Abstract
Background
Natural products are utilized globally for the management of diseases such as diabetes mellitus. Carrot seeds are ethnobotanically used in the management of diabetes mellitus. This study investigated the in vitro and in vivo antihyperglycemic activities of verbenone and L-arginine, which are compounds found in carrot seed.
Results
Verbenone, L-arginine, and their ratios combination expressed significantly in vitro α-amylase inhibitory activity with IC50 of 13.00, 12.69, and 13.30 µg/mL respectively compared with acarbose (IC50 = 12.64 µg/mL) and significant in vitro α-glucosidases inhibitory activity with IC50 of 29.01, 23.76 and 30.11 µg/mL respectively compared with acarbose (IC50 = 16.80 µg/mL). Furthermore, the compounds improved glucose uptake in yeast cells and inhibited haemoglobin glycation in vitro. In the in vivo study, the compounds significantly reduced the levels of blood glucose, the levels of liver total cholesterol, and liver triacylglycerol, the calculated cardiovascular indices, whereas the level of plasma high density lipoprotein (HDL) cholesterol was significantly elevated in all diabetic treated mice.
Conclusion
The data revealed the in vitro antidiabetic activity of verbenone and L-arginine. Also, the compounds ameliorated hyperglycemia, hyperlipidemia and other diabetic-induced biochemical alterations in NAD-STZ-induced diabetic mice model.
Highlights
-
Verbenone, a natural bicyclic ketone monoterpene, and L-arginine, a natural amino acid, are both found in Daucus carota and other medicinal plants useful in the management of diabetes mellitus.
-
Verbenone and L-arginine possess in vitro antidiabetic activities, improve glucose uptake, and inhibited haemoglobin glycation.
-
Verbenone possesses in vivo antidiabetic activities in NAD-STZ-induced diabetic mice with potential synergetic effect with L-arginine.
-
Verbenone and L-arginine possess antihyperlipidemic properties and could prevent the risk of cardiovascular diseases in NAD-STZ-treated mice.
Similar content being viewed by others
1 Background
Diabetes mellitus (DM) is a chronic metabolic disorder that causes hyperglycemia due to inherited and/or acquired deficient production of insulin by the pancreatic β-cells (type 1 diabetes), or ineffectiveness of the insulin produced due to the gradual development of resistance and/or pancreatic β-cells dysfunction (type 2 diabetes) [1]. Conventionally, diabetes is managed through exogenous insulin administration, lifestyle/diet modification as well as the use of drugs that increase endogenous insulin availability such as sulfonylurea (glibenclamide), or enhance the sensitivity of insulin-like biguanide (metformin), or drugs that inhibit carbohydrate digestion and absorption like acarbose [1,2,3]. However, attainment of controlled blood sugar levels and the alleviation of other hyperglycemia-induced alterations continued to be a challenge among diabetics, despite the availability of numerous hypoglycemic drugs. High cost, limiting drug availability/affordability, limited tolerability of patients, and/or the significant mechanism-based side effects are but a few reasons hindering attainment of sugar control [2, 4].
Therefore, researchers are focusing on the utilization of plant products containing constituents like nitrogen compounds, terpenes, phenolic compounds, and hydroxylated compounds as alternative bioactive compounds to be used in developing drugs that exert effective hypoglycemic effects. These products are also demonstrating high potential in achieving global health status, especially in emerging economies like Nigeria due to their availability, affordability, relative safety compared to synthetic agents, and profound therapeutic benefits [2, 5,6,7,8].
Daucus carota (carrot), a monocarpic perennial herb demonstrates several pharmacological and biological properties including antidiabetic activity [9,10,11,12,13,14,15,16,17,18,19]. Studies on the activities of D. carota focused mainly on its various parts, its essential oil, or the major components; carotol, daucene, and daucol [5, 13, 20]. There is a paucity of knowledge regarding the antidiabetic activity of verbenone and L-arginine, which are bioactive molecules found in the seed extracts.
Verbenone (trimethyl-bicyclo-heptenone, Fig. 1a) is a natural bicyclic ketone mono-terpene found in D. carota and other medicinal plants such as Rosmarinus officinalis (Rosemary). It can also be synthesized through the oxidation of α-pinene from turpentine oil [21]. It is a colorless, minty liquid that is only soluble in non-polar solvents and used as an anti-aggregation pheromone to control insects, as a cough suppressant, in aromatherapy because of its pleasant aroma. Other studies have reported its anticancer, anti-inflammatory, hemolytic, bronchodialating, antiviral, and antioxidant activities [22, 23].
L-arginine (2-amino-5-guanodino-pentenoic acid, Fig. 1b) is an amino acid obtained from natural dietary protein sources such as carrot, broccoli, banana, watermelon, fish, soy milk, honey or through laboratory fermentation using Corynebacterium or Escherichia coli [24]. It is a white odorless powder soluble in water, insoluble in ether, and slightly soluble in ethanol [25]. it slows down the progression of diabetic nephropathy and renal failure by increasing glomerular filtration rate, and possess direct and nitric oxide (NO)-dependent antioxidant properties that is used for the treatment of intralipid (IL)- induced non-alcoholic steatohepatitis (NASH) [24, 26, 27]. However, studies on the antidiabetic activity of verbenone and L-arginine in Nicotinamide (NAD)-Streptozotocin (STZ)-treated mice is scarce. Therefore, the present study aim at investigating the in vitro and in vivo antihyperglycemic activities of verbenone and L-arginine in NAD- STZ-induced diabetic albino mice.
