Abstract
Background
The induction of oxidative stress is one of the most important cancer etiologies. Plant essential oils contain many effective antioxidant compounds in improving oxidative stress. In the present study, the pharmacological potential of Trachyspermum ammi essential oil (TAEO) and Ferula assafoetida essential oil (FAEO) was compared in oxidative stress improvement and cytotoxic effect. TAEO and FAEO were prepared by Clevenger apparatus, and the medicinal compounds in the essential oils were evaluated by GC–MS assay. The TAEO and FAEO were also evaluated as to their phenolic and flavonoid content, monovalent reducing power, and total radical scavenging activity, respectively, by Folin–Ciocalteu, aluminum chloride, FRAP, and DPPH methods. The cytotoxic effect of the TAEO and FAEO was evaluated by MTT assay on MCF-7 (ER+) and MDA-MB-468 (ER−) breast cancer cell lines.
Results
The GC–MS analysis indicated that thymol and (E)-Sec-Butyl propenyl disulfide, respectively, were the highest components of TAEO and FAEO. The phenolic content (P < 0.0001), flavonoid content (P < 0.0001), reducing power (P < 0.0001), radical scavenging activity (P < 0.0001), and cytotoxic effect (P < 0.05) of TAEO were significantly higher than FAEO. The IC50 value of the cytotoxic effect of TAEO on MCF-7 and MDA-MB-468 after 72 h of incubation was, respectively, 192.5 ± 42.57 and 331.4 ± 7.24 ppm.
Conclusion
The cytotoxic effect of TAEO was more potent on the MCF-7 cell line, probably in an estrogen-dependent manner of cellular growth inhibition. It appears that TAEO has a high capacity for improving oxidative stress and inhibiting cell proliferation in breast cancer.
Similar content being viewed by others
1 Background
Breast cancer is a community problem and the most common non-skin cancer in the USA. About 40,000 people die each year in the USA and 400,000 worldwide from breast cancer. Breast cancer is a chronic disease caused by the uncontrolled growth of malignant epithelial cells in breast tissue. Induction of oxidative stress is one of the most important causes of cancer. Normal cells in exposure to oxidative stress undergo physiological changes such as growth signal production, inhibition of inhibitory signals, and angiogenesis, which lead to breast cancer [1]. Abortion, smoking, overweight, stress, and anxiety are the most important factors for the induction of oxidative stress. Breast cancer is a heterogeneous tumor with extensive clinical manifestations. In some cases, this tumor lacks estrogen receptor, progesterone receptor, and epidermal growth factor receptor HER2 that is called a triple-negative tumor. These tumors are very resistant to common treatments and do not have a good prognosis. Currently, the usual treatment for breast cancer is surgery, chemotherapy, radiotherapy, or hormone therapy. In addition to being costly, the treatments have such severe side effects as therapeutic resistance and damage to healthy organs [1]. Therefore, more research should be conducted on drug discovery with higher efficiency and fewer side effects.
Medicinal plants are rich sources of secondary metabolites and probably are efficient in cancer treatment or prevention. The pharmacological properties of the plants are attributed to their secondary metabolites. Phenolic and flavonoid compounds are secondary metabolites with high antioxidant effects. It has increased the use of antioxidant plants in the formulation of anticancer drugs. The secondary metabolites with high antioxidant activity improve oxidative stress more efficiently and probably are effective in cancer prevention. Recent studies have shown a direct relationship between the phenolic content and their antioxidant and cytotoxic effects. [2].
Trachyspermum ammi and Ferula assafoetida are the most widely used medicinal plants in traditional Iranian medicine, which are commonly known as natural antioxidants. Trachyspermum ammi is an herbaceous and aromatic plant and contains phenolic and flavonoid compounds such as thymol, carvacrol, and linear and annular monoterpenes. Previous studies show that thymol is the major chemical component [3]. Ferula assafoetida is also a perennial herbaceous plant of the Apiaceae family. Pharmacological and biological studies have shown its antioxidant and cytotoxic effects [4]. The purpose of the present study was to compare the pharmacological potential of TAEO and FAEO in improving oxidative stress and anticancer effects. Therefore, the essential oils were compared in the phenolic and flavonoid content, reducing activity, radical scavenging activity, and cytotoxic effect. The cytotoxic effects of the essential oils are evaluated on MCF-7 and MDA-MB-468 breast cancer cell lines.
