Abstract
Background
The prevalence of chronic kidney diseases (CKD) is higher in patients with diabetes. The American diabetes association (ADA) provides components of diabetes care, treatments, and guidelines to diagnose diabetic patients at risk of CKD. Herein we followed the ADA diagnosis guidelines to identify the Type 2 Diabetes mellitus (T2DM) patients at risk of CKD which is underestimated in the region. The study main objectives are to investigate the CKD prevalence and association with risk factors according to the ADA classification, and also to identify the T2DM patients at risk of renal diseases. A descriptive retrospective study was conducted. The data were collected using face-to-face interviews and through accessing patients’ medical records from Endocrinology and Cardiology clinics in an academic tertiary care hospital.
Results
About 40% of the 331 T2DM outpatients were at risk of developing CKD. The majority were in CKD Stage 3, then Stage 2. The estimated GFR (eGFR) values were significantly reduced in the T2DM patients who are; older than 50 years; have diabetes for more than 10 years; and have abnormally high serum and urine creatinine, proteinuria, and albumin to creatinine ratio. Further, the eGFR values were negatively associated with; the duration of T2DM; serum creatinine, proteinuria; and albumin to creatinine ratio.
Conclusions
This study provides evidence of the increasing risk of CKD among T2DM patients in the region. Hence, T2DM patients especially elders and those with the long onset of diabetes need to go under regular checks on their clinical parameters to prevent CKD progression.
Similar content being viewed by others
1 Background
According to the International Diabetes Federation (IDF) report, the prevalence of type 2 diabetes mellitus (T2DM) has been rapidly increasing worldwide [1]. Persistent hyperglycemia leads to a wide range of serious complications including macrovascular complications such as coronary artery disease, peripheral arterial disease, and stroke as well as microvascular complications such as diabetic nephropathy, neuropathy, and retinopathy [2,3,4].
Patients with T2DM are at a twofold higher risk of developing chronic kidney disease (CKD) compared to people who don't have diabetes [5]. The prevalence of CKD in diabetic patients ranges between 27.1 and 83.6% affected by the presence of risk factors [6, 7]. The presence of diabetic kidney disease (DKD) in patients increases the mortality risk by three times [8]. It can also progress to end-stage renal disease (ESRD) in which maintenance renal replacement therapy including hemodialysis, peritoneal dialysis, and kidney transplantation is the only management approach [9], 10. Additionally, DKD can result in financial strain on individuals, communities, and healthcare systems. The cost to manage kidney disease in patients with diabetes includes hospitalizations, medications, laboratory tests, consultations, dialysis treatment, transplantation surgeries, and transportation [11]. Of special note, the presence of diabetes contributes to 27% more expenditure in patients on dialysis [12]. For this reason, the early diagnosis of kidney disease and the identification of risk factors leading to kidney disease progression are essential.
Kidney disease has been evaluated using different formulas, markers, and criteria [13, 14]. The estimated glomerular filtration rate (eGFR) measures the rate of serum creatinine secretion from kidneys and is primarily used as a biomarker to assess kidney function status along with the presence of evidence of kidney damage [13]. Hence, the eGFR is considered a strong predictor of the progression to CKD and ESRD [5, 15, 16].
DKD is poorly diagnosed and underestimated in the Middle East region. Few outdated studies described the prevalence of DKD in the region [17]. A systematic review was recently published about CKD prevalence among T2DM patients in the Middle East region, they found that the CKD prevalence was about 29%, however, the data were from studies performed between the years 2009–2019, which covers a few countries in the region [17]. Since the prevalence of CKD differs from one region to another due to cultural and demographic differences, it is of great importance to have recent information about CKD prevalence in the Middle East region to establish an information baseline about CKD among the T2DM patients in the region.
We hypothesize that the early diagnosis and identification of diabetic patients who are at risk of developing CKD diseases may help in preventing kidney failure, especially in patients with poor glycemic control and the absence of adequate preventive measures [18].
2 Aims of the study
This study aims to identify diabetic patients with an increased risk of renal disease by finding the correlations between the eGFR and the clinical parameters.
