Abstract
Background
Ethnobotanical studies on plants and their active compounds take a great interest in traditional medicine. After pharmacological and toxicological studies, there will be a possibility to be used in therapy. This study aimed to examine the in vitro antioxidant and cytotoxic activity of the methanol extracts of Arbutus andrachne L. and Euphorbia rigida M.Bieb. 10, 25, 50, 75, 100 and 150 µg mL−1 concentrations of A. andrachne and E. rigida were tested for antioxidant activity by using DPPH radical scavenging assays, total antioxidant capacity (phosphomolybdate assay) and and metal ion chelating activity. In addition, in vitro cytotoxic effects of this plants methanol extracts on Hep3B and HepG2 human hepatocellular carcinoma cell lines were evaluated at 24, 48 and 72 h. The cytotoxicity test was carried using the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] assay.
Results
Methanol extract obtained from both plants showed increased antioxidant activity depending on the increase in concentration. When A. andrachne and E. rigida methanol extracts were compared in free DPPH scavenging activity, total antioxidant capacity and metal ion chelating activity, A. andrachne methanol extract was found more effective than E. rigida. Results from MTT assay revealed that except for 72 h treatment of HepG2 cells with 400 and 500 µgmL−1 extract concentrations, A. andrachne methanol extract did not show significant cytotoxic effects on either Hep3B or HepG2 cells at any concentration and treatment time. On the contrary, it significantly increased proliferation in Hep3B cells from 48 h and at a concentration of 100 µg mL−1. E. rigida methanol extract exhibited statistically significant cytotoxic activity on HepG2 cells after 48 and 72 h treatment. However, the treatment concentrations of E. rigida methanol extract were not as effective on Hep3B cells as on HepG2 cells.
Conclusions
According to our findings, it was determined that A. andrachne methanol extract did not have cytotoxic activity on neither Hep3B nor HepG2 cells, while E. rigida methanol extract had cytotoxic activity especially on HepG2 hepatocellular carcinoma cells. Further research is needed to identify and purify the active ingredients in E. rigida extracts.
Similar content being viewed by others
1 Background
Medicinal plants are widely used in developing countries because of their advantages such as dissimilarity, flexibility, easy to approach, relatively low cost, low levels of technological input and increasing economic importance. WHO estimates that 80% of the population around the world use medicinal plants for primary health care. Usually, plant seeds, berries, roots, leaves, bark, or flowers are used for medicinal purposes [1]. There are very many reports on the use of herbal medicines for the treatment of individual health conditions or multiple health conditions. Several plant parts used for herbal medicines have been extensively studied.
Cancer, an abnormal malignant growth of body tissue or cell, is main health problem in both developed and developing countries. Hepatocellular carcinoma is the sixth most widespread cancer and the third most common cause of death from cancer [2]. Today, the active substances of many drugs used in cancer treatment are obtained from medicinal plants [3]. Medicinal plants have to be screened for anticancer activity for more of use. Herbal drugs show their anticancer effects by mechanisms such as carcinogen inactivation, antiproliferation, cell cycle suspension, induction of apoptosis and differentiation, suppression of angiogenesis, antioxidation and reduction of multiple drug resistance [4]. The growing cost for conventional treatments of cancer has prompted people to depend more on traditional medicine [5, 6]. As a therapeutic alternative and a safe choice, herbal medicine might even increase the success rate of most cancer treatments by having a lower systemic toxicity indicated in chemotherapy [7]. Despite the afore-mentioned advantages, little is known about the possible medicinal application of medicinal plants or their cytotoxicity [8].
Arbutus andrachne is belonging to the Ericaceae family and distributed in the Eastern Mediterranean, mainly in Greece and Turkey [9]. The local name in Turkey is “sandalağacı” [10]. Fruits of this plant contain tannin, anthocyanin and carotenoids, and fruits are used widely in food products such as marmalade, fruit jelly and jam, and also in alcoholic beverages like wine and liquor [11]. Whole plant is used for various ethno medicinal purposes in the Mediterranean countries [12]. The antioxidant activities of methanol extracts from roots, leaves and fruits of the A. andrachne were investigated. Also, the effects of the extracts on the cardiodynamics of isolated perfused rabbit hearts. It was reported that methanol extract of the roots possesses high antioxidant activity and antihypertensive effect [13].
The genus Euphorbia is the largest in the plant family Euphorbiaceae, comprising about 2000.
known species and ranging from annuals to trees. 91. Euphorbia species are growing in Turkey [14]. All contain latex and have unique flower structures Euphorbia genus is known to contain a wide variety of terpenoids, ranging from mono-, sesqui-, and diterpenes to triterpenoids and steroids. Many of these compounds have been investigated for their toxicity or their potential therapeutic activity, promising anticancer activity and some have been used as medicines since ancient times [15]. This Euphorbia species have been used in folk medicine to treat skin diseases, migraines, inflammatory infections, respirational infections, body pain, intestinal parasites and warts [12, 16, 17]. The biological activities of the genus, including antitumor, antiviral, cytotoxic properties [18,19,20,21,22,23,24,25,26]. In this study, we investigated the antioxidant activities of the methanol extracts from Arbutus andrachne and Euphorbia rigida by measuring the free radical scavenging, total antioxidant capacity and metal chelating activity. Also, we evaluated the in vitro cytotoxic effects of the extracts on Hep3B and HepG2 human hepatocellular carcinoma cells by MTT assay. To the knowledge, there is no comparative study has been published to evaluate the cytotoxic properties of A. andrachne stem (wood and bark) methanol extract. Also, there are studies on the biological effects of various Euphorbia and Arbutus species but, we did not find any studies on the cytotoxic effects of plants in the literature review.
