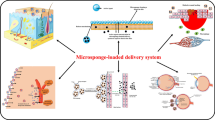
Abstract
Background
Microsponges are one of the advanced drug delivery systems that facilitates precise and controlled release of active ingredients that are suitable for topical and oral use. These porous microspheres are typically sized between 5 and 300 μm, offer benefits including controlled release, stability, and minimized side effects. Manufacturing techniques like quasi-emulsion solvent diffusion and liquid–liquid suspension polymerization are usually employed to prepare microsponges, although various challenges arise from the use of potentially hazardous organic solvents.
Main body
Microsponges possess distinct traits such as extended drug release, formulation flexibility, and high drug loading capacity. Entrapment of drugs requires considerations of solubility, stability, and miscibility, while evaluation methods encompass production yield and particle size analysis. Their applications range from dermatological to biopharmaceutical delivery, with diverse products utilizing this technology. Ongoing innovations about microsponges are evident in patents concerning medical dressings and hyaluronic acid delivery systems.
Conclusion
Microsponges present a promising avenue in drug delivery, despite many challenges. Current review addresses on limitations and diverse products highlighting commercial viability. Patent activity signifies continued interest, suggesting significant potential for enhancing patient care.
Similar content being viewed by others
1 Background
Innovative drug delivery technology is rapidly evolving and microsponges are at the forefront in innovative pharmaceutical technology. The technology associated with microsponge drug delivery holds immense potential in realizing the objective of precise and regulated drug administration at specific sites. As a result, researchers have given it a lot of attention [1]. Microsponges are non-collapsible, strongly cross-linked, porous microspheres made of polymeric materials with size ranging between 5 and 300 μm diameter. They possess the ability to load a wide range of active ingredients such as essential oils, antimicrobial agents, fragrances, sunscreens, anti-inflammatory compounds, and antifungal agents. Lately, the use of them for oral administration was also been studied [2, 3, 5]. When used as topical drug carriers, microsponges offer a steady and extended rate of drug release, minimizing discomforts and side effects without compromising the therapeutic efficacy [4]. The purpose of microsponges is to effectively administer a pharmaceutically active substance at the lowest possible dose as well as to improve the formulation stability, elegance, and flexibility, reduce side effects and alter the drug release profile in controlled or sustained manner [6]. There are several ways to produce the topical drug formulations using microsponge delivery mechanism, including lotion, gel, or cream. When the microsponge formulation is applied topically, the microsponge delivery system (MDS) releases its active ingredients in response to different stimuli, viz., friction, pH, and temperature at certain timings [7]. Excessive accumulation of drug in the dermis and epidermis may be avoided using microsponge system. As a result, it can considerably decrease irritability without compromising in producing therapeutic activity [6, 8]. Because of their sponge-like structure, microsponges offer special features like easy dissolving and quick compressing of materials. They provide better patient compliance, stable, nontoxic, non-allergic, and non-mutagenic while they offer very few adverse effects unlike other drug delivery systems [2]. Dermal delivery of drug is a broad area of use for microsponges but they are also used for oral administration, advances in bone and tissue engineering, illness detection, and RNAi (ribonucleic acid interference) silencing. The rapid progression of drug delivery technology is further propelled by the emergence of innovative categories of pharmaceuticals and biopharmaceuticals, encompassing proteins, peptides, and nucleic acid-based therapeutics. Consequently, microsponge drug delivery systems represent a burgeoning field that demands thorough exploration [9]. Like enhancement of solubility, precision in targeted organ action, augmented drug stability, targeted medication administration, controlled dispensation of drugs, controlled Release of drugs, dermal drug delivery, oral administration of drugs, advancements in engineering of bone tissue, advances in cardiovascular engineering, rebuilding the vascular walls [10]. Figure 1 shows the structure of microsponges.
2 Main text
2.1 Characteristics of microsponges [1, 11]
-
1.
Majority of components and vehicles can be used to formulate microsponges.
-
2.
Microsponges exhibit complete miscibility with a small quantity of nonpolar solvent.
-
3.
Microsponge formulations remain stable over the pH range between 1 and 11.
-
4.
Microsponges are stable at temperatures as high as 130º C.
-
5.
Microsponges demonstrate stability when exposed to the catalyst and within the environment of polymerization.
-
6.
Microsponges are self-sterilizing as they possess pores of 0.25 μm, which do not allow bacteria to permeate into them.
-
7.
Microsponge compositions can be economical and free flowing.
-
8.
Up to 50–60% of microsponge formulations exhibit substantial entrapment.
-
9.
They are flexible to formulate.
-
10.
It offers prolonged release of drug for up to 12 h.
2.2 Advantages of microsponge delivery system [9, 10]
-
1.
Microsponges can absorb oil at a ratio of up to six times their weight without experiencing desiccation.
-
2.
They offer prolonged drug release for up to 12 h.
-
3.
Enhance the robustness at chemical, physical, and thermal levels.
-
4.
Adaptability to create innovative product shapes.
-
5.
They regulate the drug release.
-
6.
Exhibit, improved patient compliance with less irritability and improved tolerance.
-
7.
Can exhibit site specific and targeted activity.
-
8.
MDS have stability over a pH range 1–11.
-
9.
They are free from harmful effects, non-irritating, non-mutagenic, and non-allergic.
-
10.
Exhibits increased drug stability and high drug loading capacity.
-
11.
Compared to other technologies such as liposomes and microencapsulation, MDS is easy to prepare, has a larger payload, and a wider spectrum of chemical stability.
-
12.
MDS allows the incorporation of immiscible products and increases drug's bioavailability.
-
13.
Compatible with all vehicles and other excipients and the solution is easy to navigate and reasonably priced.
2.2.1 Advantages over conventional formulations
Conventional topical formulations are formulated with the aim of targeting outer layers of the skin. Upon application, these formulations gradually release their active constituents that form a concentrated coating that absorbs fast. As a result, dermis and epidermis experience an accumulation or excessive buildup of drug, while the microsponges possess the capability to mitigate this issue by releasing the active ingredient to the skin in a gradual manner. Consequently, the microsponge system has the potential to significantly diminish side effects such as irritation, while maintaining its efficacy. Examples of such formulations include MDS of benzoyl peroxide, which has minimum irritation and good efficacy [12].
2.2.2 Advantages over ointments
Patient compliance is decreased by the ointment's greasy texture. Ointments are not particularly successful as drug delivery vehicles since these compounds require high concentrations of active ingredients to work, which might lead to irritation and sensitization. Bad odors, uncontrollable evaporation of active ingredients and possible drug-vehicle incompatibilities are disadvantages of topical preparations. However, the microsponge system in the epidermis or under the skin's surface prolongs the activity without any irritation or other issues faced by normal ointments [13].