3 Methods
3.1 Study design
A descriptive research methodology was used to conduct this study. The data were collected using both face-to-face interviews with patients and through accessing their electronic medical records from Jun to Sep 2020. Raw data were coded and analyzed using IBM SPSS software (2020) (SPSS Inc., Chicago, IL, USA). The needed statistical indices were extracted using quantitative data analysis techniques. The descriptive analysis was used in which continuous variables were presented as means and standard deviations, meanwhile categorical variables were presented as frequencies and percentages. The biochemical markers were compared between males and females using an independent t-test. We used Spearman's coefficient to give an idea about the degree of correlation and if the relationship is forward or inverse between the independent variables and to identify associations between sociodemographic and clinical variables with eGFR levels. A p-value < 0.05 was considered statistically significant. The study protocol is in conformity with the ethical principles of the Helsinki Declaration and approved by the Institutional Review Board committee at King Abdullah University Hospital (Reference number 48/132/2020), which was issued on 01.04.2020, the IRB approval letter is provided on the additional file. Participants provided written consent for participation. The STROBE cross-sectional reporting guidelines were checked to improve the study quality [19].
3.2 Participants
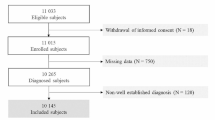
The participant’s data were collected from the Endocrinology and Cardiology clinics at King Abdullah University Hospital in Jordan. The T2DM patients were using anti-diabetic medications, aged 18 years or older, and had performed a kidney function test within the last year. However, patients who had any of the following criteria were excluded from participation in this study; patients undergoing acute renal failure, pregnant women, those who were on a purely vegetarian diet or protein-rich diet, those who had recent changes in their muscle mass such as in imputation, and those who had impaired mental capacity that might affect their answers to the interview questions.
3.3 Data collection and variables
The demographic information includes age and gender. The diabetes onset duration was shown in years. The biochemical variables include; fasting blood sugar (FBS) and glycated hemoglobin (HbA1c) to reflect the blood sugar level; The serum and urine creatinine (sCr and uCr) values were collected as an indicator and biomarker of kidney malfunction. The presence of protein in the urine (PU) was detected by using a dipstick urinalysis screening test. The albumin-to-creatinine ratio (ACR) reflects urine microalbumin.
3.4 Outcome measures
The eGFR was calculated using the Modification of Diet in Renal Disease (MDRD) formula using the equation (https://www.mdcalc.com/mdrd-gfr-equation) as expressed in mL/min per 1.73 m2 [20]. The kidney function stages were classified according to the American Diabetes Association guidelines [21] as follows; Normal kidney function: eGFR (= > 60) with no evidence of kidney damage. Stage 1 CKD: eGFR (=> 90) with evidence of kidney damage. Stage 2 CKD: eGFR (60–89) with evidence of kidney damage. Stage 3 CKD: eGFR (30–59) with or without evidence of kidney damage. Stage 4 CKD: eGFR (15 -29) with or without evidence of kidney damage. Stage 5 CKD: eGFR (< 15) with or without evidence of kidney damage [21].
4 Results
4.1 The demographic and clinical picture of the T2DM patients
The mean age of the 331 T2DM patients was 60 years old with a range of 33–99 years. The studied population consisted of 54% males and 46% females T2DM patients.
The clinical picture for the T2DM patients is shown in Table 1. About 60% of the patients had T2DM from 1 to 10 years and 40% had T2DM for more than 10 years. The HbA1C values were more than 7% in 68.1% of T2DM. The FBS values were higher than 130 mg/dL in 67.4% of the T2DM patients, indicating uncontrolled diabetes. About 21% of the T2DM patients had higher sCr values than normal (males 0.7–1.2 mg/dL, females: 0.5–1 mg/dL). Additionally, the uCr values were high (more than 2000 mg/day) in 96.2% of T2DM patients. The Albumin to Creatinine ratio (ACR) values were normal to mildly increased in 61.1%, and it was moderately to severely increased in about 40% of T2DM patients. Proteinuria (PU) is present in 22.5% of T2DM patients.
The eGFR values were also calculated for the T2DM patients (Table 1). Further analysis for the P-value using the SPSS software showed that the eGFR values were highly significant (P < 0.000) in all of the following; T2DM patients with more than 10 years of diabetes onset duration (eGFR = 68.80 compared to 84.24); T2DM patients with high sCr (eGFR = 39.79 compared to 85.65); T2DM patients with moderately to increased ACR ( eGFR = 68.56 compared to 85.4); T2DM patients with uCr values more than 2000 (eGFR = 76.42 compared to 98.38); and in T2DM patients with PU (eGFR = 60.69 compared to 82.06). However, the reduction in the eGFR values of the T2DM patients with HbA1C 7% and more, and in patients with FBS of more than 130 mg/dl was not significant (eGFR = 77.17, 75.09 compared to 80.16, and 78.33) respectively. The eGFR reduction folds in the T2DM patients were the largest (2.2 folds) with high sCr, followed by PU (1.4 folds), and in the elderly (1.3 folds) (Table 1).
