Abstract
Background
The epithelial-mesenchymal transition (EMT) affects the retinal pigment epithelium's natural homeostasis. According to observations from around the world, numerous oculopathies, including proliferative vitreoretinopathy (PVR), diabetic retinopathy (DR), and other macular degenerative illnesses such as age-related macular degeneration (AMD), have been linked to the epithelial-mesenchymal transition of retinal pigment epithelium (EMT of RPE). Retinopathy is referred to as an impairment in the retina, where AMD is characterized as an alteration in the macula region, DR as an impairment in the microvascular system, and PVR as an alteration in the subretinal bands, fibrovascular membranes, and fibrotic alteration in the detached retina. To find molecular targets and therapeutic drugs to protect and restore RPE function, a connection between EMT-related signaling pathways and RPE degeneration must be established.
Main body of abstract
Studies conducted in vivo and in vitro indicate that several signaling pathways, including the Rho pathway, the transforming growth factor-β (TGFβ) pathway, the Jagged/Notch pathway, mitogen-activated protein kinase (MAPK)-dependent pathway, and Wnt/β-catenin pathway, are activated in RPE cells during PVR and AMD. In order to discover the most suitable candidate for retinopathy therapies, it is necessary to determine the relationship between the regulators of the EMT and the degeneration of the RPE. To treat retinopathies, particularly those that are brought on by the EMT of retinal pigment epithelial cells, it is necessary to investigate prospective pharmaceutical candidates.
Conclusion
TGFβ's intracellular cascade, which comprises both canonical (SMAD-associated) and non-canonical (SMAD-nonassociated) pathways, is shown to be the most active signaling pathway in the degeneration of the RPE caused by EMT.
Similar content being viewed by others
1 Background
Cell signaling is being researched as a unique approach for better comprehending molecular processes, establishing intercellular signal network profiles, and finding biomarkers and therapeutic targets associated with cellular functions [1]. It is yet unknown exactly how these transcription factors contribute on a molecular level to the epithelial-mesenchymal transition of the retinal pigment epithelium (EMT of RPE) [2]. Growth factors and other biological agents cause RPE cells to lose their cell polarity and cell-to-cell contact, which triggers EMT through a variety of signaling pathways that encourage cell division, migration, and the synthesis of extracellular matrix (ECM). The ECM is a complex network made up of a number of multidomain macromolecules that are structured in a network and cell/tissue-specific configurations [3]. RPE cells begin to exhibit mesenchymal cell characteristics such as the ability to migrate and proliferate as they become less differentiated. This review focuses on current developments in the field of molecular understanding at the level of cell signaling and presents current thoughts on various approaches by focusing on retinal pigment epithelium-related retinopathies such as proliferative vitreoretinopathy (PVR) and age-related macular degeneration (AMD). The macula, the small region of the central retina that is important for high-acuity vision, breaks down in AMD [4]. One of the problems that poses a major threat to the health of diabetic patients is DR, a microvascular impairment that can result in loss of visual acuity[5, 6]. Proliferative vitreoretinopathy (PVR) is defined by subretinal bands, fibrovascular membranes, and fibrotic alterations in the detached retina [7].
2 Main text
2.1 The role of EMT in retinal pigment epithelium
The Bruch's membrane and photoreceptors are both supported by the retinal pigment epithelium, which plays an essential role in maintaining visual acuity. RPE is terminally differentiated, highly polarised, and located between photoreceptors and choroids [8]. The RPE controls the flow of molecules between the fenestrated choroid capillaries and the photoreceptor layer of the retina to create the outer blood-retinal barrier (BRB). BRB works in many different ways, such as membrane pumps, transporters, channels, passive but selective diffusion, transcytosis, metabolic alteration of solutes in transit, and metabolic modification of solutes after they have left the cell. A monolayered cuboidal epithelium is held together by adherens junctions and tight junctions, which regulate epithelial diffusion via spaces between neighboring cells [9,10,11,12].
EMT is the process through which healthy epithelial cells modify into mesenchymal cells [13]. At four to six weeks of gestation, the human RPE cell undergoes terminal differentiation and thereafter remains mitotically inactive [2]. Additionally, it is a well-studied fact that EMT is highly involved in embryonic organization and re-organization. Furthermore, the EMT can be divided into three types [14] (Fig. 1). There are three basic types of EMT, depending on where the process starts and how it ends. EMTs are divided into three types: type I, which is linked to embryogenesis, type II, which is linked to pathology, and type III, which is linked to carcinogenesis [14, 15]. The study employed many markers to distinguish between epithelial and mesenchymal cells despite the fact that EMT is a dynamic process [16].
The zona-occludens-1 (ZO-1), epithelial cadherin (E-cadherin), and cytokeratin are epithelial markers. N-cadherin, fibronectin, and vimentin are mesenchymal markers [17]. EMT in RPE is considered a fundamental underlying mechanism for severe diseases as it is observed in retinopathies such as AMD and PVR [17, 18]. Additionally, the in vitro, in vivo, and clinical data suggest that RPE cells undergo EMT [19,20,21, 17]. EMT of the RPE is caused by a combination of mechanisms including oxidative stress, pathological inflammation, aging, infections, tight junction loss, ECM breakdown, altered growth factor synthesis, misfolded protein accumulation, and infections [22].
2.2 Cellular signaling of EMT specifically in retinal pigment epithelium
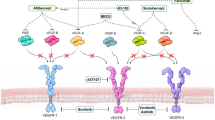
The transformation of epithelial cells into mesenchymal cells, which results in the emergence of new biochemical instructions, requires cellular reprogramming and highly complicated cellular rearrangement [23]. Extracellular signals alter the gene expression of proteins associated with epithelial and mesenchymal tissues during the EMT process in the retinal pigment epithelium. They also regulate a variety of related cellular behaviors, including cell proliferation, migration, and death. This results in the development of PVR and AMD through a network of interconnected signaling pathways [2]. Tight junctions also play a part in the regulation of signaling pathways that control cellular functions including migration, proliferation, and differentiation [2]. Current understanding suggests that the phosphoinositide-3-kinase/Protein kinase B (PI3K/Akt) pathway, TGFβ, Wnt, and Notch are only a few of the regulatory signaling pathways that are associated with the EMT of the RPE, as well as significant interactions between them [24] (Fig. 2).
