Abstract
Background
In Tunisia, the prevalence of diabetes mellitus increased from 15.5% on 2016 to 23% by 2023. While Chronic Kidney Disease (CKD) stills the most dreaded complications of diabetes, studies on the prevalence of chronic kidney disease non-dialysis diet are scarce. The aim of this study was to assess the prevalence of chronic kidney disease among the Tunisian diabetic population based on investigators’ specialty, demographic criteria (gender, age, duration of diabetes and geographic distribution) and diagnosis criteria (albuminuria and/or eGFR).
Methods
This observational, multicentric, and cross-sectional study enrolled all diabetic subjects from all regions of Tunisia with at least 3 months of follow-up before the inclusion date, from 09 January to 08 February 2023. CKD diagnosis was established based on the KDIGO guidelines. The study was carried out at medical departments and ambulatory clinics of different healthcare providers. Baseline data were collected by investigators using an electronic case report form (eCRF). Continuous variables were described by means, median, standard deviation, and quartiles. Categorical data were tabulated in frequencies and percentages.
Results
The overall prevalence of CKD among the 10,145 enrolled patients with diabetes mellitus was 38.7% with a 95%CI [37.8-39.6%]. 50.9% were male, with a mean age of 67.5 (± 11.3) years. The mean diabetes duration was 16.1 years (± 8.9). The highest CKD prevalence was noted among nephrologists (82.2%), while it was similar between the cardiologists and the primary care physicians (30.0%). CKD prevalence was highest among males (43.0% versus 35.1%) and increased proportionally with patients’ age and diabetes duration. CKD was more frequent in the Mid-East Area when compared to other regions (49.9% versus 25.3 to 40.1% in other regions). Albuminuria was present within 6.6% of subjects with CKD, and it was found an estimated glomerular filtration rate (eGFR) < 60 ml/min/1.73 m² within 13.3% of subjects wit h CKD. 18.9% had both criteria.
Conclusions
In Tunisia, CKD among diabetics had a prevalence of 38.7%, approaching European prevalence. The prevalence discrepancy worldwide of CKD can be improved with a larger population size and by implementing standardized practices.
Similar content being viewed by others
Introduction
The International Diabetes Federation reports a continuous increase in diabetes prevalence reaffirming diabetes as a major global health concern. By 2021, 537 million adults aged between 20 and 79 years are living with diabetes around the world. This number is predicted to rise to 643 million by 2030 [1]. In Tunisia, the prevalence of diabetes mellitus reached 15.5% by 2016 [2]. On 2023, the ATERA survey estimated the prevalence of type 2 diabetes at 23% [3].
Chronic Kidney Disease (CKD) is one of the most dreaded complications of diabetes. A systematic review estimated its prevalence globally to be 57% [4, 5]. Besides, diabetes mellitus has emerged as the most prevalent cause of end-stage renal disease (ESRD) [6], cardiovascular events, and early death in developing and developed countries [4].
In Africa, in a recent systematic review, the prevalence of CKD was estimated to range between 2% and 41% while the prevalence of CKD among diabetic subjects ranged from 11 to 90%. In North Africa, CKD prevalence varies from 11 to 20% [8].
CKD problem among diabetic subjects remains underestimated on the entire continent due to a lack of epidemiological information from different African countries. In Tunisia, studies on the prevalence of chronic kidney disease non-dialysis diet are scarce.
Our study aimed to assess the prevalence of chronic kidney disease among the Tunisian diabetic population based on investigators’ specialty, demographic criteria (gender, age, duration of diabetes and geographic distribution) and diagnosis criteria (albuminuria and/or eGFR).
Methods
Study design and patients’ inclusion
This observational, multicentric, and cross-sectional study enrolled all diabetic subjects with at least 3 months of follow-up before the inclusion date, from 09/01/2023 to 08/02/2023.
Diabetes mellitus was defined as a fasting plasma glucose ≥ 7 mmol/L on two or more occasions, a random blood glucose ≥ 11.1 mmol/L with symptoms of hyperglycemia or hyperglycemic crisis, HbA1C > 6.5% [9] or patients under treatment of type 2 diabetes.
The inclusion criteria were as follow:
-
Age ≥ 18 years old,
-
Type 1 or Type 2 diabetes,
-
Follow-up at the consultation for at least more than 3 months,
-
Informant consent of the patient.
Subjects with chronic dialysis, kidney transplant, and pregnant women were non-included. When informed consent was withdrawn, diagnosis of chronic renal disease was not well established, and/or missing data, subjects were excluded from the study. The recruited individuals in this non-interventional study underwent clinical evaluations and get routine medical care as decided by their treating physicians. Participation was entirely voluntary.