4.2 Kidney functions among T2DM patients according to the ADA classification
The studied groups’ kidney functions were classified according to the ADA guidelines as shown in Table 2 and Fig. 1. About 60% of the T2DM patients had no clinical evidence of CKD, 5.2% were in Stage 1 CKD, 13.6% were in Stage 2 CKD, 16.9% were in Stage 3 CKD, about 3% were in Stage 4 CKD, and 1.5% were in Stage 5 CKD.
kidney functions in T2DM patients. A Pie chart showed the kidney functions in the participating T2DM patients according to the ADA classification [21]; It was normal in 60%; 5% were in Stage 1 CKD (S1), 14% were in Stage 2 CKD (S2); 17% were in Stage 3 CKD (S3), 3% were in Stage 4 CKD (S4), 1.5% were in Stage 5 CKD (S5)
4.3 The correlation of biochemical variables with the eGFR
The correlation between the biochemical variables and the eGFR was analyzed in Table 3 using Spearman’s correlation coefficient. Results showed that eGFR values were negatively correlated (P < 0.05) with diabetes onset duration, sCr, ACR, and PU (rs = − 0.303, − 0.871, − 0.371, − 0.340) respectively. Indicating a decrease in kidney functions with the increase in diabetes onset duration, or with the increase in renal biochemical variables. Interestingly, the HbA1C values were positively correlated with the diabetes onset duration and the FBS level (rs = 0.150, 0.486) respectively. The sCr values were positively correlated with the diabetes onset duration and the HbA1C level (rs = 0.221, 0.134) respectively. This suggests a link between uncontrolled diabetes and the increase in sCr level. The ACR values were positively correlated with FBS, HbA1C, and sCr levels (rs = 0.293, 0.231, 0.366) respectively. Similarly, PU values were positively correlated with FBS, HbA1C, sCr, and ACR (rs = 0.170, 0.177, 0.399, 0.519) respectively.
5 Discussion
This study is among the first studies in the region that evaluated kidney functions in diabetic patients according to the ADA guidelines using recent data. In addition, this research demonstrated a significant association between the biochemical variables and the eGFR. Previous studies in the region evaluated kidney functions using different criteria to diagnose CKD [16, 21]. Herein, the kidney functions among T2DM patients were evaluated by following the ADA classification of CKD stages [21]. Further, the prevalence of CKD differs from one region to another, each population has its determining factors that affect CKD prevalence such as lifestyle and ethnicity [23], which make it difficult to draw a general conclusion about the CKD prevalence among diabetics. In addition, as the prevalence of T2DM is increasing worldwide, it is always important to evaluate kidney functions based on recent data obtained from T2DM patients for more accurate conclusions. Together necessitate for recent studies cover the CKD prevalence and the associated risk factors among diabetics.
The CKD prevalence in the Middle East region was poorly covered. A previous study showed that the CKD prevalence in non-diabetics was about 34% [24]. A recent systematic review covered all the studies in the region (n = 9 studies) between the years 2009–2019, and showed that the CKD prevalence average was about 29% [17]. In comparison, we found that about 40% of the studied group were at risk of developing CKD shortly. Further, the CKD stage prevalence was the highest in the S3 (17%), compared to a previous report showing that most of the T2DM patients were in S2 [25]. This indicated an increase in CKD prevalence and pathogenicity in the studied population.
The prevalence of CKD is expected to increase among T2DM patients of younger ages. A previous study from the USA evaluated kidney functions in T2DM patients, they found that the age-adjusted prevalence of CKD decreased over the years, indicating a possibility of early onset of CKD with time. Herein, we noticed a reduction in the eGFR among T2DM patients who were more than 50 years old compared to a previous report in 2016 which indicated an increase in the CKD prevalence in T2DM patients who were more than 65 years old [23].