2 Methods
2.1 Plant material and methanol extraction
The branches (anatomically stem that consists of wood and bark) of A. andrachne and aerial parts of the E. rigida were used in this study. Collections of the branches and identifications were done by Assoc. Prof. Dr. Yelda Güzel in 2018 from the natural habitats from Hatay, TURKEY. Voucher specimens were deposited in the Herbarium of the Biology Department of Hatay Mustafa Kemal University. Voucher numbers are; Y. Güzel-1104 for A. andrachne and Y. Güzel-1105 for E. rigida. Plant samples air dried. Then, stems of A. andrachne and aerial parts of E. rigida (air-dried) samples were chopped and grounded into powder using an electric blender (HR2118 Philips, Netherlands). These plant powders (20 g) were extracted with 500 mL methanol at room temperature for 24–48 h. After filtration, the extract was evaporated at 40 °C under reduced pressure (Laborota 4002, Heidolph). The crude extracts were kept at +4 °C until the experimental studies.
2.2 1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging assay
The DPPH free radical scavenging potential assay is based on the stable DPPH scavenging activity. Quantitative measurements of radical scavenging assay were carried out according to the method described by Brand-Williams et al. [27]. One milliliter of 0, 1 mM DPPH methanol solution was added to 3 mL of different concentrations (10, 25, 50, 75, 100 and 150 µg mL–1) of extracts in methanol. The mixture was vigorously shaken, then left at room temperature to stand. Using a microplate reader (Elisa Reader, Biotek Co, USA) the absorbance of the mixture was measured at λ = 517 nm after 30 min. 25 and 50 µg mL−1 ascorbic acid, the commercial known antioxidant was used as a positive control. All experiments were performed in triplicate.
The percentage of the DPPH free radical was calculated using the following equation:
where A0 was the absorbance of the control and A1 was the absorbance in the presence of the A. andrachne and E. rigida methanol extracts. The actual decrease in absorption induced by the test was compared with the positive controls.
2.3 Phosphomolybdate assay (total antioxidant capacity)
The total antioxidant capacity (TAC) assay of samples was carried out by the phosphomolybdenum method [28]. A 0.1-mL aliquot of the extract (10, 25, 50, 75, 100 and 150 µg mL−1) solution was shaken with 1 mL of reagent solution (0.6 M sulfuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate). The test tubes were covered with aluminum foil and incubated in a water bath at 95 °C for 90 min. After the samples were cooled, the absorbances were measured at 765 nm. Ascorbic acid was used as standard (25 and 50 µg mL−1) and the results were expressed as µg mL−1 of ascorbic acid equivalents. All experiments were performed in triplicate. The total antioxidant capacity (TAC) of the extracts was estimated using the following formula:
Abs: absorbance.
2.4 Metal ion (Iron II) chelating ability
The chelation of ferrous ions by extracts was estimated by method of Dinis et al. [29]. Briefly, 50 µL of 2 mM FeCl2 was added to 1 mL of different concentrations of the methanol extracts 10, 25, 50, 75, 100 and 150 µg mL−1). The reaction was initiated by the addition of 0.2 mL of 5 mM ferrozine solution. The mixture was vigorously shaken and left to stand at room temperature for 10 min. All experiments were performed in triplicate. The absorbance of the solution was thereafter measured at 562 nm. The percentage inhibition of ferrozine – Fe2+ complex formation was calculated as:
where A0 was the absorbance of the control, and As was the absorbance of the methanol extracts/standard. Na2EDTA was used as positive control (standard).
2.5 Cell culture and cytotoxicity assay
In cytotoxicity assays, Hep3B (ATCC HB-8016) and HepG2 (ATCC HB-8065) human hepatocellular carcinoma cells were used, because these cell lines are widely used hepatocellular carcinoma cells in studies about hepatotoxicity and drug metabolism. These cell lines possess well-known similarities each other. However, there are significant differences between these cell lines such as ethnic origins, intrinsic and drug-dependent gene expressions, cell growth inhibition, and signaling pathways associated with differential drug responses [30]. Hep3B and HepG2 cells were cultured in DMEM (Dulbecco’s Modified Eagle’s Medium) supplemented with 10% FBS, 1% penicilin-streptomycin and Amphotericin B under humidified atmosphere of 5% CO2 at 37 °C until confluent. The cells were trypsinized and cytotoxicity assays were carried out in 96 well-plates.