2 Methods
2.1 Chemicals and reagents
Verbenone, L-arginine, streptozotocin (STZ), nicotinamide (NAD), and Dimethyl sulfoxide (DMSO) were purchased from Carbosynth Limited, Axis House High Street, Compton, Newbury, Bekshire, London, UK, while the reagents for estimation of biochemical parameters were purchased from Randox Laboratories, Ardmore, Co. Antrim, UK. Glucometer (“Accu-Chek Active” test meter) was used to estimate blood glucose levels. Other chemicals and reagents were of analytical grade.
2.2 Experimental animals
Wister albino mice were obtained from the animal house of Department of Pharmacology, Bauchi State University, Gadau, Bauchi State, Nigeria. The mice were kept and maintained under standard laboratory conditions. They were fed on a standard laboratory diet and allowed access to water ad libitum. The University of Jos Ethical Review Committee approved the animal care and research procedures with the registration number UJ/FPS/F17-00379.
2.3 In vitro antidiabetic assay
α-Amylase and α-glucosidase inhibition assay were carried out using the methods described by Andrade-Cetto et al. [28] and Kuppusamy et al. [29] respectively at the concentrations of 20–100 µg/mL using acarbose as reference drug.
2.3.1 % increase in glucose uptake by yeast cells
Commercial baker’s yeast was dissolved in distilled water and subjected to repeated centrifugation (3000×g, for 5 min) until clear supernatant fluids were obtained and a 10% (v/v) of the suspension was prepared in distilled water. Various concentrations of the samples (20–100 μg/ml) were added to 1 ml of glucose solution (25 mM) and incubated for 10 min at 37 °C. The reaction was initiated by adding 100 μL of the prepared yeast suspension followed by vortexing and further incubation at 37 °C for 60 min. These were then centrifuged at 2500×g for 5 min and amount of glucose estimated in the supernatant using glucose oxidase method [30]. Metronidazole was used as reference drug for this assay.
2.3.2 Inhibition of haemoglobin glycosylation assay
To 1 mL of haemoglobin solution, 25 μL of gentamicin and 25 μL of the samples were pipetted into test tubes separately. The reaction was initiated by adding 1 mL of 2% glucose in 0.01 M phosphate buffer (pH 7.4) and incubated in dark at room temperature. The concentrations of glycosylated haemoglobin at the incubation period of 0, 24, and 72 h were estimated spectrophotometrically at 443 nm [31].
2.4 In vivo antidiabetic assay
2.4.1 Acute toxicity study
The acute toxicity test of verbenone was done according to Lorke’s method [32]. In the first phase, nine (9) randomly selected mice were grouped into group 1, 2 and 3. They were administered with 10, 100, and 1000 mg/kg bwt of verbenone, and placed under observation for 24 h, for behavior as well as mortality. In the second phase of the toxicity study, nine (9) randomly selected mice were grouped into group 1, 2 and 3, were given 2000, 4000, and 5,000 mg/kg bwt of verbenone and then observed for another 24 h for behavior as well as mortality, then the LD50 was calculated as follows:
where, LD50 = Median lethal dose.
D0 = Highest dose that gave no mortality.
D100 = Lowest dose that produced mortality.
2.4.2 Induction of diabetes
Diabetes was induced on overnight fasted mice by single dose intraperitoneal injection of STZ (60 mg/kg bwt) prepared in 0.1 M citrate buffer at pH 4.5, 10 min after injection with 110 mg/kg bwt of NAD normal saline solution. The mice were given 10% dextrose after 24 h of STZ administration to prevent STZ-induced hypoglycemia and mortality. After 72 h, mice with blood glucose level of 200 mg/dL and above were selected for the study.
2.4.3 Animals grouping and treatments
The animals were randomly distributed into nine (9) groups containing five (5) mice each. After diabetes induction, various doses of verbenone, L-arginine, verbenone + L-arginine were prepared in 5% DMSO used as the vehicle and at volume of 0.2 ml. The intervention period was performed for 14 days where animals were given daily doses of the respective preparations:
Group I: normal mice which received 5% DMSO (0.2 mL).
Group II: diabetic mice which received 5% DMSO (0.2 mL).
Group III: diabetic mice treated with 5 mg/kg bwt of glibenclamide.
Group IV: diabetic mice treated with 100 mg/kg bwt of verbenone.
Group V: diabetic mice treated with 200 mg/kg bwt of verbenone.
Group VI: diabetic mice treated with 100 mg/kg bwt of L-arginine.
Group VII: diabetic mice treated with 200 mg/kg bwt of L-arginine.
Group VIII: diabetic mice treated with 100 mg/kg bwt of verbenone + L-arginine (1:1 v/m).
Group IX: diabetic mice treated with 200 mg/kg bwt of verbenone + L-arginine (1:1 v/m).
2.4.4 Preparation of samples for biochemical analysis
Blood samples were collected aseptically from the tips of mice tails onto glucose test strips for measurement of blood glucose levels. However, at the end of treatment, all experimental animals were weighed and sacrificed on the fifteenth (15th) day under mild anesthesia (chloroform), and their blood samples were collected into containers containing EDTA. Plasma was separated by centrifugation at 1500×g for 10 min into appropriately labeled test tubes for analyses. Also, the liver of the mice was excised, homogenized using 0.25 M cold sucrose solution, and centrifuged at 1500×g for 5 min to obtain supernatants that were appropriately collected and labeled in test tubes for further analyses.