T2DM patients have a higher risk of developing CKD compared to nondiabetic patients [8]. The high blood sugar in diabetics alters glomerular hemodynamics and makes the vessels narrow and clogged which damages the blood vessels in the kidney causing diabetic nephropathy [15, 26]. Diabetic nephropathy is a serious complication that progresses slowly along with the diabetes onset duration [15, 26]. In agreement with our findings, the eGFR values were significantly reduced with the increase in diabetes onset duration.
The eGFR values were significantly associated with sCr in T2DM patients. This Confirms the previous findings by Nelson et al., who found that the increase in serum creatinine level was associated with a decrease in eGFR and CKD stages progression [27]. The presence of PU was also associated with the decrease in eGFR. The same finding was reported in a study from Japan, they found that the eGFR value from patients with PU was lower than that in patients without [28]. The increase in sCr and the presence of PU are considered strong predictors for reduced kidney functions.
Interesting associations were detected between HbA1C level with diabetes duration; FBS levels; sCr level; and urine ACR values, confirming previous reports about the correlations between the different biochemical variables in the T2DM patients [29,30,31,32,33] Further, an association was also detected between PU with sCr, and ACR confirming previous findings [31]. These findings encourage more investigation into the mechanism of action and the effect of the high glucose level on other clinical parameters, which eventually affect whole-body functions.
5.1 Limitations
The limitations of this research include; a group of T2DM patients who were hesitant or not interested in participating in the study. This might create a selection bias that affects the generalizability of the research results because of the low sample size. The nature of the cross-sectional studies that are susceptible to different types of bias. The information bias arises from the missing information about patients’ clinical parameters. Of note, this research is free of any potential bias since the T2DM patients were approached randomly, and their participation was not subjected to any benefits.
6 Conclusions
This study provides evidence of the increasing risk of kidney diseases among T2DM patients. The eGFR values were significantly reduced with the increase in age, diabetes onset duration, and abnormal clinical parameters. Therefore, T2DM patients have to go under regular checks on their clinical parameters especially those with other risk factors such as hypertension and hyperlipidemia. T2DM patients are highly encouraged to adopt a healthy lifestyle. Patient education is of great importance to monitor the disease and to slow down the CKD progression. More updated studies about CKD prevalence are recommended in the region to reflect the current situation in the Middle East region.
Availability of data and materials
The data is available by the corresponding author upon request.
Abbreviations
- T2DM:
-
Type 2 diabetes mellitus
- CKD:
-
Chronic kidney disease
- ADA:
-
American diabetes association
- eGFR:
-
Estimated GFR
- PU:
-
Protein urea
- sCr:
-
Serum Creatinine
- uCr:
-
Urine Creatinine
- ACR:
-
Albumin to creatinine ratio
- FBS:
-
Fasting blood sugar
References
IDF_Atlas_10th_Edition_2021.pdf. Accessed 01 Dec 2022. [https://diabetesatlas.org/idfawp/resource-files/2021/07/IDF_Atlas_10th_Edition_2021.pdf
Al-Bdour MD, Al-Till MI, Abu-Samra KM (2008) Risk factors for diabetic retinopathy among Jordanian diabetics. Middle East Afr J Ophthalmol 15(2):77–80. https://doi.org/10.4103/0974-9233.51997
Roden M (2016) Diabetes mellitus: definition, Klassifikation und Diagnose. Wien Klin Wochenschr 128(2):37–40. https://doi.org/10.1007/s00508-015-0931-3
Khawaja N, Abu-Shennar J, Saleh M, Dahbour SS, Khader YS, Ajlouni KM (2018) The prevalence and risk factors of peripheral neuropathy among patients with type 2 diabetes mellitus; the case of Jordan. Diabetol Metab Syndr 10:8. https://doi.org/10.1186/s13098-018-0309-6
Koye DN, Magliano DJ, Nelson RG, Pavkov ME (2018) The global epidemiology of diabetes and kidney disease. Adv Chronic Kidney Dis 25(2):121–132. https://doi.org/10.1053/j.ackd.2017.10.011