2 Methods
2.1 Essential oils extraction
The Trachyspermum ammi seeds and Ferula assafoetida gum were bought from the herbal store of Fasa (Fars province, Iran), and the genus and species of the plants were confirmed by the experts of Fasa Medicinal Plants Research Center (FMPRC). Trachyspermum ammi seeds and Ferula assafoetida gum were kept in the herbarium of FMPRC, and voucher numbers of FMPRC-100-29 and FMPRC-100-19 were assigned to them, respectively. This study was approved by the Medical Ethics Committee of Fasa University of Medical Sciences (Code: IR.FUMS.REC.1396.306). Essential oils were extracted from the seeds of Trachyspermum ammi and Ferula assafoetida gum by Clevenger apparatus [5].
2.2 Measurement of phenolic and flavonoid content
The phenolic content of TAEO and FAEO was evaluated by Folin–Ciocalteu assay according to the previous study [6]. Gallic acid was used as standard, and phenolic content of the essential oils was reported in microgram gallic acid equivalent (GAE) per milligram (µgGAE/mg). For the evaluation of flavonoid content of TAEO and FAEO, the aluminum chloride method was also used according to the previous study [7]. Quercetin was used as standard, and the flavonoid content of the essential oils was reported in microgram quercetin equivalent (QE) per milligram (µgQE/mg). All measurements were done three times in duplicate.
2.3 The gas chromatography–mass spectrometry analysis (GC–MS)
The GC–MS analysis of FAEO and TAEO essential oils was performed using a TRACE MS (Thermo Quest-Finnigan, USA) equipped with a DB-5 MS fused silica capillary column (30 m*0.25 mm*0.25 µm).
2.4 Monovalent reducing power
The ferric reducing antioxidant power (FRAP) assay was used for the evaluation of monovalent reducing power of TAEO and FAEO according to the previous study [8]. FeSO4 serial dilutions were also used as the standard, and the antioxidant activity of the essential oils was reported in μmolFe2+/g. All measurements were repeated three times in duplicate.
2.5 Total radical scavenging activity
The 2,2-diphenyl-1-picrylhydrazyl (DPPH) clearance assay was used for the evaluation of total radical scavenging capacity of TAEO and FAEO according to the previous study in the different concentrations of 100, 500, 1000, 2000, 3000, 4000, and 5000 ppm [7].
2.6 Cell culture
Two invasive human breast ductal carcinoma cell lines, MDA-MB-468 (triple-negative) and MCF-7, were purchased from the national cell bank of Iranian Pasteur Institute. The cell lines were cultured in DMEM and seeded in the 96-well plate for evaluation of cytotoxic effects (10,000 cells per well) and morphological changes [7].
2.7 Cytotoxic effects of TAEO and FAEO and morphological changes
MTT colorimetric assay was used to evaluate the cytotoxic effects of TAEO and FAEO according to previous study [7]. The cultured 96-well plates were treated with different concentrations of 100, 200, and 400 ppm of the essential oils based on the anticancer compounds classification criteria [9, 10]. The cytotoxic effects of essential oils were calculated by the reduction rate in cell viability of the cancer cell line. Finally, IC50 values of the cytotoxic effects of the essential oils were calculated by linear regression. The morphological changes of treated cells were also evaluated by an inverted microscope after 24, 48, and 72 h of incubation.
2.8 Statistical analysis
Statistical analysis of data was performed using a t test in GraphPad Prism 8.0.2 software. The significance level was considered less than 0.05 (P < 0.05), and the data were expressed as Mean ± SD.