Signaling cascades involved in EMT process. Many signaling pathways have been implicated in the retinal pigment EMT. either of canonical or non-canonical TGFβ signaling pathways are involved in the EMT of retinal pigment epithelium. Many additional signaling pathways, such as BMP signaling, RTK signaling, Wnt signaling, and jagged-Notch signaling, are implicated in the EMT process in retinal pigment epithelium. Furthermore, the interplay between these pathways is well documented. Diagrams developed with Microsoft PowerPoint
2.3 Rho signaling pathway
Ras homolog family member A (RhoA/Rho)-kinase is associated with ocular fibrosis. The control of cellular actomyosin cytoskeletal architecture and motility is greatly influenced by two of RhoA's main downstream effectors, the Ras-related C3 botulinum toxin substrate 1 (Rac1) and Rho-associated, coiled-coil-containing kinases, Rho-associated kinase (ROCK) [25]. The Rho pathway has been found to control the assembly and structure of the actin cytoskeleton, as well as associated gene expression. It may be critical for RPE cell fibrotic response [26]. LIM domain kinase (LIMK), an actin-binding protein, phosphorylates cofilin, hence stabilizing actin filaments [3]. Activated RhoA or its downstream effector ROCK increases LIM-kinase activity, which then phosphorylates cofilin in TGFβ1-treated ARPE-19 cells. This phosphorylation reduces cofilin function, which promotes actin polymerization and cytoskeleton remodeling, ultimately leading to fibrosis [26].
TGFβ-induced RhoA activation stimulates cell motility and increases alpha-smooth muscle actin (α-SMA) expression in primary RPE cells [27]. The RhoA/Rho-kinase pathway has been demonstrated to mediate the synthesis of type I collagen by TGFβ2 in human RPE cells [19, 25]. In an in vivo PVR animal model, matrix stiffness enhanced ARPE-19 cell activation via the RhoA/(YAP) pathway, as well as retinal fibrogenesis [28]. Yes-associated protein 1 (YAP1) is a transcription coregulator that increases the expression of genes that regulate cell differentiation and proliferation [3]. Furthermore, thrombin stimulates ROCK and Rho, leading to phosphorylation of the myosin light chain and the production of actin stress fibers in retinal pigment epithelial cells undergoing EMT [29]. Furthermore, recent research suggests that inhibiting RhoA upstream with C3 exoenzyme or inhibiting YAP downstream with verteporfin significantly reduced MMP production and collagen gel contraction in ARPE-19 cells. Blocking RhoA/YAP signaling inhibited the TGFβ/Smad pathway in vivo and reduced PVR-induced retinal fibrogenesis. This paper provides novel PVR treatment approaches that target the RhoA/YAP pathway [28]. Nicotinamide suppresses EMT in the RPE and increases RPE cell differentiation by downregulating ROCK and casein kinase 1 (CK1) [30]. The ROCK inhibitor Y27632 and the RhoA inhibitor simvastatin both decrease TGFβ2-induced type I collagen synthesis in ARPE-19 cells, demonstrating the existence of a connection between the Rho and SMAD pathways [11].
2.4 Signaling cascade Mitogen-activated protein kinase (MAPK)
MAPK is divided into three subfamilies: extracellular signal-regulated kinases (ERKs), c-Jun N-terminal kinases (JNKs), and p38 mitogen-activated protein kinases (p38s). ERKs are activated by growth factors, whereas JNKs and p38 are activated by cellular stresses or inflammatory cytokines. These signaling pathways regulate several biological processes in the fibrotic process of the eye [30]. The MAPKs cascade, which is regulated by a number of activators, is thought to be involved in the development of the epithelial-to-mesenchymal transition process. The Ras-MAPK pathway activates SNAIL1 and SNAIL2. The early growth response factor-1 (Egr-1) accelerates the transition from epithelial to mesenchymal [31]. TGFβ-induced EMT and fibrosis were mediated by ERK activation in ARPE-19 cells [32]. A recent study of induced pluripotent stem cell (hiPSC)-derived RPE cells found that inhibiting TGFβ and fibroblast growth factor (FGF/MAPK) pathways improved differentiation of RPE[33]. Furthermore, previous in vitro and in vivo studies indicate that the SNAIL is expressed at both the transcription and post-transcription levels in many complex signaling pathways such as integrin-linked kinase (ILK), phosphatidylinositol 3-kinase (P1P3-K), MAPKs, glycogen synthase kinase 3-b (GSK-3b), and nuclear factor kappa B (NF-κB) [34].
Shukal and coworkers demonstrated using an in vitro study that, the anti-epithelial to mesenchymal transition in cells of RPE by a pyruvate analog, the dichloroacetate (DCA) via (MAPK/Erk) and PI3K/Akt pathway [35]. Inhibiting keratin 8 (KRT8) phosphorylation suppresses oxidative stress-mediated epithelial to mesenchymal transition in RPE cells while avoiding potential cell death, indicating that autophagy-mediated KRT8 overexpression combined with MAPK1/3 pathway inhibition could be a potential AMD intervention strategy [18]. Saika and colleagues established the therapeutic efficacy of p38MAPK inhibitor (SB202190) in ARPE-19 cells, with reduced TGFβ2-mediated migration and extracellular matrix production via MAPK signaling [36].
2.5 Notch signaling cascade
It is believed that Notch signaling pathway may be significant in the onset and genesis of many illnesses because the Notch signaling cascade modulates the ratio of cell death to proliferation [37]. The transcriptional regulator retinol-binding proteins (RBP) is necessary for the conventional Notch cascade in the RPE. RBP-J performs a transcriptional factor role in Notch signaling [38, 39]. Throughout the development of the eye, the Notch signaling system plays a role in the regulation of cell fate, differentiation, and patterning [39]. Notch signaling has also been connected to the control of cell proliferation, specification, and differentiation during the retinogenesis and formation of the mammalian ocular lens [39, 40].