Janmohamed MN et al (2013) Prevalence of chronic kidney disease in diabetic adult out-patients in Tanzania. BMC Nephrol 14(1):183. https://doi.org/10.1186/1471-2369-14-183
Guo K et al (2016) Prevalence of chronic kidney disease and associated factors in Chinese individuals with type 2 diabetes: Cross-sectional study. J Diabetes Complicat 30(5):803–810. https://doi.org/10.1016/j.jdiacomp.2016.03.020
Afkarian M et al (2013) Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol 24(2):302–308. https://doi.org/10.1681/ASN.2012070718
Giorda CB et al (2018) Ten-year comparative analysis of incidence, prognosis, and associated factors for dialysis and renal transplantation in type 1 and type 2 diabetes versus non-diabetes. Acta Diabetol 55(7):733–740. https://doi.org/10.1007/s00592-018-1142-y
Ghaderian SB, Hayati F, Shayanpour S, Beladi-Mousavi SS (2015) Diabetes and end-stage renal disease; a review article on new concepts. J Renal Inj Prev 4(2):28–33. https://doi.org/10.12861/jrip.2015.07
Dasgupta I (2014) Cost of treating diabetic kidney disease. Indian J Nephrol 24(3):139–140. https://doi.org/10.4103/0971-4065.131999
Foley RN, Collins AJ (2009) The growing economic burden of diabetic kidney disease. Curr Diab Rep 9(6):460. https://doi.org/10.1007/s11892-009-0075-9
Narva AS, Bilous RW (2015) Laboratory assessment of diabetic kidney disease. Diabetes Spectr 28(3):162–166. https://doi.org/10.2337/diaspect.28.3.162
Umanath K, Lewis JB (2018) Update on diabetic nephropathy: core curriculum 2018. Am J Kidney Dis 71(6):884–895. https://doi.org/10.1053/j.ajkd.2017.10.026
Taderegew MM (2020) Assessment of renal impairment using estimated glomerular filtration rate among type 2 diabetes mellitus patients in North-East Ethiopia: a cross-sectional study. J Diabetes Metab Disord 19(2):1473–1481. https://doi.org/10.1007/s40200-020-00680-4
Belguith H (2012) Use of e-GFR formula to evaluate kidney function in diabetes mellitus patients in Al-Jouf area, Saudi Arabia. J Biomed Sci 1(1). Accessed 27 Jul 2021. https://www.jbiomeds.com/abstract/use-of-egfr-formula-to-evaluate-kidney-function-in-diabetes-mellitus-patients-in-aljouf-area-saudi-arabia-1941.html
Naser AY et al (2021) Prevalence of chronic kidney diseases in patients with diabetes mellitus in the middle east: a systematic review and meta-analysis. Int J Endocrinol 2021:4572743. https://doi.org/10.1155/2021/4572743
Khalil A, Abdalrahim M (2014) Knowledge, attitudes, and practices towards prevention and early detection of chronic kidney disease. Int Nurs Rev 61(2):237–245. https://doi.org/10.1111/inr.12085
von Elm E et al (2007) The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 370(9596):1453–1457. https://doi.org/10.1016/S0140-6736(07)61602-X
Munikrishnappa D (2009) Chapter 6: limitations of various formulae and other ways of assessing GFR in the elderly: is there a role for cystatin C? 2009, https://www.semanticscholar.org/paper/Chapter-6-%3A-Limitations-of-Various-Formulae-and-of-Munikrishnappa/9dd34571c14c7e17d1cf6e4b2fb47b00afe42662
Association AD (2019) 11. Microvascular complications and foot care: standards of medical care in diabetes—2019. Diabetes Care 42(Supplement 1):S124–S138. https://doi.org/10.2337/dc19-S011
Jamal-Shahwan M, Galil-Hassan NA, Shaheen RA (2019) Assessment of kidney function and associated risk factors among type 2 diabetic patients. Diabetes Metab Syndr 13(4):2661–2665. https://doi.org/10.1016/j.dsx.2019.07.025
Wu B et al (2016) Understanding CKD among patients with T2DM: prevalence, temporal trends, and treatment patterns—NHANES 2007–2012. BMJ Open Diab Res Care 4(1):e000154. https://doi.org/10.1136/bmjdrc-2015-000154
Khalil AA, Abed MA, Ahmad M, Mansour AH (2018) Under-diagnosed chronic kidney disease in Jordanian adults: prevalence and correlates. J Ren Care 44(1):12–18. https://doi.org/10.1111/jorc.12214
Belguith H (2012) Use of e-GFR formula to evaluate kidney function in diabetes mellitus patients in Al-Jouf area, Saudi Arabia. J Biomed Sci 1(1). Accessed 19 Feb 2020. http://www.jbiomeds.com/abstract/use-of-egfr-formula-to-evaluate-kidney-function-in-diabetes-mellitus-patients-in-aljouf-area-saudi-arabia-1941.html
