoid therapy has displayed significant improvement in indices of alveolar–capillary membrane permeability and mediators of inflammation and tissue repair. Few preliminary trial findings show promising results and recommend the use of methylprednisolone and dexamethasone in the severe form of the COVID-19. Few studies have reported that early administration of dexamethasone could reduce duration of mechanical ventilation and overall mortality in patients with established moderate to severe ARDS; however, there is insufficient data to prove its benefits over its risk. Routine use of corticosteroids should be favored only after a better insight is obtained, with the completion of these trials.
Availability of data and materials
The datasets analyzed during the current study will be available from the corresponding author on reasonable request.
Abbreviations
- ACE:
-
Angiotensin-converting enzyme
- ARDS:
-
Acute respiratory distress syndrome
- ChiCTR:
-
Chinese Clinical Trial Registry
- COVID-19:
-
Coronavirus disease 2019
- CRiS:
-
Clinical Research Information Service–Republic of Korea
- EU:
-
European Union
- ICU:
-
Intensive care unit
- IRCT:
-
Iranian Registry of Clinical Trials
- MERS-CoV:
-
Middle East respiratory syndrome coronavirus
- NEWS2:
-
National Early Warning Score 2
- RNA:
-
Ribonucleic acid
- SARS-CoV:
-
Severe acute respiratory syndrome coronavirus
- sCAP:
-
Severe community-acquired pneumonia
- SOFA:
-
Sequential Organ Failure Assessment
- WHO:
-
World Health Organization
References
Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W, China Novel Coronavirus Investigating and Research Team (2020) A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382(8):727–733. https://doi.org/10.1056/NEJMoa2001017
Gorbalenya AE, Baker SC, Baric RS, de Groot RJ, Drosten C, Gulyaeva AA, Haagmans BL, Lauber C, Leontovich AM, Neuman BW, Penzar D, Perlman S, Poon LLM, Samborskiy D, Sidorov IA, Sola I, Ziebuhr J (2020) Severe acute respiratory syndrome-related coronavirus: The species and its viruses – a statement of the Coronavirus Study Group. Nat Microbiol 5:536–544. https://doi.org/10.1038/s41564-020-0695-z
WHO. WHO Director-General’s remarks at the media briefing on 2019-nCoV on 11 February 2020. Available from: https://www.who.int/zh/dg/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020. Accessed 25 June 2020.
He F, Deng Y, Li W (2020) Coronavirus disease 2019 (COVID-19): what we know? J Med Virol 92(7):719–725. https://doi.org/10.1002/jmv.25766
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395(10223):497–506. https://doi.org/10.1016/S0140-6736(20)30183-5
Chan JF, Yuan S, Kok KH, To KK, Chu H, Yang J, Xing F, Liu J, Yip CC, Poon RW, Tsoi HW, Lo SK, Chan KH, Poon VK, Chan WM, Ip JD, Cai JP, Cheng VC, Chen H, Hui CK, Yuen KY (2020) A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet 395(10223):514–523. https://doi.org/10.1016/S0140-6736(20)30154-9
https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen (Accessed 29 June 2020)
Ahn DG, Shin HJ, Kim MH, Lee S, Kim HS, Myoung J, Kim BT, Kim SJ (2020) Current status of epidemiology, diagnosis, therapeutics, and vaccines for novel coronavirus disease 2019 (COVID-19). J Microbiol Biotechnol 30(3):313–324. https://doi.org/10.4014/jmb.2003.03011
Lu H (2020) Drug treatment options for the 2019-new coronavirus (2019-nCoV). Biosci Trends 14(1):69–71. https://doi.org/10.5582/bst.2020.01020
Cava C, Bertoli G, Castiglioni I (2020) In silico discovery of candidate drugs against Covid-19. Viruses 12(4):404. https://doi.org/10.3390/v12040404