In the study of the TGFβ2-induced EMT process in retinal pigment epithelium, it is discovered that the TGFβ-dependent Smad signaling cascade initiates the Jagged/Notch pathway. Further, the blocked Notch pathway inhibited the EMT process effectively [41]. Zhang and coworkers reported that the notch inhibitor (LY411575) blocked the Notch signaling in the PVR model of ARPE-19 cells [42]. One study by Niessen and coworkers targets SNAIL2 as part of Notch signaling and it has been observed that during hypoxia conditions SNAIL1 is directly induced by Notch signaling [43]. The overexpression of Jagged-1 and Notch-3 was observed in the TGFβ2-induced EMT of RPE [44]. The Jagged-1 knockout suppressed TGFβ2-mediated EMT process in RPEvia downregulation of SNAIL, SLUG, and zinc finger E-box binding homeobox 1 (ZEB1) [44]. The collagen-1 (COL1A1 and COL1A2) expression was found regulated by TGFβ2 treatment in cells (ARPE-19), as they are related to Notch/Jagged and MAPK signaling pathway [45]. Another research demonstrates the connection between the Notch/Jagged and MAPK signaling pathways in the EMT process in the RPE caused by the concomitant administration of TGFβ1 and tumor necrosis factor-alpha (TNF-alpha) [45].
2.6 Wnt-β-catenin and Hippo-YAP signaling pathway
An essential component of the Wnt-beta-catenin pathway, beta-catenin is activated by dissociating from its complex and translocating into the nucleus, where it further activates the genes SNAIL and other EMT-related genes [16]. The Wnt signaling mechanism is crucial for cancer, aging, and post-natal stem cell regeneration in addition to regulating tissue differentiation during embryogenesis [46]. Beta-catenin binds to the cytoplasmic domain of cadherins, illustrating a point at which the Wnt pathway and the cadherin adhesion mechanism converge [46, 47]. Important for the EMT, the phosphorylation statuses of beta-catenin, a key Wnt mediator, and GSK-3, which is positioned upstream of beta-catenin, are altered. By changing the expression or active status of its transcriptional regulators Snail and Smads, the Wnt/beta-catenin pathway, as well as the pathways started by FGF and TGFβ, govern the EMT process in RPE. The junction of many pathways is where GSK-3 is found [46, 48].
The Wnt-beta-catenin pathway is activated by laser-mediated coagulation, and this furthers the EMT process in RPE outcomes, according to an earlier investigation by Han and colleagues [49]. The overexpression of beta-catenin was prevented in one in vitro experiment using ARPE-19 cells by the XAV939 (Wnt-beta downregulator). The increased expression of EMT markers is caused by the overexpression of beta-catenin [50]. The stimulation of EMT by the Wnt-beta-catenin signaling pathway was also demonstrated by further excessive light exposure on the retina [51]. Pentraxin 3 (HC-HA/PTX3) is a potent, non-toxic inhibitor that, in a dose-dependent manner, inhibits Wnt signaling to suppress the EMT process in RPE [52]. So, as already established, laser photocoagulation triggers a Wnt/beta-catenin signal transduction pathway, which in turn encourages the proliferation and conversion of the epithelial cells of the retinal pigment epithelium into mesenchymal cells. It may be favorable for the regeneration of the RPE to therapeutically regulate Wnt/beta-catenin signaling.
Contact inhibition and EMT, which govern organ size, are regulated by the Hippo signaling system [50]. The Hippo signaling cascade controls the transcription factors YAP and TAZ, which link the EMT process to cell proliferation [53, 54]. Hippo-YAP was shown to be associated with cadherin and the phosphorylation of Src family members [56]. The in vitro investigation demonstrated the role of EMT in RPE suppression by reducing tight junction disintegration [17]. The Hippo-YAP pathway, whose activity is mostly dependent on tight and adheren junctions, maintains RPE differentiation [16]. The Yap, on the other hand, was not found in the primary cells (RPE) of mice [17]. More research is needed to completely understand the facts surrounding the Hippo-YAP pathway, which is linked to the EMT process in the RPE.
2.7 Transforming growth factor-beta (TGFβ) signaling pathway
TGFβ and its intracellular cascade are particularly important in the EMT process in RPE [2]. TGFβ is a crucial cytokine as an anti-inflammatory compound. Its production is associated with wound injury and inflammation [16]. TGFβ showed a role in both normal physiological as well as abnormal pathological conditions [17]. The presence of TGFβ1, TGFβ2, and TGFβ3 are reported in the human eye [55]. In general, the concentration of TGFβ cytokine is increased as an inflammatory response but if this condition remains for a prolonged period then it leads to EMT [56]. The drastic increased TGFβ2 favors a significant loss of cell–cell attachment in RPE [57]. The expression of TGFβ, epidermal growth factor (EGF), insulin-like growth factor (IGF-II), or FGF-2 initiates the process of digesting the basement membrane by binding with epithelial receptors and starts kinase activities [58]. Among these factors, the most interesting cytokine is the EGF and its receptor, epidermal growth factor (EGFR). Many research efforts have established its significance in the induction, maintenance, and control of cell proliferation, differentiation, and migration [59]. Further, it should be noted that TGFβ is a known inducer of EMT, and that EGFR has been observed to rise in EMT-affected cellular microenvironment where EGF-assisted cell plasticity occurs [60].
In previous studies, it has been found that the galactoside-binding lectin family protein (Galactin-1) is crosstalk with TGFβ signaling, in a knockout mouse it shows decreased choroidal neovascularization (CNV) severity and suppression of EMT process in RPE [61] (Table 1). Some other studies also represent the crosstalk between Smad-dependent signaling and ERK1/2 molecular interaction in RPE [41]. Troglitazone and pioglitazone, peroxisome proliferator-activated receptor-gamma (PPAR-γ) suppresses phosphorylation of Smad and thus inhibit TGFβ2-mediated EMT process in RPE [62, 63]. Inhibition of sub-retinal fibrosis is shown by the retinoic acid receptor gamma (RAR-γ) agonist through the TGFβ pathway [64].