3 Results
3.1 Phenolic and flavonoid content
The phenolic content of TAEO was 429.63 ± 25.52 µgGAE/mg, which was significantly (P < 0.0001) higher than FAEO (203.75 ± 26.34 µgGAE/mg). The flavonoid content of TAEO (467.17 ± 10.75 µg EQ/ mg) was also 5.49 times more than FAEO (85.08 ± 21.87 µg EQ/mg) and was statistically significant (P < 0.0001) (Table 1).
3.2 GC–MS analysis of the essential oils
According to Table 2, GC–MS analysis of FAEO revealed the identification of five components that were reported by retention time and area percentage. The highest components of FAEO were (E)-Sec-Butyl propenyl disulfide, IR-λ-Pinene, and (E)-Sec-Butyl propenyl disulfide, respectively, by 46.43%, 27.30%, and 17.83% peak area. Polysulfides were the major component of FAEO. GC–MS analysis of TAEO also indicated the presence of thymol and carvacrol. Thymol was the major component and made up 99.65% of the TAEO composition.
3.3 Monovalent reducing power
According to Table 1, the monovalent reducing power of TAEO was 374.40 ± 41.48 μmolFe2+/g, which was significantly (P < 0.0001) higher than FAEO (56.25 ± 3.25 μmolFe2+/g).
3.4 Total radical scavenging activity
The highest radical scavenging activity was related to ascorbic acid (IC50 = 30.99 µg/mL), TAEO (IC50 = 4934 ppm), and FAEO (IC50 > 5000 ppm), respectively. According to Table 3, the radical scavenging activity of TAEO was significantly higher than FAEO at concentrations of more than 500 ppm (P < 0.0001). The highest total radical scavenging activity of TAEO was observed at the concentration of 4000 ppm by 47.71 ± 2.19%. There was a direct relationship between the concentration of essential oils and their scavenging activity.
3.5 Cytotoxic effects of essential oils
According to Table 4, the cytotoxic effect of TAEO (400 ppm) on the MCF-7 cell line was 92.81 ± 0.52% after 24 h of incubation. The cytotoxic effect was significantly (P < 0.0001) higher than FAEO (28.69 ± 5.56). After 48 h of incubation, the cytotoxic effect of TAEO at the concentration of 100 ppm on MCF-7 was equal to 10.43 ± 2.91%, which was significantly (P = 0.008) less than FAEO (18.02 ± 1.14). At the concentrations of 200 and 400 ppm of TAEO, the cytotoxic effects were 49.50 ± 8.42% and 94.79 ± 0.70%, respectively. The cytotoxic effects were significantly higher than FAEO (respectively, 29.01 ± 3.32% (P = 0.03) and 40.05 ± 3.47% (P < 0.0001)). After 72 h of incubation, the cytotoxic effects of TAEO at the concentrations of 200 and 400 ppm were also equal to 68.43 ± 4.85% and 97.67 ± 0.57%, respectively. The cytotoxic effects were significantly higher than FAEO (respectively, 28.67 ± 1.88% (P = 0.008) and 44.16 ± 0.08% (P < 0.0001)).
The treatment of MDA-MB-468 cells with TAEO at the concentration of 100 ppm brought the cytotoxic effects of 0.42 ± 1.01%, 4.39 ± 5.86%, and 4.88 ± 0.17% after 24, 48, and 72 h of incubation, respectively. The cytotoxic effects were significantly lower than FAEO (respectively, as 9.06 ± 5.94% (P = 0.03), 14.68 ± 5.84% (P = 0.02), and 21.21 ± 6.90% (P = 0.01)). But at the concentration of 400 ppm, the cytotoxic effects of TAEO were 58.48 ± 3.31%, 58.52 ± 11.72%, and 65.95 ± 6.87% after 24, 48, and 72 h of incubation. The cytotoxic effects were significantly higher than FAEO (significantly as 19.07 ± 5.95% (P < 0.0001), 26.46 ± 6.87% (P < 0.0001), and 26.66 ± 6.08% (P < 0.0001)).