Cell viabilities were measured by MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (Sigma-Aldrich, Germany)] assay. In this assay, viable cells cleave the yellow tetrazolium salt MTT, by the mitochondrial enzyme succinate dehydrogenase [31]. For MTT assay, Hep3B and HepG2 cells were plated 1 × 103 cells/plate in 96- well plate and incubated for 24 h, until monolayer formed. After that 25, 50, 100, 150, 200, 400 and 500 µg mL−1 concentrations of E. rigida and A. andrachne methanol extracts were added for treatment. Control cells received only maintenance medium. Furthermore, anticancer agent Farmorubicin (40 µg mL−1) was used for Hep3B and HepG2 cells. As a solvent control, 0.1% DMSO (Dimethyl Sulfoxide) was used. The plates were incubated at 37 °C in a humidified incubator with 5% CO2 for 24, 48 and 72 h. At the end of treatment times cellular viabilities were determined by MTT assay [30]. The absorbance was read at 570 nm by using a microplate reader (BioTek Epoch). All experiments were performed in triplicate.
2.6 Statistical analysis
Each experiment was performed in three replicates. Results were expressed as means ± SD and analyzed by One Way ANOVA (SPSS 20.00 software package program). Statistically significant difference was considered at the level of p < 0.05.
3 Results
3.1 Antioxidant activity
3.1.1 DPPH assay
The antioxidant potential of methanol extracts of A. andrachne and E. rigida was evaluated on the basis of their ability to scavenge stable free DPPH radicals. This test is based on change in color of DPPH solution from purple to yellow, due to scavenging of stable free DPPH radicals, which from purple to yellow measured at 517 nm [32]. A stronger yellow color indicates a greater ability of the extract to scavenge free DPPH radicals and stronger antioxidant potential. In the present study, the antioxidant potential of the methanol extracts from A. andrachne and E. rigida was explored in a dose-dependent (10–150 µg mL–1) manner as shown in Table 1 and Fig. 1. An increase in DPPH scavenging ability was observed with increase in concentration of extracts. The DPPH scavenging ability of A. andrachne methanol extract was higher than that of E. rigida methanol extract at each concentration tested. E. rigida methanol extract, on the other hand, showed high DPPH scavenging activity from the concentration of 50 µg mL−1. The DPPH scavenging activity of A. andrachne stem methanol extract was even higher than the standard antioxidant ascorbic acid from a concentration of 50 µg mL–1.
3.1.2 Total antioxidant capacity assay (TAC)
The results in Table 1 and Fig. 1 show that A. andrachne stem methanol extract possess high TAC starting from 75 µg mL–1, while the highest TAC was at 150 µg mL–1 (81.30 ± 0.235%). These results were even higher than the TAC value of ascorbic acid (76.50 ± 0.059). Although E. rigida methanol extract also showed total antioxidant activity, the total antioxidant capacity of E. rigida was considerably lower than both ascorbic acid and A. andrachne stem methanol extract.
3.1.3 Metal chelating activity
Phosphomolybdate assay is based on the reduction of phosphomolybdate ion in the presence of an antioxidant resulting in the formation of a green phosphate/MoV complex which is measured spectrophotometrically [33, 34]. Metal chelating ability assay is based on the measurement of iron-ferrozine absorbance at 562 nm in presence of an antioxidant compound [29].
A. andrachne stem methanol extract was tested in the concentration range of 10–150 µg mL–1, with only three of the six concetrations (50, 100 and 150 µg mL–1) showing concentration-dependent chelating activity (Table 1; Fig. 1). However, this activity is considerably lower than the metal chelating activity of EDTA (97.55 ± 0.053%) used as a standart. Compared at the concentration of 150 mg/mL A. andrachne stem methanol extract (55.32 ± 0.055%) the strongest activities, while the weakest activities were detected in 10 µg mL–1 A. andrachne stem methanol extract (44.52 ± 0.072%). E.rigida methanol extract was also tested in the concentration range of 10 to 150 µg mL–1 and only one of the six concentrations (150 µg mL–1) showed chelating activity above 50% (51.44 + 0.023%) (Table 1; Fig. 1).
3.2 Cell proliferation assay
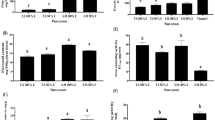
To investigate the cytotoxic potential of the A. andrachne and E. rigida methanol extracts on Hep3B and HepG2 cancer cells, the MTT colorimetric assay was performed. The cytotoxic activity of A. andrachne stem methanol extract against two cancer cell lines (Hep3B and HepG2) was evaluated (Table 2; Figs. 2 and 3). Hep3B and HepG2 cancer cells were treated with seven different concentrations of A. andrachne stem methanol extract (25, 50, 100, 150, 200, 400 and 500 µg mL–1) for 24, 48 and 72 h. Three concentrations (25, 50 and 100 µg mL–1) after 24 h of treatment slightly reduced viability in Hep3B cells. The highest decrease in viability was at 100 mg ml−1 (89.76 ± 0.057%). In contrast to these results, treatment with the other four concentrations (150, 200, 400 and 500 µg mL–1) for 24 h increased Hep3B cell viability concentration dependently. The highest viability rate was at 500 µg mL–1 (112.31 ± 0.144%). The cell viability obtained with the positive control farmorubicin was only 1.06 ± 0.014%. Farmorubicin (40 µg mL–1) showed a very high cytotoxic effect on all Hep3B cells and killed almost all Hep3B cells. The viability and proliferation enhancing effect of A. andrachne methanol extract of Hep3B cells at 24 h increased at all concentrations after 48 and 72 h of treatment, and these data were statistically significant when compared to control and solvent control (Table 2; Fig. 2).