2.2.3 Advantages over liposomes and microencapsulation
MDS offers benefits in comparison with alternative approaches such as liposomes and microencapsulation. Microcapsules often lack the ability to regulate the rate of active substance leakage. Once the wall ruptures, the active ingredients enclosed within the microcapsules are promptly released. The drawbacks associated with liposomes encompass reduced drug loading, less heat stability, complex formulation processes, limited chemical stability, and susceptibility to microbial instability. Conversely, the microsponge system exhibits robustness, enduring temperatures up to 130 °C, and remains stable within a pH range between 1 and 11, distinguishing it from the aforementioned systems. Additionally, its self-sterilizing nature is attributed to an average pore size of 0.25 µm, preventing the access of pathogens while maintaining compatibility with diverse vehicles and substances. Moreover, it retains its free-flowing characteristic and offers a higher drug load capacity ranging between 50 and 60% [14].
2.3 Limitations [11]
1. The process of microsponge formulation includes addition of organic solvents which are called porogens and are found to be harmful to the environment and public safety as some of them may be very combustible.
2. There are instances where the residual monomer traces found in microsponge formulations are poisonous and dangerous to human health.
2.4 Properties of the actives for entrapment into microsponges [15,16,17]
-
1.
Drugs used for microsponge formulation should ideally possess minimal solubility; failing which, the vehicle may degrade the microsponge before application.
-
2.
Drug must not react with monomers and must not cause the preparation's viscosity to rise while being formulated.
-
3.
It must maintain stability under conditions of polymerization.
-
4.
Should be miscible with minimum quantity of solvent.
-
5.
Drug ought to keep the microsponge's spherical structure intact.
-
6.
In order to eliminate cosmetic defects, the vehicle must be restricted to containing only 10 to 12% w/w of microsponge.
2.5 Microsponge preparation methods
2.5.1 Quasi-emulsion solvent diffusion technique
Microsponges by quasi-emulsion solvent diffusion method is prepared by dissolving the polymer in suitable solvent usually ethanol which forms an inner phase. Subsequently, the drug will be added into the inner phase and the mixture was permitted to dissolve for a duration of 15 min at 35 °C under ultrasonication. In the next step, the outer phase is prepared by dissolving polyvinyl alcohol (PVA) in distilled water at an ambient temperature. Following this, the inner phase is combined with the outer phase at room temperature and the mixture is subjected to continuous stirring for a duration of two hours at 500 rpm. This results in microsponge formation, later the preparation is filtered to isolate microsponges. Subsequently, the resultant product is cleaned and dried at 40 °C in an oven [18,19,20].
Figure 2 illustrates the quasi-emulsion solvent diffusion preparation process.
2.5.2 Quasi-emulsion solvent evaporation technique
Quasi-emulsion solvent evaporation technique is one of the very feasible methods to prepare microsponges. Dichloromethane (DCM), ethyl cellulose, and drug were used to create the internal phase and the internal phase is stirred on a magnetic stirrer for 15 min. Subsequently, the internal phase should be cautiously introduced drop by drop into a solution comprising a surfactant and plasticizer in water, which serves as the external phase. Upon the completion of the emulsification process, the mixture is subjected to continuous stirring for about 1 h. This results in the elimination of DCM that leads to the formation of microsponges. The suspension formed is thus filtered to obtain microsponges and is dried for 24 hours at 40 °C [21, 22].
Figure 3 illustrates the preparation process for quasi-emulsion solvent evaporation.
2.5.3 Liquid–liquid suspension polymerization
This technique involves the use of monomers, surfactant, and the active ingredient that are dissolved in a suitable solvent. To create a suspension, the above mixture is added with a suspending agent [23]. Once the suspension containing distinct particles of the desired size is established, polymerization is triggered either through the addition of a catalyst or by raising the temperature, occasionally supplemented with radiation. The polymerization process yields a reservoir-like configuration featuring surface perforations in specific cases, an inert liquid, which is fully miscible with the monomer but immiscible with water, is employed to establish the pore network throughout the polymerization process. Upon completion of the polymerization, the liquid is extracted, resulting in the formation of microsponges that interpenetrate with previously generated microsponges. These microsponges serve as carriers in topical treatments by including a range of active ingredients, including antifungal agents, rubefacients, anti-acne chemicals, and anti-inflammatory agents. A two-stage process is employed when the medication is vulnerable to the conditions of polymerization. Figure 4 illustrates the preparation of suspension polymerization in a liquid–liquid system using a reaction vessel.
An overview of the many steps involved in creating microsponges is provided below:
-
Step 1: Selection of monomer and the monomer mixture.
-
Step 2: Creation of chain monomers after initiating polymerization.
-
Step 3: Ladders are formed by the cross-linking of chain monomers.
-
Step 4: Production of spherical particles.
-
Step 5: Bunches of microspheres are produced when the microspheres agglomerate.
2.6 Mechanism of drug release
In reaction to one or more external stimuli, microsponges tend to release drug in a predetermined amount. Figure 5 represents the mechanism of drug release via microsponge delivery system.
Solubility The rate at which active agents are discharged from microsponges can be triggered by an aqueous media, such as sweat. The release of the active drug can be influenced by factors such as the solubility of the drug in the external medium, the concentration gradient, or the capacity of the microsponge network to expand [25].
Pressure release When the microsponge system is compressed or squeezed, fluid or the active ingredient is released, resupplying the skin with the amount of entrapped active component. The sponge's release and the microsponges' resilience may also have an impact on the amount released [26, 28].
Temperature change Temperature can be used to trigger the release of drug from microsponges. At ambient temperature, numerous of the encapsulated active ingredients may exhibit excessive viscosity, hindering their direct flow from the microsponges onto the skin. Raising the temperature of the skin also raises the flow rate, which raises the release [27].
pH triggered systems By altering the microsponge's covering or coatings, the microsponges can release drug as pH-based released [28].
2.7 Characterization and evaluation of microsponges
2.7.1 Production yield
The practical or production yield of the microsponges can be calculated using the following formula [29, 30].
2.7.2 Particle size analysis
The particle size of the produced microsponges can be analyzed by a particle size analyzer. This instrument enables sample measurement within the range of 0.020 to 2000 mm. The particle size of microsponges can also be determined by an optical microscope [31, 32].
2.7.3 Drug content
To estimate the drug content in microsponges, 100 mg equivalent microsponges are precisely weighed and mixed in 100 milliliters of phosphate buffer solution (PBS) (pH 6.8). The mixture should be filtered through a 0.45-µm membrane filter and the samples are to be analyzed at a suitable wavelength using ultraviolet–visible (UV) spectrophotometer. The drug content can be calculated using the following formula [33].
2.7.4 Entrapment efficiency
The solvent extraction method can be used to assess the drug entrapment efficiency. Ten mg of precisely weighed microsponge particles is dissolved in 5 mL of methanol using a magnetic stirrer for a duration of 20 min. 20 mL of freshly prepared phosphate buffer solution (PBS) must be added and heated to a temperature range of 45–50 °C till the formation of a clear solution. Later, methanol is allowed to evaporate, cooled to 25 °C and filtered. Following appropriate dilutions, the drug's concentration is measured using UV spectroscopy. To compute drug encapsulation efficiency (DEE%), the following formula can be used [1, 34].