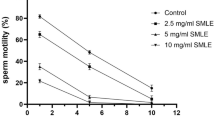
According to Table 3, the cytotoxic effects of the essential oils on the MCF-7 cell line were higher than the MDA-MB-468 cell line. The IC50 values of cytotoxic effects of TAEO on MCF-7 cell line were 228.6 ± 21.53, 209.80 ± 20, and 192.5 ± 42.57 ppm, respectively, after 24, 48, and 72 h of incubation. The IC50 values of cytotoxic effects of FAEO were also more than 400 ppm in all incubation times. About the MDA-MB-468 cell line, the IC50 values of cytotoxic effects of TAEO were 336.6 ± 20.84, 334.1 ± 36.96, and 331.4 ± 7.24 ppm, respectively, after 24, 48, and 72 h of incubation. The IC50 values of cytotoxic effects of FAEO were also more than 400 ppm in all incubation times (Fig. 1).
3.6 Morphological changes of treated cells
The morphological changes of MCF-7 and triple-negative MDA-MB-468 cell lines changed in a dose- and time-dependent manner in exposure to TAEO and FAEO. As shown in Fig. 2, MCF-7 and MDA-MB-468 cells in exposure to the essential oils were ruptured. The cell debris and cell granules were also increased significantly. TAEO caused higher morphological changes compared to FAEO. Compared to the MDA-MB-468 cell line, the morphological changes of the MCF-7 cell line were also higher. The highest amount of cell granule, cell contraction, and rupture was observed in the group of MCF-7 cells treated with TAEO (400 ppm) after 72 h of incubation (Fig. 2-20).
4 Discussion
To find a medicinal plant with the properties of oxidative stress improvement and cytotoxic effects, the pharmacologic potential of TAEO and FAEO was compared. TAEO had a high phenolic and flavonoid content showing relatively high antioxidant activity in FRAP and DPPH assays and probably having a good therapeutic effect in improving oxidative stress. Therefore, TAEO had a relatively high cytotoxic effect on MCF-7 and MDA-MB-468 cell lines. However, FAEO had relatively weak cytotoxic effects on the breast cancer cell lines. FAEO had low phenolic and flavonoid content and antioxidant effects.
The cytotoxic effects of TAEO had a concentration and time-dependent pattern. After 24 h of incubation, its cytotoxic effects on MCF-7 and MDA-MB-468 breast cancer cell lines, respectively, were 15% and 0.42% on the concentration of 100 ppm. The cytotoxic effects intensified with an increase in TAEO concentration and incubation time. Therefore, the cytotoxic effects after 72 h of incubation reached 97.67 and 65.95% on the concentration of 400 ppm, respectively, in the MCF-7 and MDA-MB-468 breast cancer cell lines.
The cytotoxic effects of traditional medicinal products are divided into four categories according to the IC50 value. Medicinal products with the IC50 values of 0–20, 20–100, 100–1000, and > 1000 ppm are classified, respectively, as very active, relatively active, weakly active, and inactive cytotoxic compounds [9]. The IC50 values of cytotoxic effects of TAEO on MCF-7 cell line were 228.6, 209.8, and 192.5 ppm, respectively, after 24, 48, and 72 h of incubation, respectively. On MDA-MB-468, the IC50 values were also 336.6, 334.1, and 331.4 ppm, respectively. According to the classification criteria of the anticancer compounds, TAEO is considered a “weakly active” compound. The IC50 values of cytotoxic effects of FAEO on MCF-7 and MDA-MB-468 cell lines were > 400 ppm. FAEO probably is an inactive anticancer compound.