The cytotoxic effect of A. andrachne stem methanol extract on the proliferation and viability of HepG2 cells is presented in Table 2 and Fig. 3. A. andrachne methanol extracts at 50, 100, 150, and 200 40 µg mL–1 increased the viability of HepG2 cells somewhat at 24 h of treatment, but slightly decreased at the other three concentrations (25, 400, and 500 40 µg mL−1) (Table 2; Fig. 3). This effect was not statistically significant when compared with solvent control and farmorubicin ((40 µg mL–1). As a result of 48 h of treatment, cell viability started to decrease due to the increase in concentration with the application of 200, 400 and 500 µg mL–1 concentrations in the viability and proliferation of HepG2 cells. The most significant decrease in cell viability occurred after 72 h of treatment with 500 µg mL−1 extract concentration (67.05 ± 0.031%). Farmorubicin used as control produced a very high cytotoxic effect on HepG2 cells at all three treatment times and killed almost all cells.
Effect of E. rigida methanol extract on the proliferation and viability of Hep3B cells are present in Table 3 and Fig. 4. After 24 h of treatment of E. rigida methanol extract, all concentrations except 500 µg mL−1 increased proliferation in Hep3B cells. This effect was found to be significantly high in 25, 50 and 100 µg mL−1 (128.40 ± 0.015%, 125 ± 0.033%, 123 ± 0.098%, respectively) extract treatments compared to the control and solvent control (DMSO) group (p < 0.05). After 48 h of treatment, no significant effect of methanol extract was observed on the cell viability and proliferation, except for a 500 µg mL−1 concentration (82.54 ± 0.285%). After 72 h of treatment, E. rigida methanol extract significantly reduced cell viability and proliferation due to the increase in concentration and showed cytotoxic effect compared to the control group, excluding 25, 50 and 100 µg mL−1 (p < 0.05). DMSO, used as solvent control, had no significant cytotoxic effect at any treatment time. Farmorubicin, which was used as an anticancer agent, produced a very high cytotoxic effect on Hep3B cells during all treatment times and killed almost all of the cells.
Effect of E. rigida methanol extract on the proliferation and viability of HepG2 cells are also present in Table 3 and Fig. 5. 400 and 500 µg mL−1 concentrations of E. rigida methanol extract reduced the viability of HepG2 cells at 24 h of treatment (p < 0.05), and all concentrations except for 25 and 50 µg mL−1 at 48 h of treatment resulted in a significant decrease in cell viability. After 48 h of treatment with E. rigida methanol extract, the concentration that most reduced HepG2 cell viability and proliferation was 500 µg mL−1 (58.92 ± 0.087%). The 72 h treatment of E. rigida methanol extract caused a concentration-dependently and significantly decrease in the viability of HepG2 cells in comparison with the control. HepG2 cell viability decreased to 45.91% at 500 µg mL−1 concentration treatment (Table 3; Fig. 5). In HepG2 cells treated with E. rigida methanol extract decreased of the cell viability and proliferation was observed at most after 72 h of treatment, and this decrease was parallel to the increase in concentration. Farmorubicin killed almost all of the cells at all treatment time.
4 Discussion
Cancer is one of the most dangerous diseases with fast progression and high mortality around the world. Today, about 60–75% of anticancer agents are derived from natural sources [33,34,35]. The chemical constituents of the plants or crude extracts are known to be biologically active ingredients. Plant polyphenols have been highlighted as potential anticancer agents as well as being chemical inhibitors [36] because of their high antioxidant activity, targeting signaling molecules, and preventing or protecting cells from further damage and/or transformation into cancer cells [36, 37]. In our literature review, we found antioxidant, anti-inflammatory and cell protective effects of Arbutus spp. but, we could not find any data regarding the cytotoxic effect of Arbutus species including A. andrachne stem (wood and bark). Furthermore, it has been shown that Euphorbia spp. is promising plants antitumor activity [38, 39]. Also, there are reports about antioxidant activities of different Euphorbia species [25, 26]. In this regard, the antioxidant activities of methanol extracts from A. andrachne and E. rigida were investigated using DPPH assay, phosphomolybdate assay and metal chelating assay. Also, cytotoxic effects of the extracts on two human hepatocellular carcinoma (Hep3B and HepG2) cell lines were investigated using MTT assay.