Wu J, Geng J, Liu L, Teng W, Liu L, Chen L (2015) The relationship between estimated glomerular filtration rate and diabetic retinopathy. J Ophthalmol 2015:e326209. https://doi.org/10.1155/2015/326209
Nelson AW, Mackinnon B, Traynor J, Geddes CC (2006) The relationship between serum creatinine and estimated glomerular filtration rate: implications for clinical practice. Scott Med J 51(4):5–9. https://doi.org/10.1258/RSMSMJ.51.4.5
Irie F et al (2006) The relationships of proteinuria, serum creatinine, glomerular filtration rate with cardiovascular disease mortality in Japanese general population. Kidney Int 69(7):1264–1271. https://doi.org/10.1038/sj.ki.5000284
Verma M, Paneri S, Badi P, Raman PG (2006) Effect of increasing duration of diabetes mellitus type 2 on glycated hemoglobin and insulin sensitivity. Indian J Clin Biochem 21(1):142–146. https://doi.org/10.1007/BF02913083
Dave M, Gupta AK, Patel P, Heernath H (2019) Correlation between fasting blood sugar level, HbA1C level and serum lipid levels in type 2 diabetes mellitus patients. IJCMR. https://doi.org/10.21276/ijcmr.2019.6.7.13
Karar T, Alniwaider RAR, Fattah MA, Al-Tamimi W, Alanazi A, Qureshi S (2015) Assessment of microalbuminuria and albumin creatinine ratio in patients with type 2 diabetes mellitus. J Nat Sci Biol Med 6(Suppl 1):S89–S92. https://doi.org/10.4103/0976-9668.166095
Farasat T, Sharif S, Naz S, Fazal S (2015) Significant association of serum creatinine with HbA1C in impaired glucose tolerant Pakistani subjects. Pak J Med Sci 31(4):991–994. https://doi.org/10.12669/pjms.314.7063
Haque N, Debnath BC, Ibrahim M, Sirajuddin K, Majumder M, Hossain MS (2011) Association of HbA1c with urinary ACR & eGFR in type-2 diabetes mellitus. Pulse. https://doi.org/10.3329/pulse.v5i1.20183
Acknowledgements
The authors would like to thank the Endocrinology Clinic at King Abdullah University Hospital, Jordan, for providing support in sample collection and patients’ data. Our gratitude is for Jordan University of Science and Technology for funding this research. A special thank is for the patients who agreed to share their information in this study.
Funding
The expenses of samples’ collection and the research assistant who collected the data from the Endocrinology and Cardiology clinics at King Abdullah University Hospital was funded by the deanship of scientific research of Jordan University of Science and Technology, Jordan. Grant no. 20200195 that was granted for Dr. Sayer Al-Azzam.
Author information
Authors and Affiliations
Contributions
EA: participated in the study design, writing the result and discussion sections, and editing the manuscript. HA: Study design, collecting data, writing the introduction, and editing the manuscript. SA: Ethical approval, collecting data and editing the manuscript. AQ: Statistical analysis and writing the method section. AA: writing and editing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study involved human participants. The study protocol is in conformity with the ethical principles of the Helsinki Declaration and approved by the Institutional Review Board committee at King Abdullah University Hospital (Reference number 48/132/2020), that was issued on 01.04.2020, the IRB approval letter is provided on the additional file 1 in the file inventory. Each patient who participated in this study provided a signed written consent form for participation, the consent form was provided on the additional file 2. The data collection form was also provided on the additional file 3. This study was an observational and didn’t involve invasive procedures or laboratory testing. In addition, the data collection was done in a confidential way.
Consent for publication
Not applicable.
Consent to publish
All authors of this study were consent for publication.
Competing interests
All authors of this study declared that there was no conflict of interest in the publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Almomani, E.Y., Almomani, H.Y., Al-Azzam, S. et al. The rising burden of chronic kidney diseases in patients with diabetes. Beni-Suef Univ J Basic Appl Sci 12, 88 (2023). https://doi.org/10.1186/s43088-023-00428-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43088-023-00428-y