Chronic kidney disease diagnosis
In the current study, based on the Kidney Disease Improving Global Outcomes (KDIGO) guidelines [10], chronic kidney disease was defined by albuminuria and/or estimated glomerular filtration rates (eGFR) < 60 ml/min/1.73 m² for three months. Normative values for albuminuria are generally expressed as the urinary loss rate and referred to as Albumin Excretion Rate (AER) or Albumin-to-Creatinine Ratio (ACR). The threshold for AER is ≥ 30 mg/24 hours sustained for > 3 months to indicate CKD (which is equivalent to an ACR > 30 mg/g or > 3 mg/mmol). Albuminuria categories are detailed in Table 1.
AER: albumin excretion rate, PER: protein excretion rate, ACR: albumin-to-creatinine ratio, PCR: protein-to-creatinine ratio.
For each patient, estimated glomerular filtration rates were calculated based on The Modification of Diet in Renal Disease Study equation [11, 12]:
GFR categories are:
-
Category G1: GFR ≥ 90 ml/min/1.73m2
-
Category G2: GFR 60–89 ml/min/1.73m2
-
Category G3a: GFR 45–59 ml/min/1.73m2
-
Category G3b: GFR 30–44 ml/min/1.73m2
-
Category G4: GFR 15–29 ml/min/1.73m2
-
Category G5: GFR < 15 ml/min/1.73m2
Sites and investigators selection
The study was carried out for one month at medical departments and ambulatory clinics of primary care physicians (general physicians and family medicine specialists), nephrologists, specialists in nutrition and metabolic diseases, internal medicine physicians, cardiologists, or other healthcare providers in charge of diabetic patients. Tunisia is divided into seven regions (Great Tunis, North East, North West, Mid-East, Mid-West, South East, and South West). For each region, a list of prospective sites was established, and all sites were invited to participate in the study.
Data collection
Informed consent was obtained from all the study participants, and data were collected at baseline by investigators using an electronic case report form (eCRF) via a web-based data capture system managed by the DACIMA Clinical Suite®. The electronic data capture platform complies with the FDA 21 CFR part 11 requirements (Food and Drug Administration 21 Code of Federal Regulations part 11), the HIPAA specifications (Health Insurance Portability and Accountability Act), and the ICH standards (International Conference on Harmonization).
Baseline data included demographic and anthropometric data, presence of comorbidities, medical history of type 2 diabetes, and diagnostic tests for chronic renal failure.
Statistical analysis
The data reviewing and data cleaning were performed through the electronic data capture system (Dacima). After the database lock, the statistical analysis was done to describe the population outcomes according to the study objectives. Continuous variables were described by means, median, standard deviation and quartiles. Categorical data were tabulated in frequencies and percentages. No inferential statistics were performed for this study. The 95% confidence interval was estimated for the primary outcome.
Results
Study design
In an observational multicentric cross-sectional study, 11,033 subjects were eligible for the national registry of Chronic Kidney Disease TUN-CKDD. 18 subjects did not give their informed consent and were non-included. 11,015 were enrolled and among them, 750 subjects were excluded for missing data.
10 265 subjects underwent the diagnostic tests, and 120 subjects were excluded for a non-well-established diagnosis of CKD. The flowchart of the participants is presented in Fig. 1.
Prevalence of chronic kidney disease
The overall prevalence of CKD in patients with diabetes mellitus was 38.7% with a 95%CI [37.8-39.6%].
Among the 10 145 subjects enrolled, 3929 had an eGFR < 60 ml/min/1.73 m² and/or a ACR ≥ 3 mg/mmol.
The cohort with CKD and diabetes mellitus was 50.9% male, with a mean age of 67.5 (± 11.3) years. The mean diabetes duration was 16.1 years (± 8.9) with a BMI of 28.6 kg/m2 and an HbA1c level of 8.2% (± 1.7). Sociodemographic and clinical data are presented in Table 2.
Prevalence based on investigators’ specialty
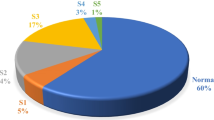
This national survey was conducted by a several groups of specialists. The highest CKD prevalence was noted among nephrologists (82.2%), while it was similar between the cardiologists and the primary care physicians (30.0%), Fig. 2.
Nephrologists reported the highest prevalence of CKD at 82.2%. This likely reflects their specific focus on kidney diseases, making them more likely to encounter and diagnose CKD patients.
Cardiologists and primary care physicians: Reported similar prevalence levels. This could be due to several factors, such as:Cardiologists: While not primarily focused on kidney health, they may encounter CKD patients due to its common co-occurrence with cardiovascular issues.Primary care physicians: As the first point of contact for many patients, they see a wide range of health conditions, including CKD.
Prevalence of chronic kidney disease based on gender
Among males included in the study population, 43.0% had CKD versus 35.1% of females.
eGFR and ACR were higher among males with a median of 44.6 (30.8; 56.7) versus 40.9 (28.9; 53.1) mL/min/1.73 m2 and 940 (300; 2000) versus 600 (135.5; 1653) mg/mmol respectively (Table 2).
Prevalence of chronic kidney disease based on age and diabetes duration
Figure 3 shows the increase of CKD prevalence proportionally with patients’ age and diabetes duration. The lowest prevalence was in the below 40 years’ age group with diabetes duration < 5 years (10.1%), while the highest was with duration above 15 years in the age group over 65 years, amounting to 62.0%.