When A. andrachne and E. rigida methanol extracts were compared in terms of antioxidant activities (DPPH free radical scavenging activity, total antioxidant capacity and metal chelating activity), A. andrachne methanol extract possess higher antioxidant activity. Differences between the antioxidant activities of the extracts may be attributed to different phytochemical contents of A. andrachne and E. rigida plants. The transition metal ion Fe2+ have the ability to maintain the formation of free radicals by gain or loss of electrons. Therefore, the decrease of reactive oxygen species genesis can be carried out by the chelation of metal ions with chelating agents. Considering the cellular damages of reactive oxygen species and their relation with various diseases, it is important that plant extracts possess metal chelating activity besides free radical scavenging activity [40, 41]. In our previous studies, we reported that diethyl ether, ethyl acetate and aqueous extracts from fruits of Arbutus unedo possess high total phenolic content and antioxidant activity [42]. Recently, it was suggested also, Arbutus unedo leaf aqueous extract has antioxidant activity, in vitro anti-inflammatory effect, and heat protective effect on the membrane integrity of human red blood cells against heat [43]. Furthermore, some studies reported vasorelaxant and antiaggregant effects of Arbutus unedo leaf extract on human platelets [44, 45] and antihaemolytic and radical scavenging activities [46, 47]. Hmaidosh et al. [48] reported that A. andrachne flowers, leaves and barks contain high amounts of total phenolic compounds. They reported also that these parts of plant possess antioxidant activity (FRAP), which was similar to the total phenolic contents. We reported also ethyl acetate, methanol and water extracts of Euphorbia platyphyllos possess high DPPH radical scavenging activity, previously [24]. Gapuz and Besagas suggested that leaf methanol extracts from three Euphorbia species (E. milii, E. trigona, and E. antiquorum) possess high total phenolic content and high DPPH radical scavenging activity [49]. They reported also that the extracts contain alkaloids, flavonoids, carbohydrates, saponin, and tannins.
In this study, it was investigated also whether A. andrachne and E. rigida methanol extracts possess cytotoxic effect on Hep3B and HepG2 cells. A. andrachne methanol extract showed no cytotoxic effect on Hep3B cells and increased cell viability and proliferation. Also, A. andrachne methanol extract showed no significant cytotoxic effect on HepG2 cells at 24 and 48 h treatments. Only 72 h treatment with 400 and 500 µg mL−1 extract concentrations significantly reduced the viability and proliferation of HepG2 cells (Table 2; Fig. 3). This may be due to different responses of the cells to the components of the extract. When the results were examined, it was understood that methanol extract had no significant cytotoxic effect on both cells. Moreover, methanol extract appears to increase cell proliferation in Hep3B cells depending on time and concentration (Table 2; Fig. 2).
In previous studies conducted with cancer cells, it was found that extracts of Arbutus species don’t possess cytotoxic and/or antiproliferative effect. Fortalezas et al. reported that hydroethanolic extract of A. unedo fruits caused no effect on viability of SK-N-MC human neuroblastoma cells [50]. Also, it was suggested that A. andrachne did not exhibit significant antiproliferative effect on breast, colorectal and skin cancer cells [4]. In addition, it was reported that arbutin, a main compound in Arbutus species, has not significant effect on cell viability, even at high concentrations on TCCSUP human bladder cancer cell line, HepG2 human hepatocellular carcinoma cells, and U937 human lymphoma cells [51,52,53]. In our study, very insignificant dose-dependent results were obtained for Arbutus extract. Because of the high antioxidant activity of the A. andrachne stem methanol extract, free radicals forming in the cell may be eradicated and cytotoxic effect is neutralized. In addition, it has been reported that the polyphenol compounds of plants to be anticancer agents [54,55,56].
Methanol extract of E. rigida exhibited statistically significant cytotoxic activity on HepG2 cells after 48 and 72 h treatment (Table 3; Fig. 5). However, the tried concentrations of E. rigida methanol extract were not as effective on Hep3B cells as on HepG2 cells (Table 3; Figs. 4 and 5). This result could be explained by different sensitivity of tumor cells to the chemical content of the extract. Previous studies suggested that triterpenoids, alkaloids, diterpene esters, organic acids, acetophenone derivatives, and flavonoids are the main chemical ingredients of Euphorbiaceae plants [57, 58]. Therefore, the cytotoxic effect of E. rigida methanol extract on HepG2 cells may be considered to be due to the presence of these chemical ingredients. It was reported that Euphorbia plants possess a various biological activities such as antioxidative, antiproliferative, cytotoxic, anti-anaphylactic, antimicrobial, and anti-arthritic activities [59, 60]. Javidnia et al. [61] reported that Euphorbia hebecarpa extracts possess cytotoxic effect on K562 and U937 cells but, they had no effects on KB cells. Sadeghi-Aliabadi et al. [62] showed that ethyl acetate extracts of E. macroclada were cytotoxic against MDA-MB-468 cells. Metin and Bürün reported that the aqueous extract of E. rigida aerial parts possess negative impacts on mitosis and, cytotoxic and genotoxic effects on Allium cepa root meristematic cells at concentrations over 50 ppm [63].