Additionally, there are many agents such as bradykinin (BK) [65], fucoidan [13], bone morphogenic protein (BMP7) [66], BMP4 [67], LY-364947 (TGFβRI- inhibitor) [68], Baicalein [69], LYTAK1 (TAK1 inhibitors) [70], salinomycin [71], protein kinase-A inhibitor (H89) [72], and resveratrol [73] showed suppression of EMT process in RPE either in vivo or in vitro investigations. Recent studies on induced pluripotent stem cell (hiPSC)-derived retinal cells discovered that suppressing the protein kinase C or BMP signaling pathways, as well as reducing FGF/MAPK signaling, improved RPE differentiation [33]. Additionally, in the Smad3-mutated mouse PVR model, TGFβ signaling triggered the downregulation of the EMT process [74].
Some other therapeutic agents are involved in the inhibition of the EMT process in RPE but by interacting with multiple signaling such as curcumin suppressing the Akt, MAPK, and TGFβ pathways in RPE cells (Table 1). The mammalian target of rapamycin (mTOR) suppressor (Trichostatin A) hindered the EMT process in RPE by down-regulating the Jagged/Notch pathway, non-canonical TGF/Akt, MAPK, and ERK1/2, as well as the standard Smad signaling pathways [75]. It has been demonstrated that several intravitreal anti-vascular endothelial growth factor (anti-VEGF) medications can diminish retinal fibrosis in patients with active neovascular AMD (n-AMD) [19, 76, 77]. Choroidal neovascularization, which results in exudation, leakage, and finally fibrosis with photoreceptor loss, can be used to characterize n-AMD [3]. In recent safety phase II research, subretinal fibrosis in n-AMD patients receiving the combination of platelet-derived growth factor [Fovista®(E10030)] and an anti-VEGF drug was investigated. Additionally, a controlled phase II trial in n-AMD is now being conducted to examine the impact of either the FGF2 antagonist RBM-007 alone or in conjunction with the anti-VEGF drug on subretinal fibrosis [19].
3 Conclusions
Age-related macular degeneration and proliferative vitreoretinopathy activate several signaling cascades in RPE cells, including the SMAD, the Rho, the MAPK, the Jagged/Notch, and the Wnt/-catenin pathways, according to studies done in vitro and in vivo. The most active signaling cascade in the process of the retinal pigment epithelium's epithelial to mesenchymal conversion is discovered to be TGFβ, namely its intracellular cascade and both SMAD and non-SMAD pathways. There are no medications on the market right now that aim to treat the EMT of the retinal pigment epithelium. Retinopathies, in particular those caused by the epithelial to the mesenchymal conversion of retinal pigment epithelium cells, may only be treated with antimetabolite pharmaceuticals, however, these drugs have severe, occasionally blinding side effects.
A link between the molecular targets and the regulators of the EMT of RPE must be established in order to choose the best candidate for retinopathy therapy. To treat retinopathies, especially those brought on by the epithelial-mesenchymal transition of retinal pigment epithelium cells, it is critical to investigate to explore potential pharmaceutical treatments.
Availability of data and materials
Not applicable.
Abbreviations
- AMD:
-
Age-Related Macular Degeneration
- BK:
-
Bradykinin
- BMP-7:
-
Bone Morphogenic Protein 7
- CK1:
-
Casein Kinase 1
- CNV :
-
Choroidal Neovascularization
- ECM:
-
Extracellular Matrix
- EGF:
-
Epidermal Growth Factor
- EGFR:
-
Epidermal growth factor (EGF) and its receptor
- EMT :
-
Epithelial-mesenchymal transition
- Erks:
-
Extracellular Signal-Regulated Kinases
- Glc-N:
-
Glucosamine
- GSK-3:
-
Glycogen synthase kinase 3
- hiPSC:
-
Human Induced Pluripotent Stem Cell
- ILK:
-
Integrin-Linked Kinase
- JNKs:
-
C-Jun N-Terminal Kinases
- KRT8 :
-
Keratin 8
- MAPKs:
-
Mitogen-activated protein kinases
- mTOR:
-
Mammalian Target of Rapamycin
- n-AMD:
-
Neovascular-Age-Related Macular Degeneration
- PPAR-γ:
-
Peroxisome proliferator-activated receptor-gamma
- PVR:
-
Proliferative Vitreoretinopathy
- RAR-γ:
-
Retinoic Acid Receptor Gamma
- ROCK:
-
Rho-Associated Protein Kinase
- RPE:
-
Retinal pigment epithelium
- Shh:
-
Sonic hedgehog
- TGFβ:
-
Transforming growth factor-β
- VEGF:
-
Vascular endothelial growth factor
- YAP:
-
Yes-Associated Protein
References
Song D, Yang D, Powell CA, Wang X, Song D, Yang D, Powell CA, Wang X, Yang D (2019) Cell–cell communication: old mystery and new opportunity. Cell Biol Toxicol 35:89–93. https://doi.org/10.1007/S10565-019-09470-Y
Zou H, Shan C, Ma L, Liu J, Yang N, Zhao J (2020) Polarity and epithelial-mesenchymal transition of retinal pigment epithelial cells in proliferative vitreoretinopathy. PeerJ 8:e10136. https://doi.org/10.7717/peerj.10136
Liukkonen MPK, Paterno JJ, Kivinen N, Siintamo L, Koskela AKJ, Kaarniranta K (2022) Epithelial–mesenchymal transition-related serum markers ET-1, IL-8 and TGF-β2 are elevated in a Finnish wet age-related macular degeneration cohort. Acta Ophthalmol 100:e1153–e1162. https://doi.org/10.1111/aos.15051