Prevalence of chronic kidney disease based on geographic distribution
CKD prevalence was higher in the Mid-East Area when compared to other regions (49.9% versus 25.3 to 40.1% in other regions) (Fig. 4).
In the Greater Tunis Area, the prevalence of CKD varies between 25.5% (Ben Arous) and 50.3% (Manouba). The lowest prevalence was noted in Tozeur (16.2%).
Prevalence of chronic kidney disease based on diagnosis criteria
As KDIGO guidelines defined Chronic Kidney Disease as albuminuria and/or eGFR < 60 ml/min/1.73 m² [10], the survey determined the prevalence of each criterion.
Albuminuria was present within 6.6% of subjects with CKD, and it was found an eGFR < 60 ml/min/1.73 m² within 13.3% of subjects with CKD. 18.9% had albuminuria and eGFR < 60 ml/min/1.73 m² (Fig. 5).
The prevalence of CKD based on GFR, and albuminuria categories is detailed in Table 3.
Discussion
This prospective multicentric study provides insights into epidemiologic data of chronic kidney disease among diabetic subjects in the Tunisian population. To the best of our knowledge, the Tunisian Chronic Kidney Disease among Diabetics (TUN-CKDD) is the first national register for diabetic kidney disease. By 2023, the prevalence of CKD in Tunisian diabetic subjects was 38.7%. Among them, 6.6% exhibited albuminuria, 13.3% had an eGFR below 60 ml/min/1.73 m² and 18.9% fulfilled both criteria. The prevalence of CKD in T2D was 39.4% while it was 27.9% among T1D. Patients with T1D are typically diagnosed at a younger age being healthier and with fewer comorbidities compared to patients with T2D. These factors may contribute to the comparatively lower prevalence of CKD observed in this patient group [4]. The prevalence of CKD among patients with T2D varies across the world.
In Africa, it ranges from 11 to 90%. In North Africa, CKD prevalence varies from 11 to 20% [8], while in Sub-Saharan Africa, its prevalence seems to be higher ranging from 4 to 24% [13]. In the Middle East, The Saudi study was the largest registry including 54.670 Saudi type 2 diabetic patients. The prevalence of diabetic nephropathy was 10.8%, among them 14% had ESRD [14]. However, data on the prevalence of the earlier stages of CKD in the Middle East is still sparse.
In the United States, diabetes mellitus is the leading cause of kidney failure. By 2014, the prevalence of CKD among diabetics was estimated at 25% [15] and would attend 54% by 2030 [16]. In Canada, CKD prevalence among T2D reaches 48% [17]. In the European Union, the prevalence of CKD among diabetics varies among countries. The Finnish registry showed a prevalence of CKD among T2D subjects of 30.1% [18], while in the Greek registry, the overall prevalence of CKD among diabetics was 45% [19]. The prevalence of CKD in Tunisia seems to be near to that of European countries in contrast to the United States and Canada, where the prevalence of CKD was higher. In Asia, reported CKD prevalence among diabetics was lower than our study and ranged from 7% (95% CI 5.1–8.9%) in South Korea to 34.3% (95% CI 0.0–71%) in Singapore [20] and the Chinese registry showed a prevalence of 29.6% [21].
Many factors may contribute to the observed differences in CKD prevalence worldwide. Either those differences are possibly due to true differences in the prevalence of CKD or due to the heterogeneity of studies. Indeed, we can attribute this variability to the registries size, ethnicity, socioeconomic disadvantage, educational attainment, lower therapy goal fulfillment, patient therapeutic education, screening rates, inadequate management of early complications, dietary and lifestyle factors, smoking, obesity, and genetic background [4]. Moreover, factors beyond diagnostic criteria can influence the prevalence of kidney disease among individuals with diabetes.
Based on the KDIGO 2012 Clinical Practice Guideline, CKD diagnosis is established when eGFR declines to < 60 ml/min/1,73 m² with/without a persistently elevated urinary albumin excretion [10]. Most studies have used only eGFR to determine the presence of CKD and therefore report on the prevalence of CKD stages 3–5. By 2020, The DISCOVER study, a global 3-year prospective observational study enrolling T2D subjects from 35 countries, defined CKD among diabetics as eGFR < 60 mL/min/1.73 m2 (estimated with the CKD-EPI formula) and found a prevalence of 56.8% in the Eastern Mediterranean Countries including Tunisia [22]. As well, the Nepali study reported CKD as the reduction of eGFR less than 60mL/min estimated by the Cockcroft-Gault equation and therefore, only stages 3–5 were considered as the presence of CKD. The frequency of CKD among Nepali T2D was then estimated up to 86.6% [23]. Although, similarly to our study, other investigators have considered the combination of albuminuria and decreased eGFR, as seen in previous studies [14, 18, 19].