2.7.5 Physical compatibility testing using differential scanning calorimetry (DSC)
This is an essential assessment method for determining any potential physical interactions between the drug and excipients through thermal analysis. Any alteration in the thermogram from that of the pure drug indicates the presence of physical incompatibility. The temperature and enthalpy scale of the DSC are calibrated using indium as the internal standard. Following hermetic sealing within an aluminum pan, the powder sample of microsponges undergoes a gradual heating process at a rate of 10 °C/min, spanning from 30 to 300 °C, while being subjected to a nitrogen atmosphere flow of 20 ml/min [6, 35].
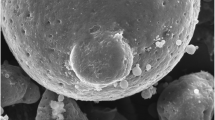
2.7.6 Scanning electron microscopy (SEM) analysis
The morphology of the microsponges prepared is studied using scanning electron microscopy (SEM) [21, 36].
2.7.7 Chemical compatibility testing using Fourier transform infrared analysis (FTIR)
Using infrared light, the FTIR spectra of the drug-loaded microsponges and the drug alone are examined to search for any chemical interactions. The FTIR analysis is done using a KBr disk standard and scanned within the range of 400 cm−1 to 4000 cm−1 [5, 21].
2.7.8 X-ray powder diffraction (XRD)
To comprehend the X-ray diffraction (XRD) pattern of both the pure drug and the optimized formulation, the XRD findings for the samples are acquired using an XRD technique fitted with a nickel filter and copper target. The powder sample is applied uniformly and smoothed out onto metal stages with glass bottoms. The XRD pattern of each sample is obtained with a step increment of 0.10 (2θ) and a dwell time of one second between successive measurements, covering a range from 10 to 500 (2θ) [37, 38].
2.7.9 Stability study
In accordance with “International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use” (ICH) recommendations, various conditions must be set to conduct stability experiments on drug-loaded microsponges. The microsponge formulations are stored under conditions of 40 °C ± 2 °C and 75% ± 5% relative humidity for a duration of three months. Three months later, the microsponges are to be subjected to in vitro drug release studies and physical properties evaluation [2, 38].
2.7.10 Polymer/monomer composition
The drug release from microspheres is controlled by polymer composition, drug loading, and microsponge size. Modifying the drug's partition coefficient between the vehicle and the microsponge system allows for direct influence by the polymer composition of the MDS on the rate of entrapped drug release. A valuable approach for investigating drug release from microsponge systems with diverse polymer compositions involves plotting the cumulative percentage of drug release against time [39].
2.7.11 Resiliency
The rheological properties of the formulation can be modified by changing the concentration of cross-linking agent and polymer composition in order to control its flow characteristics and deformation behavior. On the other hand, one should remember that increasing the concentration of cross-linking agents in order to modify viscosity may lead to the reduction or fluctuations in drug release [40, 41].
2.7.12 In vitro drug release
100 mg equivalent weight of microsponges is weighed accurately and the in vitro drug release studies can be done using USP dissolution testing apparatus type II (USP II). An aliquot of microsponge suspension is administered onto a dialysis membrane (pore size 14,000 Da, diameter 17.5 mm, HI-media) to determine the drug release. The dialysis bags must be fastened using paddles and positioned within dissolution vessels filled with buffer solution. Subsequently, the vessels are subjected to stirring at 50 rpm once the temperature is stabilized to 37 ± 1 °C. Evaluation of drug release into the surrounding solution, attributed to membrane diffusion, is conducted by periodically collecting samples from the solution at specified time intervals. UV visible spectrophotometer can be used to quantify the amount of drug released from microsponge formulation [33, 42].
2.8 Different analysis comparing the drugs with various microsponge methods
Listed in Table 1.
2.9 Applications of microsponge systems [10]
Porous polymeric microspheres known as microsponges are primarily utilized in topical applications, although recent advancements have expanded their use to oral administration. They offer a diverse range of possibilities for formulators engaged in the creation of pharmaceutical and cosmetic products. Microsponges are formulated to maximize the efficacy of delivering pharmaceutical active ingredients at minimal doses, concurrently elevating stability, mitigating side effects, and modulating drug release dynamics.
-
1.
Microsponges often demonstrate an erratic release profile of active pharmacological ingredients (API). The emergence of the enclosed API within the microsponges is promptly observed upon rupture of the capsule membrane.
-
2.
There exists a payload efficiency ranging between 50 and 60%.
-
3.
Microsponges, being minute spheres, exhibit the capability to absorb skin secretions.
-
4.
User-friendly and economically viable.
2.9.1 Pharmaceutical applications [61]
Summarized uses of microsponges in various formulations are listed in Fig. 6.
2.9.2 Microsponge for topical delivery
Microsponge systems are fabricated using polymers that demonstrate biologically inert properties. Numerous safety evaluations have confirmed that these polymers exhibit characteristics such as non-toxicity, non-mutagenicity, non-irritation, and non-biodegradability. Because of this, the body is unable to break them down or transform them into other chemicals. These systems, albeit minuscule in size, are too big to fit through the stratum corneum when they are added to topical medicines [17]. Figure 7 shows the mechanism of drug release from dermal microsponges.
Fluocinolone acetonide (FA), a corticosteroid agent, is predominantly employed in dermatology to alleviate skin irritations and improve inflammatory conditions [62]. Acne and athletes’ foot are treated with benzoyl peroxide (BPO). Common adverse effects include skin irritation, which can be lessened while lowering percutaneous absorption by carefully releasing BPO from the microsponge into the skin [63,64,65].
A research on development of the mupirocin topical microsponges using emulsion solvent diffusion technique has proved to enhance drug deposition in the skin and achieve sustained release. The impact of formulation and procedural parameters including agitation speed and volume of the internal phase, on the physical attributes of microsponges, are explored using an optimized drug/polymer ratio and a 32-factorial design. The enhanced microsponges are integrated into a base prepared with emulgel. Various aspects were examined, including the in vivo antibacterial effectiveness of formulations containing microcin, in vitro drug release, and ex vivo drug deposition. The medication and polymer molecules did not interact with the spherical, porous prepared microsponges. Preferred physical characteristics were demonstrated by emulgels incorporating microsponges. Drug release assessments conducted with cellulose-based dialysis membranes and drug deposition tests on rat abdominal skin have demonstrated a notable retention of active ingredients within the skin from formulations based on microsponges for up to 24 h. The refined formulations were confirmed to demonstrate stability and skin compatibility test through the Draize patch test. In a murine surgical wound model infected with S. aureus, emulgel formulations containing microsponges demonstrated extended effectiveness. The utilization of mupirocin in topical emulgel formulations showed improved retention and stability on the skin, suggesting the delivery system's enhanced efficacy in treating various skin infections, including primary and secondary conditions such as impetigo, atopic dermatitis, and eczema [66].