MCF-7 cell line has the estrogen receptor (ER-positive) unlike the MDA-MB-468 cell line, which lacks the estrogen receptor (ER negative). The cytotoxic effects of TAEO and FAEO on MCF-7 cells were higher than MDA-MB-468 cells. Therefore, it appears that the cytotoxic effects of TAEO and FAEO are ER-dependent. The results of morphological changes were also approved for the cytotoxic effects. The morphological changes of the MCF-7 cell line in treatment with TAEO and FAEO were also much greater than MDA-MB-468. The changes intensified with increasing concentration and time incubation. The results showed that most changes in cell morphology were observed in TAEO-treated cells.
Previous studies have also shown the high phenolic and flavonoid content of TAEO and high antioxidant effects in DPPH radical scavenging activity and Fe3+ reducing activity [11, 12]. Akhlaghi et al. reported the high phenolic content of methanolic extract of Trachyspermum ammi. The phenolic content of the extract was 164.5 ± 1.3 mgGAE/g which was lower that the phenolic content of TAEO in our study [13]. Chatterjee et al. also reported that polyphenols make up about 45% of the dry weight of the methanolic and aqueous extracts of Trachyspermum ammi. The phenolic content reported in the Chatterjee et al. study was almost the same as the present study [14]. The results of GC–MS analysis also confirm the high phenolic content of TAEO in the present study. Vitali et al. showed that TAEO has high phenolic content, flavonoid content, and antioxidant effects. TAEO was also shown to have considerable cytotoxic effects on the MDA-MB-231 triple-negative breast cancer cell line. The reported cytotoxic effects probably were due to the phenolic compounds such as thymol and carvacrol [15]. In the study of Mohammadpour et al., the cytotoxic effect of Trachyspermum copticum essential oil toward the MDA-MB-231 cell lines with the IC50 value of 236.16 µg/mL was shown. So, Trachyspermum copticum essential oil was considered a weakly active anticancer compound, similar to TAEO in the present study. Mohammadpour et al. also showed that the essential oil of Trachyspermum copticum has a lower cytotoxic effect in the normal HEK cell lines compared to the cancerous cells [16]. But the cytotoxic effects of the essential oils were not evaluated on the non-malignant breast epithelial cells in the present study. The present study focuses on the pattern of cytotoxic effects on different breast cancer cell lines.
In addition to breast cancer, numerous studies have also represented the cytotoxic effects of Trachyspermum ammi on multiple cancer cell lines. In the study of Abdel-Hameed et al., the anticancer activity of Trachyspermum ammi was shown on the hepatocellular carcinoma cell line (HepG2). The IC50 values of essential oil and n-hexane extract of Trachyspermum ammi, respectively, were 9.57 ± 0.98 and 17.42 ± 1.44 µg/mL. The higher activity of essential oil is probably related to its high contents of volatile compounds. According to Abdel-Hameed study, the TAEO is considered a very active anticancer compound on the HepG2 cell line, unlike the present study [17]. However, there is also a possibility that geographical conditions influenced the pharmacological content of the essential oils. In the study of Aruchamy et al., the cytotoxic effect of the methanol seed extract of Trachyspermum ammi on the KB cell line (an epidermal carcinoma of the mouth) with the IC50 value of 125 µg/mL was shown. This study reveals that the Trachyspermum ammi exhibited significant cytotoxic and apoptotic inducing effects through promoting reactive oxygen species (ROS) [18]. In the study of Singh et al., the chemopreventive potential of Trachyspermum ammi seeds against carcinogenesis was shown. They showed that Trachyspermum ammi has a therapeutic effect on murine skin and forestomach papilloma genesis. In the treated animals, the content of reduced glutathione was elevated. The peroxidative damage and lactate dehydrogenase activity was also reduced [19]. Therefore, it appears that TAEO can influence on cancer cell microenvironment by increasing antioxidant compounds, reducing oxidants, and improving oxidative stress. TAEO probably increases the expression of proapoptotic genes Bim, Bak, or Bax, arrests the cancer cell cycle, and inhibits cancer by producing ROS inflammatory mediators [20, 21].