One should notice that eGFR assessment methods may contribute also to the variability of CKD prevalence. Studies have shown that The MDRD study equation had the less bias and the highest accuracy to estimate eGFR in diabetic cohort trials [24, 25], while the CKD-EPI equation have less bias than the MDRD Study equation at GFR > 60 ml/min/1.73 m2 [26, 27], whilst the Cockcroft-Gault formula should be used in screening declining renal function in subjects with normal serum creatinine such as diabetic subjects with CKD stage 1 or 2 [28].
These results highlight the need for multiple sources of data from various countries to estimate and track CKD prevalence with standardized methods.
This nationwide registry involved physicians from different medical specialties, with primary care physicians being the most commonly involved. Nephrologists diagnosed the highest proportion of CKD prevalence among the diabetic subjects included in this study (82.2%), while primary care physicians and cardiologists accounted for 30% of CKD diagnoses and endocrinologists for only 15.3%. In Tunisia, the nephrologist delivers most CKD care. Like many chronic conditions, primary care physicians typically play a key role in identifying the presence of CKD in patients. Subsequently, they refer the patients to nephrologists for further evaluation and management. The KDIGO guidelines recommend referring patients to nephrologists when eGFR decreases below 30 ml/min/1.73 m² with/without increasing albuminuria [10]. Gender-related disparities have been observed in non-diabetic CKD. In many regions (excluding Japan, Singapore, and Thailand), the prevalence of CKD was higher among women compared to men. Notably, in France, Thailand, Portugal, and Turkey, CKD was twice as high in females [29].
Thereafter, several studies have explored sex differences in the prevalence of Diabetic Kidney Disease. Recent data suggest that diabetic men are at greater risk of developing CKD [30]. Indeed, our registry showed a higher prevalence of CKD among males (43%) when compared to females (35.1%). It has been shown that kidney function declines faster in men than women, possibly due to the renoprotective role of estrogens and progesterone [31] and to unhealthier lifestyles in men or the damaging effects of testosterone [32].
Furthermore, when considering specific CKD phenotypes, it is observed that diabetic men have a higher susceptibility to developing albuminuria, whereas women have a higher susceptibility to eGFR impairment and the development of ESRD [31].
It is important to acknowledge that eGFR formulas incorporate sex-specific corrections to account for disparities between men and women, such as the higher muscle mass typically found in men, resulting in higher creatinine levels and consequently lower eGFR values among women. Indeed, in our study, the median eGFR was slightly lower in women when compared to men (40.9 versus 44.6 mL/min/1.73 m2 respectively). The reasons behind these reported gender disparities in CKD are still largely unknown, but hormonal and genetic differences might be incriminated. Indeed, genetic susceptibility to CKD can be attributed to some identified genes and single nucleotide polymorphisms (SNPs) [33, 34]. Moreover, the involvement of epigenetic mechanisms along with their potential interactions with personal or environmental factors including gender, may also exert a significant influence [35, 36]. In our study, the prevalence of CKD among diabetic subjects increased proportionally with age and diabetes duration, as seen in other published data [14, 37, 38]. We report an accumulative effect of both age and duration on the prevalence of CKD, where it increases by twofold in patients with diabetes duration above 15 years, regardless of the patient’s age.
Moreover, in our study, the highest prevalence of CKD regardless of diabetes duration was among adults aged ≥ 65 years, as shown previously [14, 39]. According to the UK Prospective Diabetes Study, after a median follow-up of 15 years of diabetic subjects, approximately 28% would exhibit a decline in eGFR below 60 mL/min/1.73 m² along with albuminuria [40].
In Tunisia, in a large cohort study conducted from 2006 to 2008, the prevalence of CKD among diabetic subjects was 19.1% [41]. In our study, CKD prevalence was up to 38.7%. To the best of our knowledge, this is the first study to report the prevalence of CKD in the different regions of Tunisia. The highest prevalence was noted in the Mid-East Area (49.9% versus 25.3 to 40.1% in other regions) while the lowest was observed within the South West region (25.3%). This variability across regions in the Tunisian population might be explained by dietary and lifestyle factors or even genetic background since the different Tunisian regions are known to exhibit significant genetic diversity [42].
In our study, early stages of CKD were present among respectively 6.2% (Stage 1) and 10.8% (Stage 2) of diabetic subjects. Stages 3a and 3b exhibited higher prevalence (28.6% and 29.1%, respectively), followed by stage 4 (19.1%), while stage 5 was less common (6.2%).
In the Finnish study, early stages of CKD among diabetics were more frequent since 28.3% of patients had G1, 53.1% had G2 and G3a and G3b had a prevalence of 12.4% and 4.7% respectively [18]. As well, the Greek registry showed a higher prevalence of mild CKD [19].
Most patients with CKD, even stage 4, do not progress to the advanced stages of the disease, as mortality occurs before reaching ESRD [43,44,45].
Dalrymple et al. found that older persons with CKD were 13-fold more likely to die from any cause than to progress to ESRD [46].