*Strong chemotherapeutic medication 5-fluorouracil (5-FU) is used to treat actinic keratosis, a condition caused by chronic sun exposure that results in rigid skin and precancerous cells [67].
2.9.3 Microsponge for oral delivery
Microsponges help to maintain the drugs in a protected environment and release the drug under regulated circumstances to the lower gastrointestinal tract [25]. Through the pores of the microsponge system, weakly water-soluble drugs are captured. It has been shown that when drugs are taken orally, the microsponge system can quicken their rate of solubilization. The medicine is effectively reduced to minute particles due to the extremely small pores, which enhances their surface area and accelerates the solubilization process. An additional benefit is that the microsponge system increases the amount of medication absorbed since it takes a lot longer to transit through the small and large intestine [68]. Figure 8 shows the mechanism of drug release from oral microsponge.
A research showed that ketoprofen microsponges were prepared using quasi-emulsion solvent diffusion method and converted into table dosage forms by direct compression technique. This article proved that the plastic deformation of the microsponge structure enhanced compressibility during the physical combination of the medication and polymer, resulting in the production of mechanically robust tablets [69].
Another research used flurbiprofen (FLB) as drug and prepared microsponges using quasi-emulsion solvent diffusion method. Furthermore, FLB was encapsulated within a commercially available Microsponge® 5640 device utilizing an entrapment technique. The development of colon-specific formulations involved techniques such as compression coating, pore filling, and tabulation of microsponges with a blend of pectin and hydroxy propyl methyl cellulose (HPMC). The sponge-like structure of microsponges, allowed for the plastic deformation of the tablet, results in mechanically robust medication distribution tailored to the colon. In vitro analysis demonstrated a modified release pattern of the medication at the eighth hour, aligning with the time of arrival at the proximal colon, particularly in the presence of enzymes within compression-coated tablet formulations designed for colon-specific delivery. Nevertheless, during the eighth hour, the inclusion of enzymes resulted in a notable augmentation in the release of medication from colon-targeted formulations prepared using pore-plugging microsponges [39].
2.9.4 Microsponges for bone and tissue engineering as substitute for bone
The microsponges were formulated by combining two aqueous dispersions comprising tricalcium phosphate granules, powdered calcium hydroxyapatite, and pre-polymerized polymethyl methacrylate powders with liquid methyl methacrylate monomer. The completed composites seemed porous and worked as microsponges. Utilizing the biodegradable properties of the sponge matrix, a collagen sponge sheet encapsulating basic fibroblast growth factor (bFGF) was administered subcutaneously in mice, demonstrating locally angiogenic activity that varied in accordance with dosage form. The bolus injection of bFGF would never have been able to achieve the substantial increase in blood flow that the collagen microsponges containing bFGF produced in the ischemic hind leg of the mouse. Type I collagen acted as a depot for bFGF, which highlights its significance and its therapeutic use [70,71,72].
2.9.5 Microsponges in oral care cosmetics
By maintaining the release of volatile compounds, microsponge technology offers an exciting prospective application in oral cosmetics, where it can extend the "fresh feel." Tooth pastes and mouth washes can readily absorb microsponges of these volatile substances [73].
2.9.6 Microsponges for biopharmaceutical delivery
Biopharmaceutical delivery and tissue engineering both made use of the microsponge delivery technique. The benefits of synthetic poly(lactic-co-glycolic acid) (PLGA-A biodegradable copolymer used in biomedical applications such as drug delivery and tissue engineering) knitted mesh and natural type I collagen were combined to create hybrid 3D scaffolds. For tissue development and cell seeding, collagen microsponges were used, and a mechanically robust PLGA mesh was used as a skeleton. There were three sets of scaffolds: Collagen microsponge could be made in three different ways: sandwich-style (on both sides), semi-thickly (on one side), and thinly (in the crevices between the PLGA mesh) [23].
2.9.7 Vascular wall reconstruction with microsponge technology
A composite construct was created by integrating a collagen microsponge with a biodegradable polymeric scaffold composed of knitted mesh externally reinforced with woven polylactic acid and polyglycolic acid, resulting in the development of a tissue-engineered patch. Tissue-engineered patches devoid of pre-seeding with cells were implanted into the descending aorta of swine (n = 5), the main pulmonary artery of pigs (n = 8), or the right ventricular outflow tract of canines (n = 4). Assessments of the histology and biochemistry were carried after one, two, and six months following the implantation. In each animal, thrombus formation was not observed. Two months post-implantation, histological examination using hematoxylin/eosin staining and immunostaining revealed that all grafts exhibited robust in situ cellularization. The polymerase chain reaction technique, employed to quantify the cell population, detected a significant presence of smooth muscle and endothelial cells two months post-implantation. After six months, the tissue architecture of the patch in the large transplantation model closely resembled that of native tissue, indicating its potential as an innovative surgical material for cardiovascular system restoration [41, 74].
2.10 Different analysis showing the difference between different conventional and advanced delivery systems
2.10.1 Conventional drug delivery systems
Topical administration
-
Traditional methods such as eye drops and ointments are frequently employed in ocular drug delivery owing to their widespread acceptance among patients and the convenience they offer for self-application [75].
-
The constrained ocular bioavailability poses a substantial obstacle when utilizing conventional drug delivery systems, thereby affecting the efficiency of drug administration. In this, case microsponges offer a better bioavailability minimizing the limitations observed in conventional systems [76].
2.10.2 Advanced drug delivery systems
2.10.2.1 Novel drug delivery systems (NDDS)
Novel drug delivery systems (NDDS) have been devised to address the shortcomings associated with conventional formulations, aiming to enhance drug release kinetics, augment drug penetration, and elevate antifungal efficacy.
Research findings indicate that novel drug delivery systems exhibit superior performance compared to conventional formulations in terms of drug release, permeation, and antifungal efficacy, underscoring their advantage in ocular drug delivery [77].
2.10.3 Specific differences
2.10.3.1 Performance
Advanced delivery systems, such as microsponges, exhibit enhanced efficacy regarding drug release, permeation, and therapeutic outcomes when compared to conventional systems.
Microsponges are engineered to optimize drug administration through the augmentation of drug bioavailability and the precise targeting of particular tissues or cellular entities, thereby resulting in enhanced therapeutic efficacy [78].
2.10.3.2 Antibacterial delivery
Nano-liposomal delivery systems have surfaced as effective transporters for antibacterial agents, providing benefits in encapsulation efficiency, antibacterial mechanisms, and interactions with bioactive compounds [79].
2.10.3.3 Drug loading and release
Innovative approaches, such as supercritical CO2 impregnation, have demonstrated elevated drug loadings and extended drug release durations in contrast to conventional soaking methodologies, underscoring the significance of novel techniques in drug delivery systems [80].
2.11 Marketed products of microsponges
Listed in Table 2.