Numerous studies have revealed the cytotoxic effects of thymol as the major component of TAEO. In the study of Deb et al., the dose-dependent cytotoxic effects of thymol on acute promyelocytic leukemia (HL-60) cells were demonstrated after 24 h of exposure. However, thymol did not show any cytotoxic effect in normal human PBMC. The cytotoxic effect of thymol on HL-60 cells appears to be associated with induction of cell cycle arrest at the sub G0/G1 phase [22]. According to the literature review, it appears that thymol has a dual effect on cell viability. Thymol can induce oxidative stress-linked mitochondrial dysfunction and other intrinsic and extrinsic apoptotic cell death on cancer cells. Conversely, in the normal cells, it appears that thymol has antioxidant and cytoprotective effects [23]. In the study of Zeng et al., the in vitro and in vivo anticancer effects of thymol were demonstrated. Thymol treatment in vitro induced apoptosis and cell cycle arrest in colorectal cancer and in vivo led to cell apoptosis and a significant decrease in tumor volume through the BAX/Bcl-2 signaling pathway [24]. In the study of Seresht et al., the cytotoxic effect of thymol as the major component of TAEO was shown on the MCF-7 cell line. They represented the IC50 value of thymol on the MCF-7 cell line as 54 and 62 µg/mL, respectively, after 48 and 72 h of incubation. Furthermore, they show the apoptosis-inducing effect of thymol on the MCF-7 cell line via upregulation of P53 and P21 gene expressions [25].
About the FAEO cytotoxic effects, previous studies indicated that the ethanolic or water extract of Ferula assafoetida gum has high cytotoxic effects on the cancerous cell line. Sadooghi et al. showed that ethanolic extract of Ferula assafoetida induced morphological changes and decrease in viability of HepG2 cells with minimal cytotoxic effect on normal cells [26]. Bagheri et al. also indicated that water extract of Ferula assafoetida decreases the tumor weight and volume in an animal model of breast cancer [27]. Saleem et al. showed that pre-treatment of animals with acetone extract of Ferula assafoetida suppresses the early events of carcinogenesis [28]. It appears that the hydroethanolic extracts of Ferula assafoetida are more effective than its essential oil on the cancerous cell lines. Verma et al. have also shown that the FAEO will represent the antiproliferative activity if the essential oil has the high content of dithiolane (87.4%) [29].
5 Conclusion
The cytotoxic effects of TAEO were more than FAEO, which is probably due to higher phenolic and flavonoid contents, radical scavenging activity, and reducing power. Thymol and polysulfides, respectively, were the major components of TAEO and FAEO. The cytotoxic effects of TAEO probably are dependent on the estrogen receptor. It appears that TAEO compared to FAEO has a high capacity in improving oxidative stress and inhibiting cell proliferation. TAEO was a more appropriate drug candidate for fractionation and further research on breast cancer.
Availability of data and materials
All data are given in the current report.