Our study results should be interpreted with the consideration of a few limitations. Data analysis and results interpretation did not take into account the low recruiting potential of some sites that can prevent reaching optimal representativeness. Registry limitations include the possibility of incomplete data collection of medical history and for some patients’ albuminuria was not estimated.
The strengths of our study include its extensive scope that involved patients from across the entire national territory, encompassing both public and private healthcare systems in Tunisia. Moreover, the study was conducted by a diverse group of specialists, including primary care physicians, endocrinologists, nephrologists, and professionals from various other medical disciplines.
Prevalence of CKD among Diabetic Patients in Tunisia: Differences According to Gender and Age (BMC Nephrology, 2020): This study, while not focused on investigators’ specialty, analyzes CKD prevalence based on gender and age in the Tunisian diabetic population. It reveals higher prevalence among males and with increasing age, similar to your findings. Sex hormones and progression of chronic kidney disease in diabetic patients: (Kidney International, 2011): This review article discusses the complex interplay of sex hormones (estrogen, testosterone) and their impact on renal function and CKD progression, potentially explaining some gender differences.
In conclusion, this is the first study that carefully characterizes CKD prevalence across Tunisia. Diabetic kidney disease had a prevalence of 38.7%, approaching European prevalence. The prevalence discrepancy worldwide of CKD among diabetics can be improved with a larger population size and by implementing standardized practice. Either these differences are possibly due to true differences in the prevalence of CKD as well as to heterogeneity of the laboratory and sample selection methods, the effect of this variability about human and environmental factors, public health policies, and genetics on CKD prevalence needs further investigation. Epidemiological data allow us to better understand the Tunisian diabetic population with MRC. This will help us to assess the effectiveness of preventive measures and research to develop to improve the prognosis of these patients.
Data availability
The datasets generated and analyzed during the current study are not publicly available due to ethical concerns but are available from the corresponding author on reasonable request.
References
International Diabetes Federation. IDF Atlas, 10th edition. Available from: https://www.diabetesatlas.org
Ministry of Health, National Institute for Health. Tunisian Health Examination Survey-2016. 2016.
Jemaa R, Razgallah R, Rais L, Ben Ghorbel I, Feki M, Kallel A. Prevalence of diabetes in the Tunisian population: results of the ATERA-survey. Archives Cardiovasc Dis Supplements. 2023;15(1):131.
Thomas MC, Brownlee M, Susztak K, Sharma K, Jandeleit-Dahm KAM, Zoungas S, et al. Diabetic kidney disease. Nat Rev Dis Primers. 2015;1(1):15018.
Parving HH, Lewis JB, Ravid M, Remuzzi G, Hunsicker LG. Prevalence and risk factors for microalbuminuria in a referred cohort of type II diabetic patients: a global perspective. Kidney Int. 2006;69(11):2057–63.
Schieppati A, Remuzzi G. Chronic renal diseases as a public health problem: epidemiology, social, and economic implications. Kidney Int. 2005;68:7–10.
Reutens AT, Atkins RC. Epidemiology of diabetic nephropathy. In: Lai KN, Tang SCW, Contributions to Nephrology. Karger S. AG; 2011 [cited 2023 May 16]. p. 1–7. Available from: https://www.karger.com/Article/FullText/324934
Abd ElHafeez S, Bolignano D, D’Arrigo G, Dounousi E, Tripepi G, Zoccali C. Prevalence and burden of chronic kidney disease among the general population and high-risk groups in Africa: a systematic review. BMJ Open. 2018;8(1):e015069.
American Diabetes Association. Classification and diagnosis of diabetes: standards of medical care in diabetes—2021. Diabetes Care. 2021;44(Supplement1):15–33.
Kidney Disease. Improving global outcomes (KDIGO) CKD work group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150.
Stevens LA, Coresh J, Feldman HI, Greene T, Lash JP, Nelson RG, et al. Evaluation of the modification of diet in renal disease study equation in a large diverse population. J Am Soc Nephrol. 2007;18(10):2749–57.
Levey AS, Coresh J, Greene T, Stevens LA, Zhang Y (Lucy), Hendriksen S, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–54.
Stanifer JW, Jing B, Tolan S, Helmke N, Mukerjee R, Naicker S, et al. The epidemiology of chronic kidney disease in sub-saharan Africa: a systematic review and meta-analysis. Lancet Global Health. 2014;2(3):e174–81.
Al-Rubeaan K, Youssef AM, Subhani SN, Ahmad NA, Al-Sharqawi AH, Al-Mutlaq HM et al. Diabetic nephropathy and its risk factors in a society with a type 2 diabetes epidemic: a Saudi national diabetes registry-based study. Alvarez ML, editor. PLoS ONE. 2014;9(2):e88956.
Zelnick LR, Weiss NS, Kestenbaum BR, Robinson-Cohen C, Heagerty PJ, Tuttle K, et al. Diabetes and CKD in the United States population, 2009–2014. CJASN. 2017;12(12):1984–90.
