Abstract
Background
Liver is a complex, highly vascular organ, where anatomical variations are the norm. This study aimed to analyze all the three hepatic vascular anatomical variations in a single study which would help us understand the prevalence of hepatic vascular (arterial, portal, venous) anomalies in the population catered to by our institution.
Methods
A retrospective analysis of 545 contrast-enhanced CT scans was done from November 2019 from the institute PACS after satisfying the inclusion and exclusion criteria. The raw imaging data were processed in PACS software — Centricity™ Universal Viewer and Syngo.via Vb20 platform, for axial, coronal, and axial-oblique multiplanar reformation, maximum intensity projection (MIP), and volume rendering (VR) images. Data were analyzed in the three vascular phases to determine the anatomical variations. Analysis was done by two surgical residents in the division of HPB surgery, which was verified by a certified radiologist.
Results
There were no major differences in the prevalence rates of the vascular anomalies across gender and domicile distributions. The prevalence of normal hepatic artery and variant hepatic artery in our study was 69% and 31%, respectively. Accessory left hepatic artery (10%) was the most common hepatic artery variant in our study. Single RHV was seen in 77.4%, and other RHV variants like two RHV with common trunk, two RHV with independent drainage, and three RHV with common trunk contribute 22.6% of our study population. Accessory inferior RHV was seen in 19.8% of the study population. Normal portal vein anatomy was found in 81.1% in our study, and the most common variant in our study population was trifurcation of portal vein (16.1%).
Conclusion
This was the largest study until date from South India, studying all three hepatic vascular anatomical variations in a single study. Variations in the anatomy of hepatic arteries, portal veins, and hepatic veins are common. A good knowledge of the same is necessary especially for a hepatobiliary surgeon or for an interventional radiologist, to plan and avoid complications during a procedure. Preoperative contrast-enhanced CT scan and whenever necessary a VR or a MIP reconstruction will precisely help in identifying these variations.
Similar content being viewed by others
Background
The liver is an outstanding vascular organ that has peculiar dual afferent blood supply. The portal vein carries partially deoxygenated blood from gut accounting for 75 to 80% of blood supply to the liver [1]. The rest, 25–25% of the blood to the liver, is well-oxygenated and is carried by the right and left hepatic arteries. The hepatic veins drain directly into the inferior vena cava along the posterior surface of the liver. The three rich networks of hepatic vascular systems have a great degree of variations in terms of number, pattern, and mode of termination. In the field of hepatobiliary surgery and liver transplantation, the preoperative and intraoperative knowledge and awareness of these anatomical variations are extremely important for refinement of the preoperative planning and to reduce inadvertent intraoperative injuries and other complications, thereby improving the procedural outcomes. Even if these complicated vascular anomalies are not always reported in the regular cross-sectional imaging reports, it is very important for the surgeons to identify the vascular variants in the preoperative CTs or discuss the variants in the clinico-radiological meets for proper planning and avoid any intraoperative surprise or an unavoidable mishap. Similarly, this is also important to the interventional radiologists with the expanding indications of endovascular interventions like TACE and PVE, in this discipline.
Multidetector computed tomography (MDCT) is a noninvasive imaging modality that has rapidly emerged over the last couple of decades. It has various three-dimensional imaging techniques, such as multiplanar reconstruction (MPR), volume rendering, and maximum intensity projection (MIP), which have enhanced the imaging of venous structures. Faster imaging with high resolution and less need for contrast media has made MDCTs inevitable to interventional radiologists and surgeons for detailed evaluation of various anatomical structures before HPB surgeries and interventional procedures [2]. MDCTs precisely delineate the hepatic vascular anomalies preoperatively, which would assist the surgeon to avoid inadvertent vascular injury during surgery by anticipating the course of hepatic vascular variants and aids in preserving anomalous vessels unless their division or resection is required to get the oncological clearance. This study would help us understand the prevalence of hepatic vascular (arterial, portal, venous) anomalies in the Indian population catered to by our hospital.
Material and methods
Study setting and inclusion criteria
This was a single-center retrospective observational study done in the Department of Surgery, collaborating with the Department of Radiodiagnosis in a tertiary care teaching hospital in South India. Consecutive sampling method was used to collect data from the contrast-enhanced CTs done from November 2019, satisfying the inclusion and the exclusion criteria. The sample size was calculated by the formula for estimating a single proportion. Considering a 5% level of significance with an absolute precision of 3% and an anticipated prevalence of around 15% of hepatic vascular (arterial, portal, and venous) anomalies [3,4,5], the sample size was calculated to be 545. 545. Contrast-enhanced CTs (CECT) with at least 2 phases (arterial and portal-venous) performed in JIPMER between November 2019 and June 2020 were included in the study.
Exclusion criteria
Patients with one or more of the following were excluded from the study: arterial, portal, or hepatic venous thrombosis, large central tumors, postoperative CT scan following a liver resection, and any pathology obscuring assessment of the vascular anatomy.