2.4.5 Oral glucose tolerance test (OGTT)
Following a standard oral dose of glucose (2 g/kg bwt.), blood glucose levels were monitored at regular intervals, in order to evaluate the glucose tolerance ability of verbenone, and L-arginine. Briefly, twenty seven (27) non-diabetic mice were randomly distributed into nine (9) groups. The mice were fasted overnight and their baseline glucose levels were measured by glucometer (Accu-Chek Active). 2 g/kg bwt glucose was then administered orally to all mice before administration of 5% DMSO, glibenclamide (5 mg/kg bwt.), verbenone (100 and 200 mg/kg bwt.), L-arginine (100 and 200 mg/kg bwt.) and their ratio combination (100 and 200 mg/kg bwt.) respectively. Blood glucose levels were then measured from tail veins after 30, 60, 90 and 120 min.
2.5 Lipid profile and calculated cardiovascular indices
Total cholesterol (T-Chol), triacylglycerides (TGs), High density lipoprotein cholesterol (HDL-Chol) were determined according to the method described by Tietz [33] and Friedwald et al. [34]. Very low density lipoprotein cholesterol (VLDL-Chol.) and Low density lipoprotein cholesterol (LDL-Chol) were determined using the formula described by Friedwald et al. [34]. Atherogenic index (AI) was calculated using the formula described by Lamarche et al. [35], while cardiac index and coronary artery index were determined according to the formula described by Kang et al. [36]. Percentage increases or decreases were calculated using the formula reported by Tijjani et al. [37].
TG: triglycerides
T-Chol: total cholesterol; HDL-Chol: high density cholesterol
2.6 Estimation of markers of liver function
The method described by Reitman and Frankel [38] was used to determine the activities of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) in plasma and liver homogenates while the method described by Wright et al. [39] was used to determine alkaline phosphatase (ALP) activity in plasma.
2.7 Statistical analysis
Experimental data were expressed as mean ± standard error of mean (SEM) and were subjected to One Way Analysis of Variance (ANOVA) followed by Dunnett’s test. Significance was considered at p < 0.05. Graphs were plotted using GraphPad Prism 6 software (GraphPad Software, California, USA).
3 Results
3.1 In vitro antidiabetic activities
3.1.1 α-amylase and α-glucosidase inhibitory activities
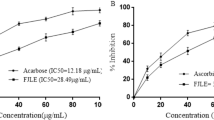
α-amylase inhibitory activity of L-arginine (IC50 = 12.69 µg/mL) expressed none significant (p > 0.05) activity compared to acarbose (IC50 = 12.64 µg/mL), while verbenone (IC50 = 13.0 µg/mL) and verbenone + L-arginine (1:1 v/m) (IC50 = 13.30 µg/mL) expressed significantly (p < 0.05) lower inhibitory activity (Fig. 2). Furthermore, verbenone + L-arginine (1:1 v/m) (IC50 = 30.11 µg/mL) expressed α-glucosidases inhibitory activity compared to verbenone (IC50 = 29.01 µg/mL) while L-arginine (IC50 = 23.76 µg/mL) and acarbose (IC50 = 16.80 µg/mL) expressed higher α-glucosidases inhibitory activity (Fig. 3).
3.1.2 % increase in glucose uptake by yeast cells
Verbenone (26.34 ± 4.75 mmol/L), and their ratio combination (22.89 mmol/L) increased glucose uptake by yeast cells higher than the metronidazole reference drug (18.66 ± 1.66 mmol/L). Verbenone and L-arginine increased glucose uptake in dose-dependent manner (Fig. 4).
Glucose uptake activity by yeast cell treated with verbenone, L-arginine and ratios of verbenone + L-arginine (1:1 v/m). Values are expressed as mean ± SEM, triplicated determinations. Values with different superscripts are significantly different at p < 0.05. Ratios (1:1) = verbenone + L-arginine (1:1 v/m)
3.1.3 % inhibition of haemoglobin glycation
The inhibitory effects of verbenone and L-arginine on haemoglobin glycation indicated a gradual increase after 24 h incubation, with a gradual decrease after 72 h incubation. However, the inhibitory effects of verbenone + L-arginine (1:1 v/m) decreased (16.45 ± 7.55%) after 72 h while the inhibitory effects of the gentamycin increased (46.07 ± 0.00) at 72 h incubation (Fig. 5).
Haemoglobin Glycosylation inhibitory activity of verbenone, L-arginine and ratios of verbenone + L-arginine (1:1 v/m) at 0, 24 and 72 h incubation. Values are expressed as mean ± SEM, triplicated determinations. Values with different superscripts are significantly different at p < 0.05. Ratios (1:1) = verbenone + L-arginine (1:1 v/m)
3.2 In vivo antidiabetic and antihyperlipidemic activities
3.2.1 Antihyperglycemic effects in glucose-loaded hyperglycemic mice
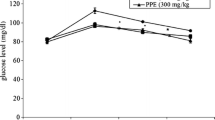
Verbenone at 200 mg/kg bwt demonstrated antihyperglycemic effects, it shows similar glucose decrease pattern with glibenclamide. Their average decreases were − 71% and − 100% respectively (Fig. 6). The control mice, verbenone (100 mg/kg bwt.), L-arginine (100 and 200 mg/kg bwt.) and ratio of verbenone + L-arginine (100 mg/kg bwt.) initially showed gradual increase in blood glucose levels after 60 min followed by a gradual decrease after 90 min.
3.2.2 Effect of verbenone and L-arginine on the blood glucose level in NAD-STZ-induced diabetic rats
Table 1 shows the blood glucose level in diabetic rats treated with verbenone and L-arginine. The diabetic group treated with verbenone, verbenone + L-arginine showed a significant reduction in the level of blood glucose with values of 244 mg/dl, and 226 mg/dl respectively, and this was comparable with the blood glucose level of diabetic rats treated with glibenclamide (158 mg/dl) (Table 1).