2.12 Patent information
Patented information is listed in Table 3.
2.13 Recent advancements of Microsponges as drug delivery systems
By altering the process of creating microsponges, several innovations have been created, including nanoferrosponges, nanosponges, and microbeads. β-CD nanosponges by using β-CD (beta-cyclodextrin—is a cyclic oligosaccharide commonly used to improve drug solubility and stability in pharmaceutical formulations) is beneficial for trapping of hydrophilic as well as hydrophobic drugs. Several drugs, including flurbiprofen, dexamethasone, doxorubicin, itraconazole, and serum albumin, were administered orally using these methods. In order to make these nanosponges, the β-CD molecule must be cross-linked using biphenyl carbonate. These nanosponge carrier drug delivery systems are particularly helpful for incorporating cytotoxic medications into targeted drug delivery for malignant cells. Additionally, they noted enhanced ribonucleic acid (RNA) stability and a somewhat successful small interfering RNA(siRNA)encapsulation procedure. This strategy could result in new therapeutic avenues for the delivery of siRNA [26, 104, 105].
2.14 Future prospects
A microsponge is made up of several interconnected voids housed in a non-collapsible framework that can hold a broad range of materials. Today, scientists are focusing more on the delivery of sunscreen, anti-acne, anti-dandruff, and agents that can also be used in the delivery of thermolabile substances like vaccines, proteins, peptides, and deoxyribonucleic acid-based therapeutics. It is also employed in the field of tissue engineering and in controlled drug release systems for medications necessitating extended therapeutic regimens. The outer surface of the sphere is typically porous, allowing substances to flow into and out of it. In these investigations, optimization techniques are used to get the greatest possible result from several formulations. Employs efficient and safe methods for delivering active substance. Moreover, parenteral and pulmonary drug administration using these porous devices has been researched. As microsponge particles find utility as a cell culture medium, their potential extends to stem cell cultivation and cellular regeneration within the organism. Prospective uses of microsponge carrier systems encompass cosmetic applications. Furthermore, the flexibility of the formulation offers advantages across diverse sectors, paving the way for innovative medication delivery systems.
3 Conclusion
Microsponges are a revolutionary drug delivery technology with versatile applications. Ranging in size from 5 to 300 μm, these porous microspheres offer controlled release, stability, and reduced side effects. Synthesized through techniques like quasi-emulsion solvent diffusion, they found use in dermatological and oral drug delivery. Advantages include prolonged drug release, adaptability, and high loading capacity, although challenges exist, such as solvent use. Evaluation methods ensure quality, and various preparation techniques contribute to their versatility. Triggered drug release mechanisms enhance effectiveness. Marketed products and patents highlight their commercial viability and ongoing innovations. Microsponges represent a promising frontier in drug delivery, with potential across pharmaceutical and cosmetic domains.
Availability of data and materials
Not applicable.
Abbreviations
- MDS:
-
Microsponge delivery system
- DCM:
-
Dichloromethane
- SEM:
-
Scanning electron microscope
- BPO:
-
Benzoyl peroxide
- DNA:
-
Deoxyribonucleic acid
- HPMC:
-
Hydroxypropyl methylcellulose
- XRD:
-
X-ray powder diffraction
- DSC:
-
Differential scanning calorimetry
- bFGF:
-
Basic fibroblast growth factor
- FA:
-
Fluocinolone acetonide
- FTIR:
-
Fourier transform infrared analysis
- Β-CD:
-
Beta-cyclodextrin
- PLGA:
-
Poly(lactic-co-glycolic acid)
- (5-FU):
-
5-Fluorouracil
- PBS:
-
Phosphate-buffered saline
- ICH:
-
International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use
- RNA:
-
Ribonucleic acid
- NDDS:
-
Novel drug delivery systems
References
Design, formulation in-vitro evaluation of herbal microsponge by using. (n.d.). CABI Databases. 4(5):1923–1940. https://doi.org/10.5555/20153225981
Bhatia M, Saini M (2018) Formulation and evaluation of curcumin microsponges for oral and topical drug delivery. Prog Biomater 7(3):239–248. https://doi.org/10.1007/s40204-018-0099-9
Pandit AP, Patel SA, Bhanushali VP, Kulkarni VS, Kakad VD (2016) Nebivolol-Loaded Microsponge gel for healing of diabetic wound. AAPS PharmSciTech 18(3):846–854. https://doi.org/10.1208/s12249-016-0574-3
Maiti S, Kaity S, Ray S, Sa B (2011) Development and evaluation of xanthan gum-facilitated ethyl cellulose microsponges for controlled percutaneous delivery of diclofenac sodium. Acta Pharm 61(3):257–270. https://doi.org/10.2478/v10007-011-0022-6
Desavathu M, Raghuveer P, Chunduru M (2017) Design, development and characterization of Valsartan microsponges by quasi emulsion technique and the impact of stirring rate on microsponge formation. J Appl Pharm Sci 193–198:70128. https://doi.org/10.7324/japs.2017.70128
Yadav V, Jadhav P, Dombe S, Bodhe A, Salunkhe P (2017) Formulation and evaluation of microsponge gel for topical delivery of antifungal drug. Int J Appl Pharm 9:30–37. https://doi.org/10.22159/ijap.2017v9i4.17760
Monika, Dua JS, Prasad D, Hans M, Kumari S (2019) Preparation and characterization of itraconazole microsponges using Eudragit RSPO and study the effect of stirring on the formation of microsponges. J Drug Deliv Therap 9:451–458. https://doi.org/10.22270/jddt.v9i3-s.2879
Kumar JR, Varatharajan R, Muthuraman A (2020) Preparation and evaluation of povidone iodine based microsponge for wound healing activity in rats. J Pharm Sci Res 12(3):436–442
Bhanse Najuka D, Shah C, Shah D (2016) Novel and innovative strategy: microsponges drug delivery system. Pharma Sci Monit 7(2):54–73
Shafi SK, Duraivel S, Bhowmik D, Kumar KS (2013) Microsponge drug delivery system. Indian J Res Pharm Biotechnol 1(2):206