Rowley WR, Bezold C, Arikan Y, Byrne E, Krohe S, Diabetes. 2030: insights from yesterday, today, and future trends. Population Health Management. 2017;20(1):6–12.
Chu L, Fuller M, Jervis K, Ciaccia A, Abitbol A. Prevalence of chronic kidney disease in type 2 diabetes: the Canadian REgistry of chronic kidney disease in diabetes outcomes (CREDO) study. Clin Ther. 2021;43(9):1558–73.
Hagnäs M, Sundqvist H, Jokelainen J, Tumminia A, Vinciguerra F, Loreto C, et al. The prevalence of chronic kidney disease and screening of renal function in type 2 diabetic patients in Finnish primary healthcare. Prim Care Diabetes. 2020;14(6):639–44.
Migdalis IN, Papanas N, Raptis AE, Ioannidis IM, Sotiropoulos AE, Dimitriadis GD. The prevalence of diabetic chronic kidney disease in adult Greek subjects with type 2 diabetes mellitus: a series from hospital-based diabetes clinics. Diabetes Res Clin Pract. 2020;166:108243.
Liyanage T, Toyama T, Hockham C, Ninomiya T, Perkovic V, Woodward M, et al. Prevalence of chronic kidney disease in Asia: a systematic review and analysis. BMJ Glob Health. 2022;7(1):e007525.
An L, Yu Q, Tang H, Li X, Wang D, Tang Q, et al. The prevalence, progress and risk factor control of chronic kidney disease in Chinese adults with type 2 diabetes mellitus in primary care. Front Endocrinol. 2022;13:859266.
Khunti K, Charbonnel B, Chen H, Cherney DZ, Cooper A, Fenici P, et al. Prevalence and progression of chronic kidney disease among patients with type 2 diabetes: insights from the DISCOVER study. Diabetes Obes Metab. 2021;23(8):1956–60.
Joshi R, Subedi P, Yadav GK, Khadka S, Rijal T, Amgain K, et al. Prevalence and risk factors of chronic kidney disease among patients with type 2 diabetes mellitus at a tertiary care hospital in Nepal: a cross-sectional study. BMJ Open. 2023;13(2):e067238.
Schwandt A, Denkinger M, Fasching P, Pfeifer M, Wagner C, Weiland J, et al. Comparison of MDRD, CKD-EPI, and Cockcroft-Gault equation in relation to measured glomerular filtration rate among a large cohort with diabetes. J Diabetes Complicat. 2017;31(9):1376–83.
Michels WM, Grootendorst DC, Verduijn M, Elliott EG, Dekker FW, Krediet RT. Performance of the Cockcroft-Gault, MDRD, and new CKD-EPI formulas in relation to GFR, age, and body size. Clin J Am Soc Nephrol. 2010;5(6):1003–9.
KDOQI clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am J Kidney Dis. 2007;49(2):12–54.
Levey AS, Stevens LA, Schmid CH, Zhang Y (Lucy), Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604.
Helou R. Should we continue to use the Cockcroft-Gault Formula? Nephron Clin Pract. 2010;116(3):c172–86.
Carrero JJ, Hecking M, Chesnaye NC, Jager KJ. Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat Rev Nephrol. 2018;14(3):151–64.
Piani F, Melena I, Tommerdahl KL, Nokoff N, Nelson RG, Pavkov ME, et al. Sex-related differences in diabetic kidney disease: a review on the mechanisms and potential therapeutic implications. J Diabetes Complicat. 2021;35(4):107841.
Giandalia A, Giuffrida AE, Gembillo G, Cucinotta D, Squadrito G, Santoro D, et al. Gender differences in diabetic kidney disease: focus on hormonal, genetic and clinical factors. IJMS. 2021;22(11):5808.
Filler G, Ramsaroop A, Stein R, Grant C, Marants R, So A, et al. Is testosterone detrimental ren function? Kidney Int Rep. 2016;1(4):306–10.
Jin H, Kim YA, Lee Y, Kwon S, hyun, Do AR, Seo S, et al. Identification of genetic variants associated with diabetic kidney disease in multiple Korean cohorts via a genome-wide association study mega-analysis. BMC Med. 2023;21(1):16.
Rizvi S. Association of genetic variants with diabetic nephropathy. WJD. 2014;5(6):809.
Kato M, Natarajan R. Diabetic nephropathy—emerging epigenetic mechanisms. Nat Rev Nephrol. 2014;10(9):517–30.
Vujkovic M, Keaton JM, Lynch JA, Miller DR, Zhou J, Tcheandjieu C, et al. Discovery of 318 new risk loci for type 2 diabetes and related vascular outcomes among 1.4 million participants in a multi-ancestry meta-analysis. Nat Genet. 2020;52(7):680–91.
Unnikrishnan R, Rema M, Pradeepa R, Deepa M, Shanthirani CS, Deepa R, et al. Prevalence and risk factors of diabetic nephropathy in an Urban South Indian population. Diabetes Care. 2007;30(8):2019–24.