3.2.3 Effects of verbenone and L-arginine on the tissue and plasma lipid profile of NAD-STZ-induced diabetic rats
All the diabetic treated groups significantly (p < 0.05) lower the liver total cholesterol levels except the diabetic group treated with verbenone + L-arginine (100 mg/kg bwt) (Fig. 7), when compared with diabetic untreated group. Furthermore, the diabetic group treated with verbenone (100 mg/kg bwt.) significantly (p < 0.05) showed a reduced level of liver triglycerides (Fig. 8) compared to the diabetic untreated mice. However, in the plasma lipids profile, no significant reduction (p > 0.05) was observed in levels of total cholesterol, triglycerides, LDL-cholesterol and VLDL-cholesterol in the diabetic treated mice compared with diabetic untreated mice except in the diabetic mice treated with gibenclamide and verbenone (100 mg/kg bwt) in triglyceride, L-arginine (100 mg/kg bwt) and ratio combination (200 mg/kg bwt.) in LDL-Chol, and gibenclamide and L-arginine (100 mg/kg bwt.) in VLDL-Chol respectively (Figs. 9 and 10). However, all the diabetic treated mice showed a significant increased (p < 0.05) in the levels of the HDL-cholesterol and calculated cardiovascular risk indices, compared to that of the diabetic untreated mice (Figs. 11 and 12).
Total cholesterol concentration in diabetic mice treated with verbenone, L-arginine and ratios of verbenone + L-arginine (1:1v/m) after 14 days treatment. Values are expressed as mean ± SEM, n = 5. Values with different superscripts are significantly different at p < 0.05. VerL-arg = verbenone + L-arginine (1:1 v/m)
Triacylglycerides concentration in diabetic mice treated with verbenone, L-arginine and ratios of verbenone + L-arginine (1:1 v/m) After 14 days treatment. Values are expressed as mean ± SEM, n = 5. Values with different superscripts are significantly different at p < 0.05. VerL-arg = verbenone + L-arginine (1:1 v/m)
Low density lipoprotein cholesterol concentration in diabetic mice treated with verbenone, L-arginine and ratios of verbenone + L-arginine (1:1 v/m) after 14 days treatment. Values are expressed as mean ± SEM, n = 5. Values with different superscripts are significantly different at p < 0.05. VerL-arg = verbenone + L-arginine (1:1 v/m)
Very low density lipoprotein cholesterol concentration in diabetic mice treated with verbenone, L-arginine and ratios of verbenone + L-arginine (1:1 v/m) After 14 days treatment. values are expressed as mean ± SEM, n = 5. Values with different superscripts are significantly different at p < 0.05. VerL-arg = verbenone + L-arginine (1:1 v/m)
High density lipoprotein cholesterol concentration in diabetic mice treated with verbenone, L-arginine and ratios of verbenone + L-arginine (1:1 v/m) After 14 days treatment. Values are expressed as mean ± SEM, n = 5. Values with different superscripts are significantly different at p < 0.05. VerL-arg = verbenone + L-arginine (1:1 v/m)
Cardiovascular disease indices in diabetic mice treated with verbenone, L-arginine and ratios of verbenone + L-arginine (1:1 v/m) after 14 days treatment. Values are expressed as mean ± SEM, n = 5. Values with different superscripts are significantly different at p < 0.05. AI = Atherogenic index, C.I = Cardiac index and C.A.I = Coronary Artery index, VerL-arg = verbenone + L-arginine (1:1 v/m)
3.2.4 Effects on marker enzymes for liver function
The plasma and liver activities of AST, ALT and ALP were determined in other to investigate the effects of verbenone, L-arginine and verbenone + L-arginine (1:1 v/m) on the liver. Results are presented in Figs. 13, 14, and 15 and we recorded a significant (p < 0.05) increases in the activities of these enzymes in the diabetic untreated mice compared to the control mice. Oral administration of gibemclimide, verbenone, L-arginine, and a combination of verbenone and L-arginine ameliorated the increase in the activities of these enzymes in the diabetic treated groups (Fig. 14).
Aspartate-amino transferase activity in diabetic mice treated with verbenone, L-arginine and ratios of verbenone + L-arginine (1:1 v/m) after 14 days treatment. Values are expressed as mean ± SEM, n = 5. Values with different superscripts are significantly different at p < 0.05. VerL-arg = verbenone + L-arginine (1:1 v/m)
Alanine-amino transferase activity in diabetic mice treated with verbenone, L-arginine and ratios of verbenone + L-arginine (1:1 v/m) after 14 days treatment. Values are expressed as mean ± SEM, n = 5. Values with different superscripts are significantly different at p < 0.05. VerL-arg = verbenone + L-arginine (1:1 v/m)
Alkaline phosphatase activity in diabetic mice treated with verbenone, L-arginine and ratios of verbenone + L-arginine (1:1 v/m) after 14 days treatment. Values are expressed as mean ± SEM, n = 5. Values with different superscripts are significantly different at p < 0.05. VerL-arg = verbenone + L-arginine (1:1 v/m)
4 Discussion
Diabetes is generally recognized as a major global health challenge with grave fatality and complications that occur as a result of non-availability or ineffective insulin [40]. Insulin is a key molecule to the metabolism of glucose, protein, and lipids. Defective insulin level or impaired insulin action can result in derangement in the metabolism of the above mentioned macromolecules, leading to several complications [41, 42]. Conventionally, different antidiabetic agents are being employed to treat and manage diabetes [1,2,3]. Over time, the effectiveness of these drugs declined as they develop secondary failure and/or side effects to patients. These failures and/or side effects associated with the intake of synthetic antidiabetic drugs posed challenges to the medical community and sparked research towards the development of alternative medicines with high efficacy, fewer side effects, and relative affordability by the poor [43, 44], hence, attention to natural product as a possible source for drug candidates emerges. Earlier studies proved that inhibition of activities of α-amylase and α-glucosidases delay the degradation of carbohydrates and caused a decrease in the absorption of glucose which in turn reduces postprandial blood glucose level [43]. Hence, researchers focus on developing therapeutics that can suppress the intestinal α-amylase and α-glucosidases as well as increase glucose uptake by skeletal cells or inhibit haemoglobin glycosylation.