Khanka PS, Hussain K (2019) Formulation and evaluation of antifungal microsponge loaded gel. Int J Res Eng Sci Manag 2(12):2581–5792
Ahmed A, Makram M, Sayed M, Louis D (2018) An overview of microsponge as a novel tool in drug delivery. MADD 2(3):1–7. https://doi.org/10.31031/madd.2018.02.000537
Jyothi KN, Kumar PD, Arshad P, Karthik M, Panneerselvam T (2019) Microsponges: a promising novel drug delivery system. J Drug Deliv Therap 9(5-s):188–194. https://doi.org/10.22270/jddt.v9i5-s.3649
Pradhan SK (2011) Microsponges as the versatile tool for drug delivery system. Int J Res Pharm Chem 1(2):243–258
Aldawsari H, Badr-Eldin SM (2013) Microsponges as promising vehicle for drug delivery and targeting: preparation, characterization and applications. Afr J Pharm Pharmacol 7(17):873–881. https://doi.org/10.5897/AJPP12.1329
Mantry S, Bagchi A, Das S, Das S (2015) Microsponge as a novel strategy of drug delivery system. Univ J Pharm Sci Res 1(1):32–38
Roy A (2015) Microsponge as a novel drug carrier system: a review. World J Pharm Res 4(12):680–701
Sharma A, Hooda A, Chaudhary H (2016) Formulation and evaluation of topical microsponges of sertaconazole. World J Pharm Res 5(11):1444–1461. https://doi.org/10.20959/wjpr201611-7332
Mahajan Aniruddha G, Jag Tap Leena S, Chaudhari Atul L, Swami Sima P, Mali Prabha R (2011) Formulation and evaluation of microsponge drug delivery system using Indomethacin. IRJP 2(10):64–69
Bhagat VS, Arote SR (2021) Formulation development and in-vitro evaluation of microsponge drug delivery system of antifungal drug. Int J Pure Med Res 5(3):654–661
Redhu S, Pawar N (2021) Development and characterization of microsponge gel for topical delivery of oregano oil. Int J Pharm Sci Res 12(2):1060–1073. https://doi.org/10.13040/IJPSR.09758232.12(2).1060-73
Deshmukh K, Poddar SS (2012) Tyrosinase inhibitor-loaded microsponge drug delivery system: new approach for hyperpigmentation disorders. J Microencapsul 29(6):559–568. https://doi.org/10.3109/02652048.2012.668955
Vitthal JP, Rajasekaran S (2022) Novel approaches of herbal microsponges design, formulation and characterization: an overview. Int J Pharm Res Appl 7(5):1280–1291. https://doi.org/10.35629/7781-070512801291
Dumbre AK, Banerjee SK, Gadhave MV, Gaikwad DD (2014) Microsponge: a novel drug delivery system. https://www.ajprd.com/index.php/journal/article/view/173
Mansi H (2019) A review on microsponge delivery system. J Drug Deliv Therap | EBSCOhost. openurl.ebsco.com. https://doi.org/10.22270/jddt.v9i3-s.2938
Jadhav N, Patel V, Mungekar S, Bhamare G, Karpe M, Kadams V (2013) Microsponge delivery system: an updated review, current status and future prospects. J Sci Innov Res 2(6):1097–1110
Thakur R, Kumar S, Gaba P (2020) A review: novel method for microsponge drug delivery system. J Pharm Biol Sci 15(4):35–44. https://doi.org/10.9790/3008-1504023544
Lalitha SK, Shankar M, Likhitha D, Dastagiri J, Babu MN (2016) A current view on microsponge drug delivery system. Eur J Mol Biol Biochem 3(2):88–95
Farsana T, Geetha VS, Jumana KK, Mubashira NP (2023) Formulation development and evaluation of antimicrobial drug loaded microsponges for topical drug delivery. World J Pharm Res. https://doi.org/10.20959/wjpr202311-28698
Mohan D (2019) Microsponge based drug delivery system of voriconazole for fungal infection: formulation development and In-vitro evaluation. jddtonline.info. https://doi.org/10.22270/jddt.v9i3.2840
Dineshmohan S, Gupta VRM (2016) Transdermal delivery of fluconazole microsponges: preparation and in vitro characterization. J Drug Deliv Therap 6(6):1334. https://doi.org/10.22270/jddt.v6i6.1334
Eshwarlal MR, Kishan CV, Krishnarao PV, Motiram CH. Formulation and evaluation of sertaconazole nitrate microsponge gel
Emerging implementation of drug loaded with microsponges technology and their antifungal activity. J Pharm Negat Results 13(S01) (2022). https://doi.org/10.47750/pnr.2022.13.s01.103
Halder S, Poddar S, Khanam J (2021) Optimization and scale-up methodology in preparing microsponge loaded with 5-fluorouracil (5-FU). Drug Deliv Transl Res. https://doi.org/10.21203/rs.3.rs-989826/v1
Rajurkar VG, Tambe AB, Deshmukh VK (2015) Topical anti-inflammatory gels of naproxen entrapped in eudragit based microsponge delivery system. J Adv Chem Eng 5(2):0122. https://doi.org/10.4172/2090-4568.1000122
Syed SM (2020) Formulation and evaluation of gel containing fluconazole microsponges. www.ajprd.com. https://doi.org/10.22270/ajprd.v8i4.753
Bansode AS (2019) Formulation, development and evaluation of Microsponge loaded Topical Gel of Nystatin. jddtonline.info. https://doi.org/10.22270/jddt.v9i2-s.2567
Osmani RAM, Aloorkar NH, Ingale DJ, Kulkarni PK, Hani U, Bhosale RR, Dev DJ (2015) Microsponges based novel drug delivery system for augmented arthritis therapy. Saudi Pharm J 23(5):562–572. https://doi.org/10.1016/j.jsps.2015.02.020
Shinkar DM, Bhamare BS, Saudagar RB (2016) Microsponges. Asian J Res Pharm Sci 6(2):77–84. https://doi.org/10.5958/2231-5659.2016.00011.4
Das A, Chakraborty P, Khatiwara B, Dhakal J, Sarangi S, Singh S, Chakrabarti S (2022) Herbal microsponge incorporated sunscreen gel: A novel strategy. Biomedicine 42(5):844–850. https://doi.org/10.51248/.v42i5.2016
Pb M, Sg K, Vs H, Yogita S (2015) Recent advances in microsponges drug delivery system. J Crit Rev 3(1):2016
Wadhwa G, Kumar S, Mittal V, Rao R (2019) Encapsulation of babchi essential oil into microsponges: physicochemical properties, cytotoxic evaluation and anti-microbial activity. J Food Drug Anal 27(1):60–70. https://doi.org/10.1016/j.jfda.2018.07.006
Kothai S, Umamaheswari R (2019) Fabrication and characterisation of honey loaded microsponges. jddtonline.info. https://doi.org/10.22270/jddt.v9i4.3125
Amrutiya N, Bajaj A, Madhu MN (2009) Development of microsponges for topical delivery of mupirocin. AAPS PharmSciTech 10(2):402–409. https://doi.org/10.1208/s12249-009-9220-7
Vitthal P, Anuradha S (2020) A review on microsponges drug delivery system. IJRAR-Int J Res Anal Rev 7:961–974