Viswanathan V, Tilak P, Kumpatla S. Risk factors associated with the development of overt nephropathy in type 2 diabetes patients: a 12 years observational study. Indian J Med Res. 2012;136(1):46–53.
Wu B, Bell K, Stanford A, Kern DM, Tunceli O, Vupputuri S, et al. Understanding CKD among patients with T2DM: prevalence, temporal trends, and treatment patterns—NHANES 2007–2012. BMJ Open Diab Res Care. 2016;4(1):e000154.
Retnakaran R, Cull CA, Thorne KI, Adler AI, Holman RR, for the UKPDS Study Group. Risk factors for renal dysfunction in type 2 diabetes. Diabetes. 2006;55(6):1832–9.
Bouzid C, Smida H, Kacem A. L’insuffisance rénale Chez Des diabétiques de type 2 Tunisiens hospitalisés: fréquence et facteurs associés. 2011;89(01):10–5.
Cherni L, Pakstis AJ, Boussetta S, Elkamel S, Frigi S, Khodjet-El‐Khil H, et al. Genetic variation in Tunisia in the context of human diversity worldwide. Am J Phys Anthropol. 2016;161(1):62–71.
Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med. 2004;164(6):659.
Peralta CA, Shlipak MG, Fan D, Ordon[Combining Tilde]ez J, Lash JP, Chertow GM, et al. Risks for end-stage renal disease, cardiovascular events, and death in hispanic versus non-hispanic white adults with chronic kidney disease. J Am Soc Nephrol. 2006;17(10):2892–9.
Foley RN, Murray AM, Li S, Herzog CA, McBean AM, Eggers PW, et al. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States medicare population, 1998 to 1999. J Am Soc Nephrol. 2005;16(2):489–95.
Dalrymple LS, Katz R, Kestenbaum B, Shlipak MG, Sarnak MJ, Stehman-Breen C, et al. Chronic kidney disease and the risk of end-stage renal disease versus death. J Gen Intern Med. 2011;26(4):379–85.
Acknowledgements
We do acknowledge the help of the « Tunisian Society of General medicine and Family medicine », the « Society of General Physicians of Tunisia», the «Tunisian Society of Family medicine», the «Tunisian Association of Nutrition Sciences», the «Tunisian Society of Endocrinology Diabetes and Metabolic Diseases», the «Tunisian Society of Internal Medicine», the «Tunisian Society of Cardiology and Cardiovascular Surgery», and all the investigators: Hanène Bouafif, Sana Sanai, Imed Eddine Abichou, Ghofrane Bel Haj Ali, Salem Abdesselem, Ibtissem Haddad, Rimeh Ben Brahim, Faouzi Addad, Maher Beji, Lilia Ben Fatma, Farhat Guetat, Azza Khedhiri, Meriam Khadhar, Arbi Besseghaier, Aouatef Chadi, Chiheb Doss, Abdelaziz Hamdene, Rym Ben Othman, Ines Kammoun, Saoussen Zaghden, Amir Jouida, Fouad Mazeh, Faten Benzarti, Melika Chihaoui, Houssem Thabet, Ali Ameur, Mohamed Moncef Mahfoudhi, Ibtissem Ben Nacef, Yousra Houidi, Haithem Ben Haj Salah, Houcem Elomma Mrabet, Manel Jemel, Mohamed Karim Zouaghi, Moahmed Abdallah, Alia Koubaa, Mellouki Salem, Habib Jerbi, Afifa Mannai, Hajer Kandara, Lotfi Omri, Najla Zran, Aida Bibi, Kmar Mnif, Rim Laroui, Abir Gana, Haifa Tounsi, Hiba Ghabi, Taieb Ach, Fatma Balegi, Najla Bchir, Lotfi Bouzrara, Janina Kooli, Emna El Feleh, Naourez Abid, Dorra Hsairi, Chadia Zouaoui, Emna Bornaz, Bilel Ben Amor, Mejda Bel hadj amor, Salem Bououmrani, Henda Jamoussi, Manel Neji, Haroun Ouertani, Wafa Fehri, Bechir Chetoui, Najla Dammak, Radhouen Gharbi, Amani Jouini, Jihen Ouali, Jihed Anoun, Ichraf Chokri, Ilhem Fessi, Meriem Marrakchi, Mounira Akkari, Naouel El Afrit, Fadia Boubaker, Slim Nigrou, Nahla Lahmar, Halima Chalghaf, Hèla Damak, Mouna Fradi, Riadh Ben Hassouna, Nada El’Aoud, Hejer Khouja, Raja Khecharem, Monia Smiti, Saoussen Antit, Dhouha Maaoui, Abdelhamid Ben Ahmed, Hamza Elfekih, Monia Bahri, Tayssir Ben Achour, Ines Bayar, Imen Ben Abdallah, Khadija Zitoun, Samir Gzizou, Sarra Tira, Zohra El Ati, Asma Ahmed, Zied Bettaieb, Rajaa Aoudia, Monia Ammar, Samia Barbouch, Mohamed