Abbreviations
- TAEO:
-
Trachyspermum ammi essential oil
- FAEO:
-
Ferula assafoetida essential oil
- GAE:
-
Gallic acid equivalent
- QE:
-
Quercetin equivalent
- FRAP:
-
Ferric reducing antioxidant power
- DPPH:
-
2,2-Diphenyl-1-picrylhydrazyl
References
Keren L, Bosse M, Marquez D, Angoshtari R, Jain S, Varma S et al (2018) A structured tumor-immune microenvironment in triple negative breast cancer revealed by multiplexed ion beam imaging. Cell 174:1373–1387
Aryal S, Baniya MK, Danekhu K, Kunwar P, Gurung R, Koirala N (2019) Total phenolic content, flavonoid content and antioxidant potential of wild vegetables from Western Nepal. Plants 8:96
Dhaiwal K, Chahal KK, Kataria D, Kumar A (2017) Gas chromatography-mass spectrometry analysis and in vitro antioxidant potential of ajwain seed (Trachyspermum ammi L.) essential oil and its extracts. J Food Biochem 41:e12364
Deveci E, Tel-Çayan G, Duru ME (2018) Phenolic profile, antioxidant, anticholinesterase, and anti-tyrosinase activities of the various extracts of Ferula elaeochytris and Sideritis stricta. Int J Food Prop 21:771–783
Vongsak B, Sithisarn P, Mangmool S, Thongpraditchote S, Wongkrajang Y, Gritsanapan W (2013) Maximizing total phenolics, total flavonoids contents and antioxidant activity of Moringa oleifera leaf extract by the appropriate extraction method. Ind Crops Prod 44:566–571
Moulazadeh A, Ranjbar R, Ardestani AD, Hekmat M, Azarnia S, Najafipour S (2021) Antioxidant activity, phenolic and flavonoid content of Lawsonia inermis and Haplophyllum vermiculare. Physiol Pharmacol 25:261–269
Moulazadeh A, Ranjbar R, Hekmat M, Sedaghat F, Yousefzadi M, Najafipour S (2021) Comparison the cytotoxic effects of Ulva fasciata and Ulva lactuca on the MCF-7 and MDA-MB-231 breast cancer cell lines. Physiol Pharmacol 25:373–383
Moulazadeh A, Ranjbar R, DakhiliArdestani A, Keshavarzi A, Karimzadeh F, Rahnavard M et al (2021) Evaluation of phenolic content, antioxidant activity and cytotoxic effects of Ulva lactuca and Hypnea musiformis marine algae on MDA-MB-468 cell line. J Fasa Univ Med Sci 11:3921–3928
Baharum Z, Akim AM, Taufiq-Yap YH, Hamid RA, Kasran R (2014) In vitro antioxidant and antiproliferative activities of methanolic plant part extracts of Theobroma cacao. Molecules 19:18317–18331
Moulazadeh A, Kouhpayeh SA (2020) Suitable concentration of anti-inflammatory herbal extracts in cell culture. J Fasa Univ Med Sci 10:2396–2399
Korani M, Jamshidi M (2020) The effect of aqueous extract of trachyspermum ammi seeds and ibuprofen on inflammatory gene expression in the cartilage tissue of rats with collagen-induced arthritis. J Inflamm Res 13:133
Saraswat N, Sachan N, Chandra P (2020) A review on ethnobotanical, phytochemical, pharmacological and traditional aspects of indigenous Indian herb Trachyspermum ammi (L). Curr Tradit Med 6:172–187
Akhlaghi H, Mahdavi B, Rezaei H (2014) Characterization of chemical composition and antioxidant properties of Trachyspermum ammi seed as a potential medicinal plant. J Chem Health Risks 4:9–16
Chatterjee S, Goswami N, Bhatnagar P (2012) Estimation of Phenolic Components and in vitro Antioxidant Activity of Fennel (Foeniculum vulgare) and Ajwain (Trachyspermum ammi) seeds. Adv Biores 3:109–118
Vitali LA, Beghelli D, Nya PCB, Bistoni O, Cappellacci L, Damiano S et al (2016) Diverse biological effects of the essential oil from Iranian Trachyspermum ammi. Arab J Chem 9:775–786
Mohammadpour G, Tahmasbpour R, Rahmani A, Esfahani AA (2018) Chemical compounds, in vitro antitumor and antibacterial activities of Trachyspermum copticum L essential oil. Iran J Pharmacol Ther 16:1–6
Abdel-Hameed E-SS, Bazaid SA, Al Zahrani O, El-Halmouch Y, El-Sayed MM, El-Wakil E (2014) Chemical composition of volatile components, antimicrobial and anticancer activity of n-hexane extract and essential oil from Trachyspermum ammi L. seeds. Orient J Chem 30:1653–1662