Nagula RL, Wairkar S (2020) Cellulose microsponges based gel of naringenin for atopic dermatitis: design, optimization, in vitro and in vivo investigation. Int J Biol Macromol 164:717–725. https://doi.org/10.1016/j.ijbiomac.2020.07.168
Ravi R, Kumar SS, Parthiban S (2013) Formulation and evaluation of the microsponges gel for an anti-acne agent for the treatment of acne. Indian J Pharm Sci Res 3:32–38
Thavva VEDAVATHI, Baratam SR (2019) Formulation and evaluation of terbinafine hydrochloride microsponge gel. Int J Appl Pharm 11(6):78–85
Jain SK, Kaur M, Kalyani P, Mehra A, Kaur N, Panchal N (2019) Microsponges enriched gel for enhanced topical delivery of 5-fluorouracil. J Microencapsul 36(7):677–691. https://doi.org/10.1080/02652048.2019.1667447
Bothiraja C, Gholap AD, Shaikh K, Pawar A (2014) Investigation of ethyl cellulose microsponge gel for topical delivery of eberconazole nitrate for fungal therapy. Ther Deliv 5(7):781–794. https://doi.org/10.4155/tde.14.43
Avoxa-Mediengruppe Deutscher Apotheker GmbH (n.d.) Enhancement of ketoprofen bioavailability by formation of microsp.: Ingenta Connect. www.ingentaconnect.com. https://doi.org/10.1691/ph2007.1.6016
Jain V, Singh R (2011) Design and characterization of colon-specific drug delivery system containing paracetamol microsponges. Arch Pharmacal Res 34(5):733–740. https://doi.org/10.1007/s12272-011-0506-4
Orlu M, Cevher E, Araman A (2006) Design and evaluation of colon specific drug delivery system containing flurbiprofen microsponges. Int J Pharm 318(1–2):103–117. https://doi.org/10.1016/j.ijpharm.2006.03.025
Patel D, Gohil D, Patel D, Shah H, Patel S, Pandya K, Shah C (2016) Formulation and evaluation of floating microsponges of allopurinol. Pharma Sci Monit 7(3):135–154
Charagonda S, Puligilla RD, Ananthula MB, Bakshi V (2016) Formulation and evaluation of famotidine floating microsponges. Int Res J Pharm 7(4):62–67
Yang M, De Cui F, You BG, Fan Y, Wang L, Peng Y, Yang H (2003) Preparation of sustained-release nitrendipine microspheres with Eudragit RS and Aerosil using quasi-emulsion solvent diffusion method. Int J Pharm 259(1–2):103–113. https://doi.org/10.1016/s0378-5173(03)00209-6
He Y, Majid K, Maqbool M, Hussain T, Yousaf AM, Khan IU, Mehmood Y, Aleem A, Arshad MS, Younus A, Nirwan JS, Ghori MU, Rizvi SAA, Shahzad Y (2020) Formulation and characterization of lornoxicam-loaded cellulosic-microsponge gel for possible applications in arthritis. Saudi Pharm J 28(8):994–1003. https://doi.org/10.1016/j.jsps.2020.06.021
Swetha A, Rao MG, Ramana KV, Basha BN, Reddy VK (2011) Formulation and in vitro evaluation of etodolac entrapped in microsponge based drug delivery system. Int J Pharm 1(2):73–80
Osmani RAM, Aloorkar NH, Thaware BU, Kulkarni PK, Moin A, Hani U, Srivastava A, Bhosale RR (2015) Microsponge based drug delivery system for augmented gastroparesis therapy: formulation development and evaluation. Asian J Pharm Sci 10(5):442–451. https://doi.org/10.1016/j.ajps.2015.06.003
Rajab NA, Jawad MS (2016) Formulation and in vitro evaluation of piroxicam microsponge as a tablet. Int J Pharm Pharm Sci 8(2):104–114
Afnan T, Chakraborty P, Chakraborty DD, Chhetri P (2022) Microsponge based drug delivery systems: a critical update on its preparation, dermatological applications_and_patent_information. J Chengdu Univ Technol 26:24
D’souza JI, More HN (2008) Topical anti-inflammatory gels of fluocinolone acetonide entrapped in eudragit based microsponge delivery system. https://rjptonline.org/AbstractView.aspx?PID=2008-1-4-101
Jelvehgari M, Siahi-Shadbad MR, Azarmi S, Martin GP, Nokhodchi A (2006) The microsponge delivery system of benzoyl peroxide: preparation, characterization and release studies. Int J Pharm 308(1–2):124–132. https://doi.org/10.1016/j.ijpharm.2005.11.001
Nokhodchi A, Jelvehgari M, Siahi MR, Mozafari MR (2007) Factors affecting the morphology of benzoyl peroxide microsponges. Micron 38(8):834–840. https://doi.org/10.1016/j.micron.2007.06.012
Wester RC, Patel R, Nacht S, Leyden JJ, Melendres J, Maibach HI (1991) Controlled release of benzoyl peroxide from a porous microsphere polymeric system can reduce topical irritancy. J Am Acad Dermatol 24(5):720–726. https://doi.org/10.1016/0190-9622(91)70109-f
Jain N, Sharma PK, Banik A (2011) Recent advances on microsponge delivery system. Int J Pharm Sci Rev Res 8(2):13–23
Mandava SS, Thavva V (2012) Novel approach: microsponge drug delivery system. Int J Pharm Sci Res 3(4):967. https://doi.org/10.13040/IJPSR.0975-8232.3(4).967-80
Aloorkar NH, Kulkarni AS, Ingale DJ, Patil RA (2012) Microsponges as innovative drug delivery systems. Int J Pharm Sci Nanotechnol 5(1):1597–1606
Thakur I, Sharma N (2021) A review on innovative and novel strategy-floating microsponges. Zenodo. https://doi.org/10.5281/zenodo.4879516
Kaity S, Maiti S, Ghosh AK, Pal D, Ghosh A, Banerjee S (2010) Microsponges: a novel strategy for drug delivery system. Agric Policy Pap 1(3):283. https://doi.org/10.4103/0110-5558.72416
Kappor D, Patel MP, Vyas R, Lad C, Tyagi B (2014) A review on microsponge drug delivery system. J Drug Deliv Therap 4(5):978. https://doi.org/10.2270/jddt.v4i5.978
Hussain H, Juyal D, Dhyani A (2014) Microsponges: an overview. Int J Drug Deliv Technol 4(4):58–66
Shah CN, Shah DP (2014) Microsponges: a revolutionary path breaking modified drug delivery of topical drugs. Int J Pharm Res 6(2):1–13
Iwai S, Sawa Y, Ichikawa H, Taketani S, Uchimura E, Chen G, Hara M, Miyake J, Matsuda H (2004) Biodegradable polymer with collagen microsponge serves as a new bioengineered cardiovascular prosthesis. J Thorac Cardiovasc Surg 128(3):472–479. https://doi.org/10.1016/j.jtcvs.2004.04.013
Maurya P, Fatma S, Sharma D, Mishra JN, Kushwaha A (2022) Ocular drug delivery systems: an overview. IJRDO J Appl Sci 8(11):26–30. https://doi.org/10.53555/as.v8i11.5439
Mehrandish S, Mirzaeei S (2020) A review on ocular novel drug delivery systems of antifungal drugs: functional evaluation and comparison of conventional and novel dosage forms. Adv Pharm Bull 11(1):28–38. https://doi.org/10.34172/apb.2021.003
Duxfield L, Sultana R, Wang R, Englebretsen V, Deo S, Rupenthal ID, Al-Kassas R (2015) Ocular delivery systems for topical application of anti-infective agents. Drug Dev Ind Pharm 42(1):1–11. https://doi.org/10.3109/03639045.2015.1070171