In the present in vitro antidiabetic study, all treatments with verbenone, L-arginine and the combination of the two compounds demonstrated potent inhibitory effect on α-amylase and α-glucosidases activities. L-arginine inhibitory action against α-amylase activity was comparable to acarbose and higher than verbenone and their combination. However, the combination of the two compounds used in this study inhibited α-glucosidases better compared with verbenone, L-arginine and at higher doses compared with acarbose. Suggesting that verbenone, L-arginine, and their combination possess inhibitory activity on these enzymes and would delay degradation of carbohydrates and reduce postprandial blood glucose, this is in agreement with the results of Rhabaso and Chiasson [43].
Glucose is an essential fuel in the biological system [45] and its uptake is due to facilitated diffusion and/or elevated glucose metabolism that causes its low level in cells. In our study, verbenone, L-arginine, and their ratio combination (1:1 v/m) increased glucose uptake by yeast cells in a dose dependent manner even better than metronidazole. Suggesting that verbenone can enhance glucose uptake, higher compared with L-arginine or their ratio combination (1:1 v/m). Studies have shown that poorly managed diabetes resulted to increased concentration of glucose in the blood leading to increased irreversible glycation of proteins such as hemoglobin [46]. More so, prolonged hyperglycemia results to formation of reactive oxygen species (ROS) associated with diabetic micro and macro-vascular complications [31, 47,48,49]. Verbenone, L-arginine, and their ratio combination (1:1 v/m) inhibited glycated hemoglobin formation at varying dosages and time in this work. This action of verbenone and L-arginine could be linked to their antioxidant activities [21, 27]. Hence, this shows that the compounds can reduce oxidation of the haem protein/ROS formation [49].
Based on our toxicity study (LD50), verbenone was found to be safe up to a dose of 2000 mg/kg bwt. Thus, doses for the study were scaled down to 1:20 and 1:10 respectively. Apart from protecting mice from the lethality of STZ, administration of the NAD prior to STZ treatment produced a required level of hyperglycemia [50]. In glucose-loaded hyperglycemic mice, verbenone exhibited the greatest antihyperglycemic activity at a dose of 200 mg/kg bwt compared to the other treatments. The onset of its antihyperglycemic action and that of glibenclamide (5 mg/kg bwt.) began from 30 min after treatment and steadily increased up to 120 min while other treatments began exhibiting their actions after 90 min. This action could be because it inhibits glucose metabolic enzymes or increased glucose uptake for metabolism as demonstrated in the in vitro study or because it increases glucose uptake by peripheral cells [51].
In NAD-STZ induced diabetic mice, verbenone demonstrated significant (p < 0.05) hypoglycemic effects. The verbenone at a dose of 200 mg/kg bwt, provided considerable hypoglycemic effects. After fourteen (14) days of intervention, verbenone 100 mg/kg bwt effectively reduced the glycemic index by 38.23%, slightly lower than the ratio of verbenone + L-arginine (1:1 v/m) at 200 mg/kg bwt with 38.42% reduction. L-arginine demonstrated poor hypoglycemic effect at the doses used apparently because it is low. Previous reports have indicated that L-arginine improves glucose tolerance, insulin sensitivity, and secretion in diabetes [26, 52, 53]. Even though verbenone exerted antidiabetic activity better than L-arginine at the doses used in the present study, L-arginine improved its dissolution in 5% DMSO and showed the potential of balancing its hypoglycemic effects [25].
Diabetes is also known to be associated with elevated levels of liver and plasma lipids which represent a high-risk factor for cardiovascular disease (CVD) [54, 55], which is the number one cause of death globally [56]. This is often linked to alterations in metabolic and regulatory mechanisms due to insulin deficiency or insulin resistance, all of which have been proven to enhance release of fatty acids from adipose tissues into the plasma by decreasing the lipoprotein lipases activities [57], thus resulting to insulin resistance, and hyperglycemia with subsequent β-cell damage. The NAD-STZ-induced diabetic mice developed hyperlipidemia which is in agreement with the reports of Adisa et al. [31] and Pandey et al. [58]. Treatment with verbenone and L-arginine reduced significant (p < 0.05) level of HDL-cholesterol and cardiovascular indices. These findings implied that verbenone, L-arginine and their ratio combination (1:1 v/m) can reduce diabetes-associated complications such as cardiovascular disease by enhancing lipids metabolism.
In diabetic conditions AST, ALT, and ALP activities are reportedly increased in plasma. Treatment with verbenone, L-arginine and their ratio combination (1:1 v/m) resulted in a reduction in levels of these enzymes in both liver and plasma except in the higher dose of L-arginine. The hepato-protective potentials of treatments at the doses could be due to their antioxidant properties, which can reduce peroxidation in the liver, and reduce formation of ROS, which are known to affect liver and kidney functions in diabetics [59].