Study procedure
Consecutive 545 contrast-enhanced CTs, satisfying the inclusion and the exclusion criteria, were identified from the hospital’s picture archiving and communication system (PACS) server from November 2019. The raw imaging data were processed in PACS software — Centricity™ Universal Viewer and Syngo.via Vb20 platform, for axial, coronal, and axial-oblique multiplanar reformation (MPR), maximum intensity projection (MIP), and volume-rendering (VR) images. Data were analyzed in the arterial phase to determine the hepatic artery variations. The portal and venous phase data were analyzed to determine the portal vein and hepatic vein variants. The images were analyzed by trainee surgical residents from the hepatopancreaticobiliary (HPB) surgical division and were subjected to review by a radiologist with at least 5 years of experience.
Definitions
The anatomical variation of the hepatic artery was enumerated based on Michel’s classification [6]. An anomalous or aberrant right or left hepatic artery is of two types — replaced and accessory. The term “replaced” is used when the normal right or left hepatic artery is absent, and the replacing vessel arises from a different source. When the normal right or left hepatic artery is present and there is an extra artery from other sources, the term “accessory” is used. The portal vein variations are listed based on Nakamura et al. [7] and Cheng et al. [8]. Classification of hepatic vein variants was based on the study published by Soyer et al., Cheng et al., and Fang et al. [9,10,11].
Results
A retrospective review of MDCTs was performed between November 2019 and June 2020. The study population of 545 patients was included after meeting inclusion and exclusion criteria, of whom 369 (67.7%) were males and 176 (32.3%) were females and of which 525 (96.3%) were South Indians and 20 (3.7%) were North Indians with a median age of 45 (Table 1). The age ranged between 2 years and 93 years.
Hepatic artery variations
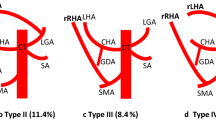
The normal anatomy (type 1) was observed in 376 (69%), of which 258 were males, 118 were females, 363 were South Indians, and 13 were North Indians. In comparison, the anomalous hepatic artery was detected in 169 (31 %) cases out of 545 CTs. A total of 111 males and 58 females was seen to have an anomalous hepatic arterial pattern. The hepatic artery variants were distributed as follows: type 2 in 28 (5.1%) cases, type 3 in 40 (7.3%) cases, types 4 in 7 (1.3%), type 5 in 55 cases for each (10%), type 6 in 7 (1.3%) cases, and types 8 and 9 in 9 patients (1.7%) each. Types 7 and 10 were not observed in our study (Fig. 1). Ten (1.8%) unclassified cases were observed. 3-dimensional (3-D) volume-rendered images of these rare unclassified hepatic artery variations found in the study have been shown in Fig. 2A–F:
-
1.
Celiacomesenteric trunk (Fig. 2A)
-
2.
Replaced RHA (Right hepatic artery) from the celiac trunk (Fig. 2B)
-
3.
Replaced RHA arising from aorta separately (Fig. 2C)
-
4.
Accessory RHA from the celiac trunk (Fig. 2D)
-
5.
Replaced RHA from the celiac trunk and accessory LHA (Left hepatic artery) (Fig. 2E)
-
6.
Replaced RHA from the celiac trunk and CHA (Common hepatic artery) from LGA (Left gastric artery) (Fig. 2F)
3-dimensional reconstruction of the rare hepatic artery variants. A Celiacomesenteric trunk. B Replaced right hepatic artery (RHA) from the celiac trunk. C Replaced RHA arising from aorta separately. D Accessory RHA from the celiac trunk. E Replaced RHA from the celiac trunk and accessory left hepatic artery (LHA). F Replaced RHA from the celiac trunk and common hepatic artery (CHA) from left gastric artery (LGA)
Hepatic veins
Right hepatic veins
Single RHV was seen in 422 (77.4%) out of 545 patients in our study, of which 283 were males, 139 were females, 407 were South Indians, and 15 were north Indians. Two RHVs with common trunk were seen in 33 (6.1%), two RHVs with independent drainage into IVC were seen in 9 (1.7%), 1 (0.2%) patient had three RHVs with common trunk, and 1 (0.2%) patient had three RHVs with independent drainage. Early branching of RHV was found in 79 (14.5%) patients (Fig. 3).
Schematic representation of right hepatic vein variants in our study. A Single right hepatic vein (RHV). B Early branching RHV. C Two RHV common trunks. D Two RHV independent drainage. E Three RHV common trunks. F Three RHV independent drainage. G Single accessory inferior RHV. H Two accessory inferior RHVs. I Common trunk of drainage of the middle hepatic vein (MHV) and left hepatic vein (LHV). J Independent drainage of MHV-LHV
Accessory inferior RHV
It was detected in 108 (19.8%) patients. Out of 108 patients, one and two accessory inferior RHVs were seen in 89 (16.3%) and 19 (3.5%), respectively (Fig. 3).