Crabb JW, Miyagi M, Gu X, Shadrach K, West KA, Sakaguchi H, Kamei M, Hasan A, Yan L, Rayborn ME, Salomon RG, Hollyfield JG (2002) Drusen proteome analysis: an approach to the etiology of age-related macular degeneration. Proc Natl Acad Sci U S A 99:14682–14687. https://doi.org/10.1073/pnas.222551899
Fletcher E, Phipps J, Ward M, Puthussery T, Wilkinson-Berka J (2007) Neuronal and glial cell abnormality as predictors of progression of diabetic retinopathy. Curr Pharm Des 13:2699–2712. https://doi.org/10.2174/138161207781662920
Li C, Miao X, Li F, Wang S, Liu Q, Wang Y, Sun J (2017) Oxidative Stress-Related Mechanisms and Antioxidant Therapy in Diabetic Retinopathy. Oxid Med Cell Longev. https://doi.org/10.1155/2017/9702820
Nagasaka Y, Kaneko H, Ye F, Kachi S, Asami T, Kato S, Takayama K, Hwang SJ, Kataoka K, Shimizu H, Iwase T, Funahashi Y, Higuchi A, Senga T, Terasaki H (2017) Role of caveolin-1 for blocking the epithelial- mesenchymal transition in proliferative vitreoretinopathy. Investig Ophthalmol Vis Sci 58:221–229. https://doi.org/10.1167/iovs.16-20513
Strauss O (2011) The retinal pigment epithelium, Webvision Organ. Retin Vis Syst. https://doi.org/10.1007/s00347-008-1868-y
Rizzolo LJ, Peng S, Luo Y, Xiao W (2011) Integration of tight junctions and claudins with the barrier functions of the retinal pigment epithelium. Prog Retin Eye Res 30:296–323. https://doi.org/10.1016/j.preteyeres.2011.06.002
Rizzolo LJ (2007) Development and role of tight junctions in the retinal pigment epithelium. Int Rev Cytol 258:195–234. https://doi.org/10.1016/S0074-7696(07)58004-6
Narimatsu T, Ozawa Y, Miyake S, Kubota S, Hirasawa M, Nagai N, Shimmura S, Tsubota K (2013) Disruption of cell-cell junctions and induction of pathological cytokines in the retinal pigment epithelium of light-exposed mice. Investig Ophthalmol Vis Sci 54:4555–4562. https://doi.org/10.1167/iovs.12-11572
Narimatsu T, Negishi K, Miyake S, Hirasawa M, Osada H, Kurihara T, Tsubota K, Ozawa Y (2015) Blue light-induced inflammatory marker expression in the retinal pigment epithelium-choroid of mice and the protective effect of a yellow intraocular lens material invivo. Exp Eye Res 132:48–51. https://doi.org/10.1016/j.exer.2015.01.003
Zhang Y, Zhao D, Yang S, Yao H, Li M, Zhao C, Zhang J, Xu GT, Li H, Wang F (2018) Protective effects of fucoidan on epithelial-mesenchymal transition of retinal pigment epithelial cells and progression of proliferative vitreoretinopathy. Cell Physiol Biochem 46:1704–1715. https://doi.org/10.1159/000489246
Kalluri R, Neilson EG (2003) Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest 112:1776–1784. https://doi.org/10.1172/JCI200320530
Kalluri R (2009) EMT: when epithelial cells decide to become mesenchymal-like cells. J Clin Invest 119:1417–1419. https://doi.org/10.1172/JCI39675
Zhou M, Geathers JS, Grillo SL, Weber SR, Wang W, Zhao Y, Sundstrom JM (2020) Role of epithelial-mesenchymal transition in retinal pigment epithelium dysfunction. Front Cell Dev Biol 8:501. https://doi.org/10.3389/fcell.2020.00501
Shu DY, Butcher E, Saint-Geniez M (2020) EMT and ENDMT: emerging roles in age-related macular degeneration. Int J Mol Sci 21:1–26. https://doi.org/10.3390/ijms21124271
Baek A, Yoon S, Kim J, Baek YM, Park H, Lim D, Chung H, Kim DE (2017) Autophagy and KRT8/keratin 8 protect degeneration of retinal pigment epithelium under oxidative stress. Autophagy 13:248–263. https://doi.org/10.1080/15548627.2016.1256932
Mallone F, Costi R, Marenco M, Plateroti R, Minni A, Attanasio G, Artico M, Lambiase A (2021) Understanding drivers of ocular fibrosis: current and future therapeutic perspectives. Int J Mol Sci. https://doi.org/10.3390/ijms222111748
Shukal DK, Malaviya PB, Sharma T (2022) Role of the AMPK signalling pathway in the aetiopathogenesis of ocular diseases. Hum Exp Toxicol 41:1–28. https://doi.org/10.1177/09603271211063165
KJSr Dhaval Shukala, b, Kinjal Bhadreshaa, Bhoomi Shastria, Deval Mehtaa, Abhay Vasavadaa, Dichloroacetate prevents TGFβ-induced EMT.pdf (n.d.).
Yang IH, Lee JJ, Wu PC, Kuo HK, Kuo YH, Huang HM (2020) Oxidative stress enhanced the transforming growth factor-β2-induced epithelial-mesenchymal transition through chemokine ligand 1 on ARPE-19 cell. Sci Rep 10:1–10. https://doi.org/10.1038/s41598-020-60785-x
Thiery JP, Acloque H, Huang RYJ, Nieto MA (2009) Epithelial-mesenchymal transitions in development and disease. Cell 139:871–890. https://doi.org/10.1016/j.cell.2009.11.007
Wang Y, Chang T, Wu T, Ye W, Wang Y, Dou G, Du H, Hui Y, Guo C (2021) Connective tissue growth factor promotes retinal pigment epithelium mesenchymal transition via the PI3K/AKT signaling pathway. Mol Med Rep 23:1–13. https://doi.org/10.3892/mmr.2021.12028
Blasiak J, Koskela A, Pawlowska E, Liukkonen M, Ruuth J, Toropainen E, Hyttinen JMT, Viiri J, Eriksson JE, Xu H, Chen M, Felszeghy S, Kaarniranta K (2021) Epithelial-mesenchymal transition and senescence in the retinal pigment epithelium of nfe2l2/pgc-1α double knock-out mice. Int J Mol Sci 22:1–17. https://doi.org/10.3390/ijms22041684