5 Conclusions
The present study demonstrated that verbenone and L-arginine possess in vitro antidiabetic activities by enhancing glucose uptake and inhibiting glycated hemoglobin formation. Furthermore, the compounds exerted in vivo antidiabetic action through amelioration of hyperglycemia and hyperlipidemia, as well as reversal of diabetes-induced alterations in biochemical and cardiovascular indices.
Availability of data and materials
Not applicable.
Abbreviations
- NAD:
-
Nicotinamide
- STZ:
-
Streptozotocin
- DMSO:
-
Dimethyl sulfoxide
- OGTT:
-
Oral glucose tolerance test
- DNPH:
-
Dinitrophenylhydrazine
- IC50 :
-
Half maximal inhibitory concentration
- ALP:
-
Alkaline phosphatase
- ALT:
-
Alanine Amino Transferase
- AST:
-
Aspartate Amino Transferase
- HDL-Chol.:
-
High Density Lipoprotein Cholesterol
- LDL-Chol.:
-
Low Density Lipoprotein Cholesterol
- VLDL-Chol.:
-
Very Low Density Lipoprotein Cholesterol
- GLUT:
-
Glucose transporter
References
Olukoba AB, Obateru OA, Olukoba LB (2012) Type 2 diabetes mellitus: a review of current trends. Oman Med J 27(4):269–273
Ezuruike UF, Prieto JM (2014) The use of plants in the traditional management of diabetes in Nigeria: pharmacological and toxicological considerations. J Ethnopharmacol 155:857–924
Tijjani H, Mohammed A, Idris ZL, Adegunloye PA, Luka CD, Alhassan AJ (2017) Current status of antidiabetic plants in the Lamiaceae family. OmniScriptum GmbH & Co., Germany, pp 1–64
Dimple KA, Kumar V, Tomer V (2018) Traditional medicinal systems for treatment of diabetes mellitus: a review. Int J Pharm Pharm Sci 14(5):7–17
Sharma M, Chahal KK, Kaur R, Singh R, Kataria D (2019) Antifungal potential and structure activity relationship of carrot seed constituents. J Food Biochem 43(9):e12971
Ali M, Diso SU, Minjibir AA, Sani JA, Nas FS, Yahaya A (2017) Assessments of medicinal plants used in treatment of various ailments in Kano city, Nigeria. Asian J Biotechnol Bioresour Technol 2(4):1–11
Al-Snafi AE (2017) Nutritional and therapeutic importance of Daucus carota-a review. IOSR J Pharm 7(2):72–88
Ayeni EA, Abubakar A, Ibrahim G, Atinga V, Muhammad Z (2018) Phytochemical, nutraceutical and antioxidant studies of the aerial parts of Daucus carota L. (Apiaceae). J Herbmed Pharmacol 7(2):68–73
Chatatikun M, Chiabchalard A (2013) Phytochemical screening and free radical scavenging activities of orange baby carrot and carrot (Daucus carota linn.) root crude extracts. J Chem Pharm Res 5(4):97–102
Yousefbeyk F, Golfakhrabadi F, Amouei A, Ghasemi A, Amin M, Gohari A, Samadi N, Amini M, Amin G (2015) Phytochemical investigation and antifungal activity of Daucus littoralis Smith sub sp. hyrcanicus Rech. f. Res J Phytochem 9(1):33–40
Kataria D, Chahal KK, Kaur P, Kaur R (2016) Carrot plant- a potential source of high value compounds and biological activities: a review. Proc Indian Natl Sci Acad 82(4):1237–1248
Brochot A, Guilbot A, Haddioul L, Roques C (2017) Antibacterial, antifungal, and antiviral effects of three essential oil blends. Microbiol Open 6(4):e00459
Prajna Shenoy A, Hedge KG (2018) Pharmacological health benefits of Daucus carota: a review. Int J Pharma Chem Res 4(21):77–82
Scarano A, Gerardi C, D’Amico L, Accogli R, Santino A (2018) Phytochemical analysis and antioxidant properties in coloured tiggiano carrots. Agriculture 8(7):102
Tijjani H, Mohammed A, Adegunloye AP (2019) Phytochemicals and in vitro antioxidant studies of Daucus carota L. seed extracts. In: 5th International electronic conference on medicinal chemistry. Accessed on 5 Jan, 2021 from https://sciforum.net/paper/view/conference/6278, pp 1–30 Nov 2019
Tijjani H, Mohammed A, Muktar S, Musa S, Abubakar Y, Adegunloye AP, Ishola AA, Joel EB, Luka CD, Alhassan AJ (2020) Antioxidant and antihyperlipidemic effects of aqueous seed extract of Daucus carota L. in triton ×100-induced hyperlipidemic mice. J App Biol Biotech 8(1):76–83
Tijjani H, Adegunloye AP, Uba H, Joel EB, Olatunde A (2020) Antioxidant activities of aqueous and ethyl acetate fractions of Daucus carota L seed against triton X-100 induced oxidative stress in mice. Sci Afr 8:e00429
Tijjani H, Mohammed A, Ahmed F, Yahaya H, Zakka N (2020) In vitro antioxidant activity-guided fractionation of Daucus carota L. seed extract. Proc Niger Acad Sci 13(2):74–85
Tijjani H, Imam SA (2021) Inhibition of haemoglobin glycosylation, glucose uptake and in vitro antidiabetic activities of solvent fractions from Daucus carota seed. Ann Sci Technol 6(1):26–33
Kaur A, Chahal KK, Kataria D, Urvashi KA (2018) Assessment of carrot seed essential oil and its chemical constituents against meloidogyne incognita. J Pharmacogn Phytochem 7(1):896–903