Fadhel Rabeh, Mariem Saihi, Sonia Nafti, Saoussen Batti, Amel Ayed, Insaf Hadj Ali, Maha Ben Moallem Hachicha, Dorsaf Zellama, Ahmed Sahli, Monia Ajmi, Wassila Raouadi, Riadh Bouftira, Houda Feriani, Ikram Agrebi, Mohamed Belkhiria, Hamza Rjili, Rim Sfaxi, Sonia Grine, Afef Slimi, Dorra Ben Aich Jaffel, Meriam Hajji, Nadia Ben Abdelhafidh, Sonia Dhifallah, Sana Abid, Besma Marghli, Samir Chriti, Abdelkarim Derbel, Mohamed Habib Sfar, Hanen Ben Djemaa, Madiha Krid, Raja Boukadida, Farès Azaiez, Yosr Ghrairi, Hanen Chaker, Ines Ketata, Najoua Ben Atitallah, Yosra Hamzi, Adel Nahdi, Samia Akermi, Boutheina Ben Rhaiem, Yosra Selmi, Hela Ghezaiel, Dalel Touati, Amel Hmida, Imene Ben Hafsa, Zied Ibn El Hadj, Amel Barhoumi, Hichem Denguir, Imen Khaled, Soumaya Beji, Mohamed Mongi Bacha, Nour El Houda Driss, Mehdi Ben Miled, Taycir Alila, Wided Smaoui, Fatma Aouini, Mouna Dey, Soumaya Dridi, Sarra Mekni, Fatma Chaker, Kais Hajji, Salah Saied, Riadh Ben Othman, Asma Zammouri, Amal Hammami, Imen Aouachri, Lassaad Jeridi, Khawla Kamoun, Lilia Zakhama, Sana Sellami, Zaineb Ajra, Wafa Ben Saada, Adnen Neji, Amani Hamdouni, Asma Fradi, Hanene Sayadi, Hayet Kaaroud, Hend Kallel Bekri, Nabil Barghouthi, Sadok Anoun, Wafa Skouri, Sondes Dahnes, Mouna Titey, Raja Fsili, Emna Kharrat, Aycha Hachana, Ines Chaari, Neila Abid, Fatma Said, Thouraya Nabli, Ilhem Naily, Emna Kharrat, Hanene El Kateb, Imen Ben Chedly, Mohamed Dammak, Nizar Nasrallah, Haithem Rhouma, Sirine Khedhaouria, Siwar Amamou, Amina Jebali, Fatma Ben Fredj Ismail, Nawres Brahmi, Donia Chebbi, Mourad Bouricha, Hanene Machfer, Inès Rdhaounia, Mohamed Abdelhakim Korchid, Marwa Trabelsi, Rim Goucha, Abdelmajid Abid, Nouha Ben Mahmoud, Neila Taboubi Fathalli, Chadia Sidhom, Achraf Jaziri, Eya Cherif, Farah Laatiri, Fatma Ben Salem, Imen Rojbi, Sabeh Zouari, Sana Toujani, Lilia Affes, Bilel Arfaoui, Ammar Chebbi, Hanene Gaied, Nejiba Farhat, Hafedh Hedri, Imen Gorsane, Karima khiari, Hajer Rzig, Sameh Alibi, Nesrine Thabet, Abdessalem Alimi, Anissa Joulak, Khaoula Ben Abdelghani, Jamila Hassine, Mohamed Salah Hamdi, Ines Jaafar, Ines Naceur, Sawssen Ben Hamida, Sondes Ouerghi, Zohra Sassi Jallali.
Funding
This work was supported by the Tunisian Society of Nephrology Dialysis and Transplantation.
Author information
Authors and Affiliations
Contributions
JL: investigation, methodology, formal analysis, writing, reviewing; AH: investigation, methodology, writing, reviewing; BBK: investigation, reviewing; IM: investigation, reviewing; SA: investigation, reviewing; AA: investigation, reviewing; SC: investigation, reviewing; MHB: investigation, reviewing; MH: investigation, reviewing; SA: investigation, reviewing; ST: investigation, reviewing; OL: investigation, reviewing; HA: investigation, reviewing; YH: investigation, reviewing; SC: investigation, reviewing; FC: investigation, reviewing; LR: investigation, reviewing; HS: project redaction, supervision, editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The protocol complies with the International Conference on Harmonization of Good Clinical Practice’s Declaration of Helsinki. The study was approved by the Ethics committee “The local clinical research ethics committee of the Military Hospital of Tunis” under the number 27/2022/CLPP/Hôpital Militaire de Tunis. This study was registered in the clinical trials database under the number NCT05577650 (13/10/2022).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Labidi, J., Harzallah, A., Kaab, B.B. et al. Prevalence of chronic kidney disease in Tunisian diabetics: the TUN-CKDD survey. BMC Nephrol 25, 67 (2024). https://doi.org/10.1186/s12882-024-03501-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12882-024-03501-5