Lee J, Ko M, Joo CK (2008) Rho plays a key role in TGF-β1-induced cytoskeletal rearrangement in human retinal pigment epithelium. J Cell Physiol 216:520–526. https://doi.org/10.1002/jcp.21424
Tsapara A, Luthert P, Greenwood J, Hill CS, Matter K, Balda MS (2010) The RhoA activator GEF-H1/Lfc is a transforming growth factor-β target gene and effector that regulates α-smooth muscle actin expression and cell migration. Mol Biol Cell 21:860–870. https://doi.org/10.1091/mbc.E09-07-0567
Zhang W, Han H (2021) Targeting matrix stiffness-induced activation of retinal pigment epithelial cells through the RhoA/YAP pathway ameliorates proliferative vitreoretinopathy. Exp Eye Res. https://doi.org/10.1016/j.exer.2021.108677
Ruiz-Loredo AY, López E, López-Colomé AM (2011) Thrombin promotes actin stress fiber formation in RPE through Rho/ROCK-mediated MLC phosphorylation. J Cell Physiol 226:414–423. https://doi.org/10.1002/jcp.22347
Cargnello M, Roux PP (2011) Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev 75:50–83. https://doi.org/10.1128/mmbr.00031-10
Grotegut S, Von Schweinitz D, Christofori G, Lehembre F (2006) Hepatocyte growth factor induces cell scattering through MAPK/Egr-1-mediated upregulation of Snail. EMBO J 25:3534–3545. https://doi.org/10.1038/sj.emboj.7601213
Kim SJ, Kim YS, Kim JH, Jang HY, Da Ly D, Das R, Park KS (2020) Activation of ERK1/2-mTORC1-NOX4 mediates TGF-β1-induced epithelial-mesenchymal transition and fibrosis in retinal pigment epithelial cells. Biochem Biophys Res Commun 529:747–752. https://doi.org/10.1016/j.bbrc.2020.06.034
Kuroda T, Ando S, Takeno Y, Kishino A, Kimura T (2019) Robust induction of retinal pigment epithelium cells from human induced pluripotent stem cells by inhibiting FGF/MAPK signaling. Stem Cell Res. https://doi.org/10.1016/j.scr.2019.101514
Franco DL, Mainez J, Vega S, Sancho P, Murillo MM, De Frutos CA, Del Castillo G, López-Blau C, Fabregat I, Nieto MA (2010) Snail1 suppresses TGF-β-induced apoptosis and is sufficient to trigger EMT in hepatocytes. J Cell Sci 123:3467–3477. https://doi.org/10.1242/jcs.068692
Shukal D, Bhadresha K, Shastri B, Mehta D, Vasavada A, Johar K (2020) Dichloroacetate prevents TGFβ-induced epithelial-mesenchymal transition of retinal pigment epithelial cells. Exp Eye Res. https://doi.org/10.1016/j.exer.2020.108072
Saika S, Yamanaka O, Ikeda K, Kim-Mitsuyama S, Flanders KC, Yoo J, Roberts AB, Nishikawa-Ishida I, Ohnishi Y, Muragaki Y, Ooshima A (2005) Inhibition of p38MAP kinase suppresses fibrotic reaction of retinal pigment epithelial cells. Lab Investig 85:838–850. https://doi.org/10.1038/labinvest.3700294
Wang Z, Li Y, Kong D, Sarkar FH (2010) The role of notch signaling pathway in epithelial-mesenchymal transition (EMT) during development and tumor aggressiveness. Curr Drug Targets 11:745–751. https://doi.org/10.2174/138945010791170860
Dou G, Wang Y, Hu X, Hou L, Wang C, Xu J, Wang Y, Liang Y, Yao L, Yang A, Han H (2008) RBP-J, the transcription factor downstream of Notch receptors, is essential for the maintenance of vascular homeostasis in adult mice. FASEB J 22:1606–1617. https://doi.org/10.1096/fj.07-9998com
Schouwey K, Aydin IT, Radtke F, Beermann F (2011) RBP-Jκ-dependent Notch signaling enhances retinal pigment epithelial cell proliferation in transgenic mice. Oncogene 30:313–322. https://doi.org/10.1038/onc.2010.428
Rowan S, Conley K, Le T, Donner A, RM-D, undefined 2008, Notch signaling regulates growth and differentiation in the mammalian lens. Elsevier. (n.d.). https://www.sciencedirect.com/science/article/pii/S001216060800924X. Accessed July 26, 2023.
Chen X, Xiao W, Wang W, Luo L, Ye S, Liu Y (2014) The complex interplay between ERK1/2, TGFβ/Smad, and Jagged/Notch signaling pathways in the regulation of epithelial-mesenchymal transition in retinal pigment epithelium cells. PLoS ONE 9:1–9. https://doi.org/10.1371/journal.pone.0096365
Zhang J, Yuan G, Dong M, Zhang T, Hua G, Zhou Q, Shi W (2017) Notch signaling modulates proliferative vitreoretinopathy via regulating retinal pigment epithelial-to-mesenchymal transition. Histochem Cell Biol 147:367–375. https://doi.org/10.1007/s00418-016-1484-x
Niessen K, Fu YX, Chang L, Hoodless PA, McFadden D, Karsan A (2008) Slug is a direct Notch target required for initiation of cardiac cushion cellularization. J Cell Biol 182:315–325. https://doi.org/10.1083/jcb.200710067
Chen X, Xiao W, Liu X, Zeng M, Luo L, Wu M, Ye S, Liu Y (2014) Blockade of jagged/notch pathway abrogates transforming growth factor 2-induced epithelial-mesenchymal transition in human retinal pigment epithelium cells. Curr Mol Med 14:523–534. https://doi.org/10.2174/1566524014666140331230411
Kimoto K, Nakatsuka K, Matsuo N, Yoshioka H (2004) p38 MAPK mediates the expression of type I collagen induced by TGF-β2 in human retinal pigment epithelial cells ARPE-19. Investig Ophthalmol Vis Sci 45:2431–2437. https://doi.org/10.1167/iovs.03-1276
Burke JM (2008) Epithelial phenotype and the RPE: is the answer blowing in the Wnt? Prog Retin Eye Res 27:579–595. https://doi.org/10.1016/j.preteyeres.2008.08.002
Nelson WJ, Nusse R (2004) Convergence of Wnt, β-catenin, and cadherin pathways. Science 303:1483–1487. https://doi.org/10.1126/science.1094291