Hu Q, Lin G-S, Duan W-G, Huang M, Lei F-H (2017) Synthesis and biological activity of novel (z)- and (e)- verbenone oxime exters. Molecules 22(10):1678
Ju C, Song S, Hwang S, Kim C, Kim M, Gu J, Oh YK, Lee K, Kwon J, Lee K, Kim WK, Choi Y (2013) Discovery of novel (1S)-(-)-verbenone derivatives with anti-oxidant and anti-ischemic effects. Bioorg Med Chem Lett 23(19):5421–5425
Mander S, Kim DH, Nguyen HT, Yong HJ, Pahk K, Kim EY, Lee K, Seong JY, Kim W-K, Hwang J-I (2019) SP-8356, a (IS)-(-)-verbenone derivative, exerts in-vitro and in-vivo anti-breast cancer effects by inhibiting nf-ӄb signaling. Sci Rep 9(6595):1–12
Böger RH (2014) The pharmacodynamics of L-arginine. Altern Ther Health Med 20(3):48–54
Tijjani H (2022) L-arginine improves the solubility of verbenone in polar and non-polar solvent in vitro. Acad Lett. https://doi.org/10.20935/AL4811
Claybaugh T, Decker S, McCall K, Slyvka Y, Steimle J, Wood A, Schaefer M, Thuma J, Inman S (2014) L-Arginine Supplementation in Type II Diabetic Rats Preserves Renal Function and Improves Insulin Sensitivity by Altering the Nitric Oxide Pathway. Int J Endocrinol 2014: https://doi.org/10.1155/2014/171546
Abu-Serie MM, El-Gamal BA, El-Kersh MA et al (2015) Investigation into the antioxidant role of arginine in the treatment and the protection for intralipid-induced non-alcoholic steatohepatitis. Lipids Health Dis 14:128
Andrade-Cetto A, Becerra-Jimennez J, Cardenes R (2008) Alpha glucosidase inhibiting activity of some Mexican plants used in treatment of type 2 diabetes mellitus. J Ethnopharmacol 116:27–32
Kuppusamy A, Muthusamy U, Swashanmugum T, Subhadradevi V, Kalyanasubramaniam R, Sambathkumar R (2011) In vitro (α-glucosidase and α-amylase) inhibition and in vivo antidiabetic property of phytic acid (ip6) in streptozotocin-nicotinamide-induced type 2 diabetes mellitus (NIDDM) in rats. J Complement Integr Med 8:1553–3840
Cirilla VP (1962) Mechanism of glucose transport across the yeast cell membrane. J Bacteriol 84(3):485–491
Adisa RA, Oke J, Olomu SA, Olorunsogo O (2004) Inhibition of human hemoglobin glycosylation by flavonoid containing leaf extract of Cnestis ferruginea. J Cameroon Acad Sci 4:351–359
Lorke D (1982) A new approach to practical acute toxicity testing. Arch Toxicol 54(4):275–287
Tietz NW (1995) Clinical guide to laboratory tests (3rd edn). W.B. Saunders Co., Philadelphia, pp 268–273
Friedwald WT, Levy RI, Frederickson DS (1972) Estimation of the concentration of low density lipoprotein cholesterol (LDL-Cholesterol) in plasma, without use of the preparative ultracentrifuge. Clin Chem 18(6):499–502
Lamarche B, Moorjani S, Lupien PJ, Cantin B, Bernard PM, Dagenais GR, Despres JP (1996) Apolipoprotein A-1 and B levels and the risk of ischemic heart disease during a five-year follow-up of men in the Quebec cardiovascular study. Circulation 94(3):273–278
Kang M, Lee EK, Lee SS (2004) Effects of two P/S ratios with same peroxidizability index value and antioxidants supplementation on serum lipid concentration and hepatic enzyme activities of rats. Clin Chim Acta Int J Clin Chem 350:79–87
Tijjani H, Banbilbwa Joel E, Luka CD (2020) Modulatory effects of some fruit juices on lipid profile in rats fed with high lipid diet. Asian J Biochem Genet Mol Biol 3(2):1–8
Reitman S, Frankel S (1957) A Colorimetric method for determination of serum glutamic oxaloacetic and glutamic pyruvic transaminases. Am J Clin Pathol 28:56–63
Wright PJ, Leathwood PD, Plummer DT (1972) Enzymes in rat urine. Alkaline phosphatase. Enzymologia 42(4):317–327
American Diabetes Association (2009) Diagnosis and classification of diabetes mellitus. Diabetes Care 32(1):s62–s67
Narkhede MB, Ajimire PV, Wagh AE, Mohan M, Shivashanmugam AT (2011) In vitro antidiabetic activity of Caesalpina digyna (R.) methanol root extract. Asian J Plant Sci Res 1(2):101–106
Gandhi GR, Sasikumar P (2012) Antidiabetic effect of Marremia emarginata Burm, F. in streptozotocin induced diabetic rats. Asian Pac J Trop Biomed 2(4):281–286
Ríos JL, Francini F, Schinella GR (2015) Natural products for the treatment of Type 2 diabetes mellitus. Planta Med 81(12–13):975–994
Doan HV, Riyajan S, Iyara R, Chudapongse N (2018) Antidiabetic activity, glucose uptake stimulation and α-glucosidase inhibitory effect of Chrysophyllum cainito L. stem bark extract. BMC Complement Altern Med 18(1):267
Navale A (2019) Glucose transporters and their cellular forms, roles and functions. ScienceDirect. Editor Vinood B. Patel, Molecular nutrition: carbohydrates, 21–34. https://doi.org/10.1016/b978-0-12-849886-6.00003-3.