5 Conclusions
Turkey has a great history of folk medicine, but this knowledge has not been documented extensively so far. In the recent years researchers have carried out many studies about traditional medicine in Turkey. Now we have important information, documents and great opportunities to study on medicinal plants. According to our findings it could be conclude that constituents extracted by methanol from E. rigida possess cytotoxic activity on HepG2 hepatocellular carcinoma cells and it required further investigations for the identification and purification of the active components. However, A. andrachne stem methanol extract possess cytotoxic activity neither on Hep3B nor on HepG2 cells. For this reason, different studies can be carried out on this plant as well as other potential effects other than anticancer effects in the future.
Availability of data and materials
Not applicable.
Abbreviations
- AA::
-
Ascorbic acid
- AME::
-
A andrachne methanol extract
- EME::
-
E rigida methanol extract
References
Telles S, Pathak S, Singh N, Balkishna A (2014) Research on traditional medicine: what has been done, the difficulties, and possible solutions. Evid Based Complement Altern Med 2014:1–3
Forner A, Llovet JM, Bruix J (2012) Hepatocellular carcinoma. Lancet 379(9822):1245–1255
Calixto JB (2019) The role of natural products in modern drug discovery. Ann Acad Bras Ciênc 91(3):20190105
Bozyel ME, Bozyel Merdamert E, Canlı K, Altuner EM (2019) Anticancer uses of medicinal plants in Turkish traditional medicine. KSU J Agric Nat 22(2):465–484
Sheldon JW, Balick MJ, Laird SA (1997) Advances in economic botany. In: The New York botanical garden. New York: Bronx; Medicinal plants can utilization and conservation coexist?
Tabrizi FHA, Irian S, Amanzadeh A, Heidarnejad F, Gudarzi H, Salimi M (2016) Anti-proliferative activity of Fumaria vaillantii extracts on different cancer cell lines. Res Pharm Sci 11(2):152–159
Gao Y, Su Y, Qu L, Xu S, Meng L, Cai SQ et al (2011) Mitochondrial apoptosis contributes to the anticancer effect of Smilax glabra Roxb. Toxicol Lett 207(2):112–120
Mahasneh AM, El-Oqlah AA (1999) Antimicrobial activity of extracts of herbal plants used in the traditional medicine of Jordan. J Ethnopharmacol 64:271–276
Delgado-Pelayo R, Gallardo-Guerrero L, Hornero-Méndez D (2016) Carotenoid composition of strawberry tree (Arbutus unedo L.) fruits. Food Chem 199:165–175
Güner A, Aslan S, Ekim T, Vural M, Babaç MT (edlr.) (2012) Türkiye Bitkileri Listesi (Damarlı Bitkiler). Nezahat Gökyiğit Botanik Bahçesi ve Flora Araştırmaları Derneği Yayını İstanbul
Baskan C, Kılıc DD, Sırıken B, Tanrikulu G, Gül M, Ertük O (2019) In vitro study on antioxidant, antibacterial and DNA interaction activities of extracts from Arbutus andrachne L. Eurasian J Forest Sci 7(3):293–300
Güzel Y, Güzelşemme M, Miski M (2015) Ethnobotany of medicinal plants used in Antakya: a multicultural district in Hatay Province of Turkey. J Ethnopharmacol 174:118–152
Abidi E, Habib J, Yassine A, Chahine N, Mahjoub T, Elkak A (2016) Effects of methanol extracts from roots, leaves, and fruits of the Lebanese strawberry tree (Arbutus andrachne) on cardiac function together with their antioxidant activity. Pharm Biol 54(6):1035–1041
Özbilgin S, Saltan Çitoğlu G (2012) Uses of some Euphorbia species in traditional medicine in Turkey and their biological activities. Turk J Pharm Sci 9(2):241–256
Gherraf N, Zellagui A, Mohamed N, Hussien T, Mohamed TAF, Hegazy ME, Rhouati S, Moustafa MFM, El-Sayed MA, Mohamed AEM (2010) Triterpenes from Euphorbia rigida. Pharmacognos Res 2(3):159–162
Singla A, Pathak K (1990) Phytoconstituents of Euphorbia species. Fitoterapia 61:483–516
Kemboi D, Peter X, Langat M, Tembu J (2020) A review of the ethnomedicinal uses, biological activities, and triterpenoids of Euphorbia species. Molecules 25:4019
Hohmann J, Evanics F, Dombi G, Szabó P (2001) Salicifoline and Salicinolide, new diterpene polyesters from Euphorbia salicifolia. Tetrahedron Lett 42:6581–6584
Appendino G, Spagliardi P, Ballero M, Seu G (2002) Macrocyclic diterpenes from Euphorbia hyberna L. subsp Insularis and reaction with oxyphilc reagents. Fitoterapia 3:576–582
Haba H, Lavaud C, Harkat H, Alabdul Magid A, Marcourt L, Benkhaled M (2007) Diterpenoids and triterpenoids from Euphorbia guyoniana. Phytochemistry 68:1255–1260