Katoh M, Katoh M (2006) Cross-talk of WNT and FGF signaling pathways at GSK3β to regulate β-catenin and SNAIL signaling cascades. Cancer Biol Ther 5:1059–1064. https://doi.org/10.4161/cbt.5.9.3151
Han JW, Lyu J, Park YJ, Jang SY, Park TK (2015) Wnt/β-catenin signaling mediates regeneration of retinal pigment epithelium after laser photocoagulation in mouse eye. Investig Ophthalmol Vis Sci 56:8314–8324. https://doi.org/10.1167/iovs.15-18359
Chen HC, Zhu YT, Chen SY, Tseng SC (2012) Wnt signaling induces epithelial-mesenchymal transition with proliferation in ARPE-19 cells upon loss of contact inhibition. Lab Investig 92:676–687. https://doi.org/10.1038/labinvest.2011.201
Iriyama A, Iriyama T, Tamaki Y, Yanagi Y (2008) Effects of white light on β-catenin signaling pathway in retinal pigment epithelium. Biochem Biophys Res Commun 375:173–177. https://doi.org/10.1016/j.bbrc.2008.07.158
He H, Kuriyan AE, Su CW, Mahabole M, Zhang Y, Zhu YT, Flynn HW, Parel JM, Tseng SCG (2017) Inhibition of proliferation and epithelial mesenchymal transition in retinal pigment epithelial cells by heavy chain-hyaluronan/pentraxin 3. Sci Rep. https://doi.org/10.1038/srep43736
Liu Y, Xin Y, Ye F, Wang W, Lu Q, Kaplan HJ, Dean DC (2010) Taz-tead1 links cell-cell contact to zeb1 expression, proliferation, and dedifferentiation in retinal pigment epithelial cells. Investig Ophthalmol Vis Sci 51:3372–3378. https://doi.org/10.1167/iovs.09-4321
Zeng Q, Hong W (2008) The emerging role of the hippo pathway in cell contact inhibition, organ size control, and cancer development in mammals. Cancer Cell 13:188–192. https://doi.org/10.1016/j.ccr.2008.02.011
Connor TB, Roberts AB, Sporn MB, Danielpour D, Dart LL, Michels RG, De Bustros S, Enger C, Kato H, Lansing M, Hayashi H, Glaser BM (1989) Correlation of fibrosis and transforming growth factor-β type 2 levels in the eye. J Clin Invest 83:1661–1666. https://doi.org/10.1172/JCI114065
Yang L, Pang Y, Moses HL (2010) TGF-β and immune cells: an important regulatory axis in the tumor microenvironment and progression. Trends Immunol 31:220–227. https://doi.org/10.1016/j.it.2010.04.002
Hirsch L, Nazari H, Sreekumar PG, Kannan R, Dustin L, Zhu D, Barron E, Hinton DR (2015) TGF-β2 secretion from RPE decreases with polarization and becomes apically oriented. Cytokine 71:394–396. https://doi.org/10.1016/j.cyto.2014.11.014
Strutz F, Zeisberg M, Ziyadeh FN, Yang CQ, Kalluri R, Müller GA, Neilson EG, Renziehausen A, Sisic Z (2002) Role of basic fibroblast growth factor-2 in epithelial-mesenchymal transformation. Kidney Int 61:1714–1728. https://doi.org/10.1046/j.1523-1755.2002.00333.x
Schneider MR, Wolf E (2009) The epidermal growth factor receptor ligands at a glance. J Cell Physiol 218:460–466. https://doi.org/10.1002/jcp.21635
Derynck R, Zhang YE (2003) Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature 425:577–584. https://doi.org/10.1038/nature02006
Wu D, Kanda A, Liu Y, Kase S, Noda K, Ishida S (2019) Galectin-1 promotes choroidal neovascularization and subretinal fibrosis mediated via epithelial-mesenchymal transition. FASEB J 33:2498–2513. https://doi.org/10.1096/fj.201801227R
Cheng HC, Ho TC, Chen SL, Lai HY, Hong KF, Tsao YP (2008) Troglitazone suppresses transforming growth factor beta-mediated fibrogenesis in retinal pigment epithelial cells. Mol Vis 14:95–104
Hatanaka H, Koizumi N, Okumura N, Kay EDP, Mizuhara E, Hamuro J, Kinoshita S (2012) Epithelial-mesenchymal transition-like phenotypic changes of retinal pigment epithelium induced by TGF-γ Are prevented by PPAR-γ agonists. Investig Ophthalmol Vis Sci 53:6955–6963. https://doi.org/10.1167/iovs.12-10488
Kimura K, Orita T, Liu Y, Yang Y, Tokuda K, Kurakazu T, Noda T, Yanai R, Morishige N, Takeda A, Ishibashi T, Sonoda KH (2015) Attenuation of EMT in RPE cells and subretinal fibrosis by an RAR-γ agonist. J Mol Med 93:749–758. https://doi.org/10.1007/s00109-015-1289-8
Wei Q, Liu Q, Ren C, Liu J, Cai W, Zhu M, Jin H, He M, Yu J (2018) Effects of bradykinin on TGF-β1-induced epithelial-mesenchymal transition in ARPE-19 cells. Mol Med Rep 17:5878–5886. https://doi.org/10.3892/mmr.2018.8556
Yao H, Ge T, Zhang Y, Li M, Yang S, Li H, Wang F (2019) BMP7 antagonizes proliferative vitreoretinopathy through retinal pigment epithelial fibrosis in vivo and in vitro. FASEB J 33:3212–3224. https://doi.org/10.1096/fj.201800858RR
Yao H, Li H, Yang S, Li M, Zhao C, Zhang J, Xu G, Wang F (2016) Inhibitory effect of bone morphogenetic protein 4 in retinal pigment epithelial-mesenchymal transition. Sci Rep 6:1–10. https://doi.org/10.1038/srep32182
Nassar K, Grisanti S, Tura A, Lüke J, Lüke M, Soliman M, Grisanti S (2014) A TGF-β receptor 1 inhibitor for prevention of proliferative vitreoretinopathy. Exp Eye Res 123:72–86. https://doi.org/10.1016/j.exer.2014.04.006
Bin Park G, Kim D (2018) Cigarette smoke-induced EGFR activation promotes Epithelial mesenchymal migration of human retinal pigment Epithelial cells through regulation of the fak-mediated Syk/Src pathway. Mol Med Rep 17:3563–3574. https://doi.org/10.3892/mmr.2017.8355