Kleandrova VV, Speck-Planche A (2020) The QSAR paradigm in fragment-based drug discovery: from the virtual generation of target inhibitors to multi-scale modeling. Mini reviews in medicinal chemistry 20(14):1357–1374. https://doi.org/10.2174/1389557520666200204123156
Redkar S, Mondal S, Joseph A, Hareesha KS (2020) A machine learning approach for drug-target interaction prediction using wrapper feature selection and class balancing. Molecular informatics 39(5):e1900062. https://doi.org/10.1002/minf.201900062
Singh R, Bhardwaj V, Das P, Purohit R (2019) Natural analogues inhibiting selective cyclin-dependent kinase protein isoforms: a computational perspective. J Biomol Struct Dyn 4:1–10. https://doi.org/10.1080/07391102.2019.1696709
Liu B, He H, Luo H, Zhang T, Jiang J (2019) Artificial intelligence and big data facilitated targeted drug discovery. Stroke Vasc Neurol 4(4):206–213. https://doi.org/10.1136/svn-2019-000290
Martinez-Mayorga K, Madariaga-Mazon A, Medina-Franco JL, Maggiora G (2020) The impact of chemoinformatics on drug discovery in the pharmaceutical industry. Expert Opin Drug Discov 15(3):293–306. https://doi.org/10.1080/17460441.2020.1696307
Fenteany G, Gaur P, Sharma G, Pintér L, Kiss E, Haracska L (2020) Robust high-throughput assays to assess discrete steps in ubiquitination and related cascades. BMC Mol Cell Biol 12(21):1–6. https://doi.org/10.1186/s12860-020-00262-5
García-Serradilla M, Risco C, Pacheco B (2019) Drug repurposing for new, efficient, broad spectrum antivirals. Virus Res 264:22–31. https://doi.org/10.1016/j.virusres.2019.02.011
Senanayake SL (2020) Drug repurposing strategies for COVID-19. Future Drug Discov 0(0) fdd-2020-0010. https://doi.org/10.4155/fdd-2020-0010
Shah B, Modi P, Sagar SR (2020) In silico studies on therapeutic agents for COVID-19: drug repurposing approach. Life sciences 252:117652. https://doi.org/10.1016/j.lfs.2020.117652
Zhou W, Liu Y, Tian D, Wang C, Wang S, Cheng J, Hu M, Fang M, Gao Y (2020) Potential benefits of precise corticosteroids therapy for severe 2019-nCoV pneumonia. Signal Transduct Target Ther 5(1):18. https://doi.org/10.1038/s41392-020-0127-9
Bhimraj A, Morgan RL, Shumaker AH, Lavergne V, Baden L, Cheng VC, Edwards KM, Gandhi R, Muller WJ, O'Horo JC, Shoham S, Murad MH, Mustafa RA, Sultan S, Falck-Ytter Y (2020) Infectious Diseases Society of America Guidelines on the treatment and management of patients with COVID-19. Clin Infect Dis 27:ciaa478. https://doi.org/10.1093/cid/ciaa478
Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y (2020) Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 8(5):475–481. https://doi.org/10.1016/S2213-2600(20)30079-5
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B (2020) Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395(10229):1054–1062. https://doi.org/10.1016/S0140-6736(20)30566-3
Liu J, Zhang S, Dong X, Li Z, Xu Q, Feng H, Cai J, Huang S, Guo J, Zhang L, Chen Y, Zhu W, Du H, Liu Y, Wang T, Chen L, Wen Z, Annane D, Qu J, Chen D (2020) Corticosteroid treatment in severe COVID-19 patients with acute respiratory distress syndrome. J Clin Invest 130(12):6417–6428. https://doi.org/10.1172/JCI140617