Hsieh WT, Lin HY, Chen JH, Kuo YH, Fan MJ, Wu RSC, Wu KC, Bark WG, Chung JG (2011) Latex of Euphorbia antiquorum induces apoptosis in human cervical cancer cells via c-jun N-terminal kinase activation and reactive oxygen species production. Nutr Cancer 63(8):1339–1347
Mota MF, Benfica PL, Valadares MC (2012) Synadenium umbellatumpax. Promotes cell cycle arrest and induces apoptosis in K-562 leukemia cells. Braz J Pharm Sci 48:497–506
Lin J, Dou J, Xu L, Aisa HA (2012) Chemical composition, antimicrobial and antitumor activities of the essential oils and crude extracts of Euphorbia macrorrhiza. Molecules 17(5):5030–5039
Assaf AM, Haddadin RN, Aldouri NA, Alabbassi R, Mashallah S, Mohammad M, Bustanji Y (2013) Anti-cancer, anti-inflammatory and anti-microbial activities of plant extracts used against hematological tumors in traditional medi-cine of Jordan. J Ethnopharmacol 145(3):728–736
Aslantürk ÖS, Aşkın Çelik T (2013) Antioxidant, cytotoxic and apoptotic activities of extracts from medicinal plant Euphorbia platyphyllos L. J Med Plants Res 7(19):1293–1304
Camargo Luz LE, Kanunfre CC, Paludo KS, da Silva JA, Kubaski Petry V, Lemes BM, Barison A, Nepel A, Wang M, Avula B, Khan IA, Beltrame FL (2016) Cytotoxic biomonitored study of Euphorbia umbellata (Pax) Bruyns. J Ethnopharm 183:29–37
Brand-Williams W, Cuvelier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. Lebensm Wiss Technol 28:25–30
Ghafoor K, Choi YH (2009) Optimization of ultrasound-assisted extraction of phenolic compounds and antioxidants from grape peels through response surface methodology. J Korean Soc Appl Biol Chem 52:295–300
Dinis TCP, Madeira VMC, Almedia LM (1994) Action of phenolic derivatives (Acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys 315(1):161–169
Qui GH, Xie X, Xu F, Shi X, Wang Y, Deng L (2015) Distinctive pharmacological differences between liver cancer cell lines HepG2 and Hep3B. Cytotechnology 67:1–12
Mossman T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Blois MS (1958) Antioxidant determinations by the use of a stable free radical. Nature 181:1199–1200
Prieto P, Pineda M, Aguilar M (1999) Spectrophotometric quantification of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application of vitamin E. Anal Biochem 269(2):337–341
Newman DJ, Cragg GM (2016) Natural products as sources of new drugs from 1981 to 2014. J Nat Prod 79:629–661
Grigalius I, Petrikaite V (2017) Relationship between antioxidant and anticancer activity of trihydroxyflavones. Molecules 22:2169
Khan N, Afaq F, Mukhtar H (2007) Apoptosis by dietary factors: the suicide solution for delaying cancer growth. Carcinogenesis 28(2):233–239
Liang QL, Dai CC, Jiang JH, Tang YP, Duan JA (2009) A new cytotoxic casbane diterpene from Euphorbia pekinensis. Fitoterapia 80:514–516
Jafarain A, Asghari G, Ghassami E (2014) Evaluation of cytotoxicity of Moringa oleifera Lam callus and leaf extracts on Hela cells. Adv Biomed Res 3:194
Gapus MCD, Besagas RL (2018) Phytochemical profiles and antioxidant activities of leaf extracts of Euphorbia species. J Bio Env Sci 12(4):59–65
Aparadth VT, Naik VV, Karadge BA (2012) Antioxidative properties (TPC, DPPH, FRAP, metal chelating ability, reducing power and TAC) within some Cleome species. Annali di Botanica 2:49–56
Aşkın Çelik T, Aslanturk ÖS, Aktaş Uygun D (2008) Determination of total phenolic content and antioxidant activity of strawberry trees, Arbutus unedo L. fruits. In: International symposium food nutrition and cancer abstracts booklet. Ege University, İzmir, pp 48–49
Moualek I, Aiche GI, Guechaoui NM, Lahcene S, Houali K (2016) Antioxidant and anti-inflammatory activities of Arbutus unedo aqueous extract. Asian Pac J Trop Biomed 6(11):937–944
Legssyer A, Ziyyat A, Mekhfi H, Bnouham M, Herrenknecht C, Roumy V, Fischmeister R (2004) Tannins and catechin gallate mediate the vasorelaxant effect of Arbutus unedo on the rat isolated aorta. Phytother Res 18:889–894
El Haouari M, López JJ, Mekhfi H, Rosado JA, Salido GM (2007) Antiaggregant effects of Arbutus unedo extracts in human platelets. J Ethnopharmacol 113:325–331
Oliveira I, Coelho V, Baltasar R, Pereira JA, Baptista P (2009) Scavenging capacity of strawberry tree (Arbutus unedo L.) leaves on free radicals. Food Chem Toxicol 47:1507–1511