Chen Z, Ni N, Mei Y, Yang Z (2017) LYTAK1 attenuates proliferation of retinal pigment epithelial cells through TGF-β-mediated epithelial mesenchymal transition via the ERK/AKT signaling pathway. Exp Ther Med 14:4951–4957. https://doi.org/10.3892/etm.2017.5187
Heffer AM, Proaño J, Roztocil E, Phipps RP, Feldon SE, Huxlin KR, Sime PJ, Libby RT, Woeller CF, Kuriyan AE (2019) The polyether ionophore salinomycin targets multiple cellular pathways to block proliferative vitreoretinopathy pathology. PLoS ONE 14:e0222596. https://doi.org/10.1371/journal.pone.0222596
Lyu Y, Xu W, Zhang J, Li M, Xiang Q, Li Y, Tan T, Ou Q, Zhang J, Tian H, Xu JY, Jin C, Gao F, Wang J, Li W, Rong A, Lu L, Xu GT (2020) Protein kinase A inhibitor H89 attenuates experimental proliferative vitreoretinopathy. Investig Ophthalmol Vis Sci. https://doi.org/10.1167/iovs.61.2.1
Junling L, Xiaorong L, Bowen X, Jianguo W (2017) Resveratrol modulates the proliferation and migration of retinal pigment epithelial cells through TGFβ1-induced EMT signal pathway. Biomed Res 28:6546–6550
Saika S, Kono-Saika S, Tanaka T, Yamanaka O, Ohnishi Y, Sato M, Muragaki Y, Ooshima A, Yoo J, Flanders KC, Roberts AB (2004) Smad3 is required for dedifferentiation of retinal pigment epithelium following retinal detachment in mice. Lab Investig 84:1245–1258. https://doi.org/10.1038/labinvest.3700156
Xiao W, Chen X, Liu X, Luo L, Ye S, Liu Y (2014) Trichostatin A, a histone deacetylase inhibitor, suppresses proliferation and epithelial-mesenchymal transition in retinal pigment epithelium cells. J Cell Mol Med 18:646–655. https://doi.org/10.1111/jcmm.12212
Daniel E, Shuang Ying G, Kim BJ, Toth CA, Ferris F, Martin DF, Grunwald JE, Jaffe GJ, Dunaief JL, Pan W, Maguire MG (2019) Five-year follow-up of nonfibrotic scars in the comparison of age-related macular degeneration treatments trials. Ophthalmology 126:743–751. https://doi.org/10.1016/j.ophtha.2018.11.020
Naylor A, Hopkins A, Hudson N, Campbell M (2020) Tight junctions of the outer blood retina barrier. Int J Mol Sci 21:211. https://doi.org/10.3390/ijms21010211
Meng Y, Ren Z, Xu F, Zhou X, Song C, Wang VYF, Liu W, Lu L, Thomson JA, Chen G (2018) Nicotinamide promotes cell survival and differentiation as kinase inhibitor in human pluripotent stem cells. Stem Cell Reports 11:1347–1356. https://doi.org/10.1016/j.stemcr.2018.10.023
Zhou L, Shi DP, Chu WJ, Song S, Hao XH, Yang LL, Xu HF (2021) Nicotinamide suppresses bevacizumab-induced epithelial-mesenchymal transition of ARPE-19 cells by attenuating oxidative stress. Int J Ophthalmol 14:481–488. https://doi.org/10.18240/ijo.2021.04.01
Zhou X, Kuang X, Long C, Liu W, Tang Y, Liu L, Liu H, He J, Huang Z, Fan Y, Zhang Q, Shen H (2017) Curcumin inhibits proliferation and epithelial-mesenchymal transition of retinal pigment epithelial cells via multiple pathways. Curr Mol Med. https://doi.org/10.2174/1566524017666171106115655
Zhang XY, Ng TK, Brelén ME, Wu D, Wang JX, Chan KP, Yung JSY, Cao D, Wang Y, Zhang S, Chan SO, Pang CP (2016) Continuous exposure to non-lethal doses of sodium iodate induces retinal pigment epithelial cell dysfunction. Sci Rep 6:1–13. https://doi.org/10.1038/srep37279
Liang CM, Tai MC, Chang YH, Chen YH, Chen CL, Lu DW, Chen JT (2011) Glucosamine inhibits epithelial-to-mesenchymal transition and migration of retinal pigment epithelium cells in culture and morphologic changes in a mouse model of proliferative vitreoretinopathy. Acta Ophthalmol 89:505–514. https://doi.org/10.1111/j.1755-3768.2011.02147.x
Herbst RS, Prager D, Hermann R, Fehrenbacher L, Johnson BE, Sandler A, Kris MG, Tran HT, Klein P, Li X, Ramies D, Johnson DH, Miller VA (2005) TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol 23:5892–5899. https://doi.org/10.1200/JCO.2005.02.840
Tan X, Chen C, Zhu Y, Deng J, Qiu X, Huang S, Shang F, Cheng B, Liu Y (2017) Proteotoxic stress desensitizes TGF-beta signaling through receptor downregulation in retinal pigment epithelial cells. Curr Mol Med 17:189–199. https://doi.org/10.2174/1566524017666170619113435
Ishikawa K, He S, Terasaki H, Nazari H, Zhang H, Spee C, Kannan R, Hinton DR (2015) Resveratrol inhibits epithelial-mesenchymal transition of retinal pigment epithelium and development of proliferative vitreoretinopathy. Sci Rep. https://doi.org/10.1038/srep16386
Acknowledgements
Not applicable.
Funding
No funding was obtained for this study.
Author information
Authors and Affiliations
Contributions
BG and PM took part in the acquisition, analysis and interpretation, manuscript preparation and reviewing. PR, BP, KT, PC and KJ commented on previous version of manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
All the authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gelat, B., Malaviya, P., Rathaur, P. et al. Regulation of epithelial-mesenchymal transition in retinal pigment epithelium and its associated cellular signaling cascades: an updated review. Beni-Suef Univ J Basic Appl Sci 12, 94 (2023). https://doi.org/10.1186/s43088-023-00435-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43088-023-00435-z