Dequin PF, Heming N, Meziani F, Plantefève G, Voiriot G, Badié J, François B, Aubron C, Ricard JD, Ehrmann S, Jouan Y, Guillon A, Leclerc M, Coffre C, Bourgoin H, Lengellé C, Caille-Fénérol C, Tavernier E, Zohar S, Giraudeau B, Annane D, Le Gouge A, CAPE COVID Trial Group, the CRICS-TriGGERSep Network (2020) Effect of hydrocortisone on 21-day mortality or respiratory support among critically ill patients with COVID-19: a randomized clinical trial. JAMA 324(13):1298–1306. https://doi.org/10.1001/jama.2020.16761
Ramiro S, Mostard RLM, Magro-Checa C, van Dongen CMP, Dormans T, Buijs J, Gronenschild M, de Kruif MD, van Haren EHJ, van Kraaij T, Leers MPG, Peeters R, Wong DR, Landewé RBM (2020) Historically controlled comparison of glucocorticoids with or without tocilizumab versus supportive care only in patients with COVID-19-associated cytokine storm syndrome: results of the CHIC study. Ann Rheum Dis 79(9):1143–1151. https://doi.org/10.1136/annrheumdis-2020-218479
Angus DC, Derde L, Al-Beidh F, Annane D, Arabi Y, Beane A et al (2020) Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial. JAMA 324(13):1317–1329. https://doi.org/10.1001/jama.2020.17022
Mason RJ (2020) Pathogenesis of COVID-19 from a cell biology perspective. Eur Respir J 55(4):2000607. https://doi.org/10.1183/13993003.00607-2020
Steinberg KP, Hudson LD, Goodman RB, Hough CL, Lanken PN, Hyzy R, Thompson BT, Ancukiewicz M National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network (2006) Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med 354(16):1671–1684. https://doi.org/10.1056/NEJMoa051693
Lee N, Allen Chan KC, Hui DS, Ng EK, Wu A, Chiu RW, Wong VW, Chan PK, Wong KT, Wong E, Cockram CS, Tam JS, Sung JJ, Lo YM (2004) Effects of early corticosteroid treatment on plasma SARS-associated coronavirus RNA concentrations in adult patients. J Clin Virol 31(4):304–309. https://doi.org/10.1016/j.jcv.2004.07.006
Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Zhou X, Du C, Zhang Y, Song J, Wang S, Chao Y, Yang Z, Xu J, Zhou X, Chen D, Xiong W, Xu L, Zhou F, Jiang J, Bai C, Zheng J, Song Y (2020) Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 180(7):934–943. https://doi.org/10.1001/jamainternmed.2020.0994
Shang L, Zhao J, Hu Y, Du R, Cao B (2020) On the use of corticosteroids for 2019-nCoV pneumonia. Lancet 395(10225):683–684. https://doi.org/10.1016/S0140-6736(20)30361-5
Khilnani GC, Hadda V (2011) Corticosteroids and ARDS: a review of treatment and prevention evidence. Lung India 28(2):114. https://doi.org/10.4103/0970-2113.80324
RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ (2020) (2020) Dexamethasone in hospitalized patients with covid-19 - preliminary report. N Engl J Med NEJMoa2021436. https://doi.org/10.1056/NEJMoa2021436
El Hadidi S, Rosano G, Tamargo J, Agewall S, Drexel H, Kaski JC, Niessner A, Lewis BS, Coats AJS (2020) Potentially inappropriate Prescriptions in Heart Failure with Reduced Ejection Fraction (PIP-HFrEF). Eur Heart J Cardiovasc Pharmacother:pvaa108. https://doi.org/10.1093/ehjcvp/pvaa108
Acknowledgements
Not applicable
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
RR and PSB contributed in the literature search, data collection, data analysis, and writing. PV did the data analysis, data interpretation, figures, and writing. SJUCJ is responsible for the concept, design, methods, data interpretation, writing, and proof reading. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Raju, R., V., P., Biatris, P.S. et al. Therapeutic role of corticosteroids in COVID-19: a systematic review of registered clinical trials. Futur J Pharm Sci 7, 67 (2021). https://doi.org/10.1186/s43094-021-00217-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43094-021-00217-3