Aruchamy M, Duraisamy P (2021) In vitro antioxidant and anticancer activity of methanolic extract of Trachyspermumammiseed on human oral cancer (KB) cell line. J Univ Shanghai Sci Technol 23:426–436
Singh B, Kale RK (2009) Chemomodulatory effect of Trachyspermum ammi on murine skin and forestomach papillomagenesis. Nutr Cancer 62:74–84
Bouchmaa N, Mrid RB, Kabach I, Zouaoui Z, Karrouchi K, Chtibi H et al (2022) Beta vulgaris subsp. maritima: a valuable food with high added health benefits. Appl Sci 12:1–16
Mrid RB, Bouchmaa N, Kabach I, Zouaoui Z, Chtibi H, Maadoudi ME et al (2022) Dittrichia viscosa L. leaves: a valuable source of bioactive compounds with multiple pharmacological effects. Molecules 27:1–16
Deb DD, Parimala G, Devi SS, Chakraborty T (2011) Effect of thymol on peripheral blood mononuclear cell PBMC and acute promyelotic cancer cell line HL-60. Chem Biol Interact 193:97–106
Islam MT, Khalipha ABR, Bagchi R, Mondal M, Smrity SZ, Uddin SJ et al (2019) Anticancer activity of thymol: a literature-based review and docking study with emphasis on its anticancer mechanisms. IUBMB Life 71:9–19
Zeng Q, Che Y, Zhang Y, Chen M, Guo Q, Zhang W (2020) Thymol Isolated from Thymus vulgaris L. inhibits colorectal cancer cell growth and metastasis by suppressing the Wnt/β-catenin pathway. Drug Des Dev Ther 14:2535
Seresht HR, Albadry BJ, Al-mosawi AKM, Gholami O, Cheshomi H (2019) The cytotoxic effects of thymol as the major component of Trachyspermum ammi on breast cancer (MCF-7) cells. Pharm Chem J 53:101–107
Sadooghi SD, NezhadShahrokhAbadi K, Zafar BS (2013) Investigating the cytotoxic effects of ethanolic extract of Ferula assa-foetida resin on HepG2 cell line. KAUMS J (FEYZ) 17:323–330
Bagheri SM, Abdian-Asl A, Moghadam MT, Yadegari M, Mirjalili A, Zare-Mohazabieh F et al (2017) Antitumor effect of Ferula assa foetida oleo gum resin against breast cancer induced by 4T1 cells in BALB/c mice. J Ayurveda Integr Med 8:152–158
Saleem M, Alam A, Sultana S (2001) Asafoetida inhibits early events of carcinogenesis: a chemopreventive study. Life Sci 68:1913–1921
Verma S, Khambhala P, Joshi S, Kothari V, Patel T, Seshadri S (2019) Evaluating the role of dithiolane rich fraction of Ferula asafoetida (apiaceae) for its antiproliferative and apoptotic properties: in vitro studies. Exp Oncol 4141:90–94
Acknowledgements
The authors are grateful for the cooperation and assistance of the Noncommunicable Diseases Research Center of Fasa University of Medical Sciences and special thanks to the ladies, Mahboubeh Bordbar and Soroush Dadvari.
Funding
Fasa University of Medical Sciences supported this study, Grant No. 95143.
Author information
Authors and Affiliations
Contributions
AM designed the study, performed the MTT tests, and analyzed data. FY wrote the introduction. RR performed the MTT tests and phytochemical assays. ADA performed the phytochemical assays. KR collaborated in the extraction of plants and phytochemical assays. AF designed the study. AK designed the study. AA designed the study. SN designed the study. All authors contributed to the drafting of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Medical Ethics Committee of Fasa University of Medical Sciences (Code: IR.FUMS.REC.1396.306).
Consent for publication
Not applicable.
Competing interests
Researchers have no conflict of interest in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Moulazadeh, A., Ranjbar, R., Dakhili Ardestani, A. et al. Cytotoxic effects of Trachyspermum ammi and Ferula assafoetida on MCF-7 and MDA-MB-468 breast cancer cell lines. Beni-Suef Univ J Basic Appl Sci 11, 147 (2022). https://doi.org/10.1186/s43088-022-00322-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43088-022-00322-z