Shi P, Cheng Z, Zhao K, Chen Y, Zhang A, Gan W, Zhang Y (2023) Active targeting schemes for nano-drug delivery systems in osteosarcoma therapeutics. J Nanobiotechnol 21(1):103. https://doi.org/10.1186/s12951-023-01826-1
Yousefi M, Andishmand H, Assadpour E, Barzegar A, Kharazmi MS, Jafari SM (2023) Nanoliposomal delivery systems of natural antibacterial compounds; properties, applications, and recent advances. Crit Rev Food Sci Nutr. https://doi.org/10.1080/10408398.2023.2170318
Coutinho IT, Maia-Obi LP, Champeau M (2021) Aspirin-loaded polymeric films for drug delivery systems: comparison between soaking and supercritical CO2 impregnation. Pharmaceutics 13(6):824. https://doi.org/10.3390/pharmaceutics13060824
Chandramouli Y, Firoz S, Yasmeen BR, Vikram A, Mahitha B, Aruna U (2012) Microsponges: a novel drug delivery system for controlled delivery of topical drugs. Int J Pharm Res Appl 2(2):79–86
Tiwari A, Tiwari V, Palaria B, Kumar M, Kaushik D (2022) Microsponges: a breakthrough tool in pharmaceutical research. Future J Pharm Sci 8(1):10. https://doi.org/10.1186/s43094-022-00421-9
Vishwakarma P, Choudhary R (2019) Microsponges: A novel strategy to control the delivery rate of active agents with reduced skin irritancy. J Drug Deliv Therap 9(6-s):238–247. https://doi.org/10.22270/jddt.v9i6-s.3757
Shaha V, Jain H, Krishna J, Patel P (2010) Microsponge drug delivery: a review. Int J Res Pharm Sci 1(2):212–218
Mahant S, Kumar S, Nanda S, Rao R (2020) Microsponges for dermatological applications: perspectives and challenges. Asian J Pharm Sci 15(3):273–291. https://doi.org/10.1016/j.ajps.2019.05.004
CN107469141A—A kind of microsponge medical dressing and preparation method thereof—Google Patents. https://patents.google.com/patent/CN107469141A/en
JP6688386B2—Hyaluronic acid microsponge and method for producing the same Google Patents. https://patents.google.com/patent/JP6688386B2/en
KR101900387B1—Microsponges having controlled solubility and improved redissolution property—Google Patents. https://patents.google.com/patent/KR101900387B1/en
Love FS III (n.d.) US7426776B2—Nonwoven towel with microsponges -Google Patents. https://patents.google.com/patent/US7426776
Straub J (n.d.). WO2000072827A2—Porous drug matrices and methods of manufacture thereof—Google Patents. https://patents.google.com/patent/WO2000072827A2/en
Won R (n.d.) US5955109A—Methods and compositions for topical delivery of retinoic acid—Google Patents. https://patents.google.com/patent/US5955109A/en
Fanchon C (n.d.) US5679374A—Anti-acne composition for the simultaneous treatment of the surface layers and deep layers of the skin, and use thereof—Google Patents. https://patents.google.com/patent/US5679374A/en
Lo RJR, Ltd Z (n.d.) US5725869A—Microsphere reservoirs for controlled release application—Google Patents. https://patents.google.com/patent/US5725869A/en
Eury RP (n.d.) US5316774A—Blocked polymeric particles having internal pore networks for delivering active substances to selected environments—Google Patents. https://patents.google.com/patent/US5316774A/en
Schaefer H (n.d.) US5292512A—Cosmetic or pharmaceutical composition containing microspheres of polymers or of fatty substances filled with at least one active product—Google Patents. https://patents.google.com/patent/US5292512A/en
Won R (n.d.) US5145675A—Two step method for preparation of controlled release formulations—Google Patents. https://patents.google.com/patent/US5145675A/en
Khule PK, Nitalikar MM, More VV, Gilhotra RM (2019) Microsponge drug delivery: a review. SGVU J Pharm Res Educ 4(1):359–365
Dean RC Jr. US4997753A—Weighted collagen microsponge for immobilizing bioactive material—Google Patents. https://patents.google.com/patent/US4997753A/en
Dean RC Jr, Berg RA, Phillips PG, Runstadler PW Jr, Silver FH, Corp V (n.d.) CA1288370C—Weighted collagen microsponge—Google Patents. https://patents.google.com/patent/CA1288370C/en
Dean RC, Phillips PG, Runstadler PW Jr, Silver FH, Berg RA, Cahn F, Corporation V (n.d.) WO1986005811A1—Weighted microsponge for immobilizing bioactive material—Google Patents. https://patents.google.com/patent/WO1986005811A1/en
Dean RC Jr, Silver FH, Berg RA, Phillips PG, Runstadler PW Jr, Maffia GJ, Corp V (n.d.) US4863856A—Weighted collagen microsponge for immobilizing bioactive materials—Google Patents. https://patents.google.com/patent/US4863856A/en
Dean RC Jr, Silver FH, Berg RA, Phillips PG, Runstadler PW Jr, Corp V (n.d.) US4861714A—Weighted collagen microsponge for immobilizing bioactive material—Google Patents. https://patents.google.com/patent/US4861714
Won R (n.d.) US4690825A—Method for delivering an active ingredient by controlled time release utilizing a novel delivery vehicle which can be prepared by a process utilizing the active ingredient as a porogen- Google Patents. https://patents.google.com/patent/US4690825A/en
Cavalli R, Trotta F, Tumiatti W (2006) Cyclodextrin-based nanosponges for drug delivery. J Incl Phenom Macrocycl Chem 56(1–2):209–213. https://doi.org/10.1007/s10847-006-9085-2
Valluru R, Ravi G, Bose SP, Damineni S (2019) Microsponges-a comprehensive review: success and challenges. Indo Am J Pharm Res 9(7):3056–3067
Acknowledgements
Not applicable.
Funding
No funding was obtained for this study.
Author information
Authors and Affiliations
Contributions
Srinatha N: The majority of the manuscript has been cited from various journals, penned and formatted. Sowjanya Battu: Engaged in the refinement, formatting, and ultimate approval of the preliminary manuscript, and endorsing the final version. Vishwanath B.A: Affirmed the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
All the authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Srinatha, N., Battu, S. & Vishwanath, B.A. Microsponges: a promising frontier for prolonged release-current perspectives and patents. Beni-Suef Univ J Basic Appl Sci 13, 60 (2024). https://doi.org/10.1186/s43088-024-00519-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43088-024-00519-4