Mendes L, de Freitas V, Baptista P, Carvalho M (2011) Comparative antihemolytic and radical scavenging activities of strawberry tree (Arbutus unedo L.) leaf and fruit. Food Chem Toxicol 49:2285–2291
Hmaidosh D, Ali M, Salame R (2020) Evaluation of antioxidant activity and the phenolic composition of Syrian Arbutus andrachne L Future of Food. J Food Agric Soc 8(3):1–7
Fortalezas S, Tavares L, Pimpão R, Tyagi M, Pontes V, Alves PM, McDougall G, Stewart D, Ferreira RB, Santos CN (2010) Antioxidant properties and neuroprotective capacity of strawberry tree fruit (Arbutus unedo). Nutrients 2:214–229
Sudan R, Bhagat M, Gupta S, Singh J, Koul A (2014) Iron (II) chelation, ferric reducing antioxidant power, and immune modulating potential of Arisaema jacquemontii (Himalayan Cobra lily). BioMed Res Int 2014:179865
Abu-Rish EY, Kasabri VN, Hudaib MM, Mashalla SH, AlAlawi LH, Tawaha KA, Mohammad MK, Mohamed YS, Bustanji YK (2016) Evaluation of antiproliferative activity of some traditional anticancer herbal remedies from Jordan. Trop J Pharm Res 15(3):469–474
Li H, Jeong YM, Kim SY, Kim MK, Kim DS (2011) Arbutin inhibits TCCSUP human bladder cancer cell proliferation via up-regulation of p21. Pharmazie 66:306–309
Seyfizadeh N, Mahjoub S, Zabihi E, Moghadamnia A, Pouramir P, Mir H, Khosravifarsani M, Elahimanesh F (2012) Cytoprotective effects of arbutin against tert-butyl hydtoperoxide induced toxicity in HepG2 cell line. Worl Appl Sci J 19:163–167
Wu LH, Li P, Zhao QL, Piao JL, Jiao YF, Kadowaki M, Kondo T (2014) Arbutin, an intracellular hydroxyl radical scavenger, protects radiation- induced apoptosis in human lymphoma U937 cells. Apoptosis 19:1654–1663
Dai J, Mumper RJ (2010) Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules 15:7313–7352
Asgar A (2013) Anti-diabetic potential of phenolic compounds: a review. Int J Food Prop 16:91–103. https://doi.org/10.1080/10942912.2011.595864
Benayad Z, Martinez-Villaluenga C, Frias J, Gomez-Cordoves C, Es-Safi NE (2014) Phenolic composition, antioxidant and anti-inflammatory activities of extracts from Moroccan Opuntia Ficus indica flowers obtained by different extraction methods. Ind Crops Prod 62:412–420. https://doi.org/10.1016/j.indcrop.2014.08.046
Fu GM, Qin HL, Yu SS, Yu BY, Yuexiandajisu D (2006) A novel 18-nor-rosane-type dimeric diterpenoid from Euphorbia ebracteolata Hayata. J Asian Nat Prod Res 8:29–34
Ghanadian M, Rahiminejad M, Saeidi H, Ayatollahi A, Shamsabadipour S (2012) Triterpenes from Euphorbia denticulata. Res Pharm Sci 7:S960
Bani S, Kaul A, Khan B, Gupta VK, Satti NK, Suri KA et al (2007) Anti-arthritic activity of a biopolymeric fraction from Euphorbia tirucalli. J Ethnopharmacol 110:92–98
Chaabi M, Freund Michel V, Frossard N, Randriantsoa A, Andriantsitohaina R, Lobstein A (2007) Antiproliferative effect of Euphorbia stenoclada in human airway smooth muscle cells in culture. J Ethnopharmacol 109:134–139
Javidnia K, Miri R, Amirghofran Z, Jafari A, Amoozegar Z (2004) Cytotoxicity and antimicrobial assessment of Euphorbia hebecarpa. Iran J Pharm Res 3:75–82
Sadeghi-Aliabadi H, Sajjadi SE, Khodamoradi M (2009) Cytotoxicity of Euphorbiamacroclada on MDA-MB-468 breast cancer cell line. Iran J Pharm Sci 5:103–108
Metin M, Bürün B (2020) Investigation of the cytotoxic and genotoxic effects of the Euphorbia rigida Bieb. Extract Caryologia 73(4):65–75
Acknowledgements
Not applicable.
Funding
This study was partly supported by HMKU Scientific Research Project Fund (18.YL.010).
Author information
Authors and Affiliations
Contributions
ÖSA, EŞY, and YG have designed the study and collected the data. ÖSA and TAÇ have performed Laboratory analysis and statistical analysis of the study. ÖSA has written manuscript; TAÇ, EŞY and YG have reviewed and edited manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aslantürk, Ö.S., Yılmaz, E.Ş., Aşkın Çelik, T. et al. Evaluation of the antioxidant and cytotoxic potency of Euphorbia rigida and Arbutus andrachne methanol extracts in human hepatocellular carcinoma cell lines in vitro. Beni-Suef Univ J Basic Appl Sci 10, 51 (2021). https://doi.org/10.1186/s43088-021-00143-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43088-021-00143-6