Nabi SA, Kasetti RB, Sirasanagandla S, Tilak TK, Kumar MVJ, Rao CA (2013) Antidiabetic and antihyperlipidemic activity of Piper longum root aqueous extract in STZ induced diabetic rats. BMC Complement Altern Med 13(37):46. https://doi.org/10.4314/tjpr.v8i5.48082
Asmat U, Abad K, Ismail K (2016) Diabetes mellitus and oxidative stress—a concise review. Saudi Pharm J 24(5):547–553
Son SM (2012) Reactive oxygen and nitrogen species in pathogenesis of vascular complications of diabetes. Diabetes Metab J 36(3):190–198
Nair SS, Kavrekar V, Mishra A (2013) Evaluation of in vitro antidiabetic activity of selected plant extracts. Int J Pharm Sci Invent 2(4):12–19
Nayak Y, Hillemane V, Daroji VK, Jayashree BS, Unnikrishan MK (2014) Antidiabetic activity of benzopyrone analogues in nicontinamide-streptozotocin induced type 2 diabetes in rats. Sci World J. https://doi.org/10.1155/2014/854267
Gunjal A, Walunj M, Aghera H, Nariya M, Goyal MR (2016) Hypoglycemic and antihyperglycemic activity of triphaladi granules in mice. Anc Sci Life 35(4):207–211
Clemmensen C, Madsen AN, Smajilovic S, Holst B, Bräuner-Osborne H (2012) L-Arginine improves multiple physiological parameters in mice exposed to diet-induced metabolic disturbances. Amino Acids 43(3):1265–1275
Monti LD, Casiraghi MC, Setola E, Galluccio E, Pagani MA, Quaglia L, Bosi E, Piatti P (2013) L-arginine enriched biscuits improve endothelial function, and glucose metabolism: a pilot study in healthy subjects and a crossover study in subjects with impaired glucose tolerance and metabolic syndrome. Metab Clin Exp 62(2):255–264
Luka CD, Tijjani H (2013) Comparative studies of the aqueous extracts of Ocimum gratissimum, Aloe vera, Brassica oleracea and Ipomoea batatas on some biochemical parameters in diabetic rats. Int Organ Sci Res J Pharm Biol Sci 6(3):23–29
Luka CD, Tijjani H, Joel EB, Ezejiofor UL, Onwukike P (2013) Hypoglycaemic properties of aqueous extracts of Anacardium occidentale, Moringa oleifera, Vernonia amygdalina and Helianthus annuus: a comparative study on some biochemical parameters in diabetic rats. Int J Pharm Sci Invent (IJPSI) 2(7):16–22
Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, Barengo NC, Beaton AZ, Benjamin EJ, Benziger CP, Bonny A, Brauer M, Brodmann M, Cahill TJ, Carapetis J, Catapano AL, Chugh SS, Cooper LT, Coresh J, Criqui M, DeCleene N, Eagle KA, Emmons-Bell S, Feigin VL, Fernández-Solà J, Fowkes G, Gakidou E, Grundy SM, He FJ, Howard G, Hu F, Inker L, Karthikeyan G, Kassebaum N, Koroshetz W, Lavie C, Lloyd-Jones D, Lu HS, Mirijello A, Temesgen AM, Mokdad A, Moran AE, Muntner P, Narula J, Neal B, Ntsekhe M, Moraes de Oliveira G, Otto C, Owolabi M, Pratt M, Rajagopalan S, Reitsma M, Ribeiro ALP, Rigotti N, Rodgers A, Sable C, Shakil S, Sliwa-Hahnle K, Stark B, Sundström J, Timpel P, Tleyjeh IM, Valgimigli M, Vos T, Whelton PK, Yacoub M, Zuhlke L, Murray C, Fuster V (2020) GBD-NHLBI-JACC global burden of cardiovascular diseases writing group. Global burden of cardiovascular diseases and risk factors: update from the GBD 2019 study. J Am Coll Cardiol 76(25):2982–3021
Sears B, Perry M (2015) The role of fatty acids in insulin resistance. Lipids Health Dis 14:121
Pandey D, Pandey S, Hemalatha S (2010) Hypolipidemic activity of aqueous extract of Melothria maderaspatana. Pharmacologyonline 3:76–83
Nabi, S.A., Kasetti, R.B., Sirasanagandla, S., Tilak TK, Kumar MVJ, Rao CA (2013). Antidiabetic and antihyperlipidemic activity of Piper longum root aqueous extract in STZ induced diabetic rats. BMC Complement Altern Med 13: 37.https://doi.org/10.1186/1472-6882-13-37
Acknowledgements
Not applicable.
Funding
There was no funding for the current report.
Author information
Authors and Affiliations
Contributions
HT, AMD, AUK designed the study, AMD, AO, AUK, HT perform the various assay, and interpreted the data, AMD, HT drafted the manuscript, All authors read and approved the final manuscript for submission.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The University of Jos Ethical Review Committee approved the animal care and research procedures with the registration number UJ/FPS/ F17-00379.
Consent for publication
Not applicable.
Competing interests
All authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tijjani, H., Danyaro, A.M., Olatunde, A. et al. Antihyperglycemic activity of verbenone and L-arginine in nicotinamide-streptozotocin-induced diabetic mice: in vitro and in vivo studies. Beni-Suef Univ J Basic Appl Sci 11, 94 (2022). https://doi.org/10.1186/s43088-022-00271-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43088-022-00271-7