Middle and left hepatic veins
Common trunk of LHV-MHV was found in 418 (76.3%), of which 283 were males, 139 were females, 400 South Indians, and 16 were North Indians. Independent drainage of LHV-MHV into the IVC was found in 129 (23.7%) patients in our study (Fig. 3).
Portal vein variations
Normal branching anatomy was detected in 442 patients (81.1%) out of 545 patients in our study. Variant anatomy was seen in 103 patients (18.9%). Two-hundred ninety-six males and 146 females have normal branching anatomy. Seventy-three males and 30 females have variant portal anatomy. Trifurcation of the portal vein was seen in 88 (16.1%) of the cases. In the next common variant, there was early branching of the right posterior sectoral branch (RPPV) with the right anterior sectoral branch (RAPV) joining the LPV. In this, there were 6 (1.1%) cases where the RAPV joined LPV in the extrahepatic portion and 3 (0.6%) cases in the intrahepatic portion (Fig. 4).
Schematic representation of portal vein branching variants in our study (excluding the miscellaneous variants). Type A Normal bifurcation into right portal vein (RPV) and left portal vein (LPV). Type B Trifurcation. Type C Early branching of right posterior portal vein (RPPV) with extrahepatic bifurcation between right anterior portal vein (RAPV) and left portal vein (LPV). Type D Early branching of right posterior portal vein (RPPV) with intrahepatic bifurcation between right anterior portal vein (RAPV) and left portal vein (LPV)
The six rare miscellaneous variants found in the study population were as follows:
-
1.
Trifurcation of right portal vein in 3 (0.5%) patients (Fig. 5B, C)
-
2.
Early separate origin of the segment 7 branch from the RPV in two patients (0.4%) (Fig. 5A)
-
3.
Non-bifurcation of the RPV with small segmental branches to the right lobe was seen in 1 patient (0.2%) (Fig. 5D, E)
Rare portal vein anatomical variants found in the study. A Early separate origin of the segment 7 branch from Right portal vein (RPV). B and C Trifurcation of RPV. D and E Non-bifurcation of the RPV with small segmental branches to right lobe (arrows). (A, B, D - 3-dimensional reconstructions of contrast enhanced CTs. C, E - Maximum intensity projections of contrast enhanced CTs)
Table 2 summarizes all the hepatic vascular variants found in our study population.
Discussion
In this current study, we have analyzed a cohort of 545 contrast-enhanced CTs to look for the prevalence of variations of all three major vessels of the liver, namely hepatic artery, portal vein, and hepatic veins in the population catered by this hospital. In the study population, 67.7% were males, and 32.3% were females. Our institute is a major tertiary care teaching hospital in South India, and likewise, 96.3% of the study cohort was South Indians, and 3.7% was North Indians. The prevalence rates of the normal and anomalous variants of each of the arterial, portal venous, and venous anatomies were similar across the sex and domicile distributions (Table 3).
Hepatic artery
The conventional arterial supply or the type 1 anatomy is celiac trunk branching into the CHA (common hepatic artery), which further divides into the proper hepatic artery and the gastroduodenal artery (GDA). Originally, Michel found 55% of his study population having this “normal” conventional variant [6]. In our study, the prevalence of this type 1 variant was 69%, which means 31% of our study population had variant anatomy. Various studies around the world have found the rate of this normal variant from 55 to 79% [12,13,14,15,16,17]. The most common aberrant arterial variant detected in our study was the type 5 variant or the accessory left hepatic artery (aLHA) in 10% of the population. The “replaced” variant of the LHA (rLHA) or the type 2 variant was present in 5% of the cases. Various studies have quoted the prevalence rates of rLHA/aLHA in 2.5–10% and 1–10% cases, respectively. In a systematic review of 57 studies, evaluating the prevalence rates of the aberrant LHAs in 19,284 patients, the pooled prevalence rates of aLHA and rLHA were 5.55% and 8.26%, respectively [18]. Usually, transient liver dysfunction is reported after a rLHA ligation which normalizes within 7 days after the surgery [19]. During liver transplantation, the extrahepatic collateral pathways are absent after the new organ is implanted, and arterial supply solely depends on the reconstructed hepatic artery and occasional intrahepatic collaterals [20]. In a deceased donor liver transplantation though an aLHA can be ignored, in the presence of a rLHA, it is preferable to reconstruct using the GDA or the celiac trunk. In case of a left lobe living donor liver transplantation, identification and reconstruction of any aberrant LHA anatomy are of paramount importance, because of the lack of both intrahepatic and extrahepatic collaterals. A replaced right hepatic artery (rRHA) from SMA (type 3 variant) was the second most common anomalous variant in the study, found in 7.3% of cases. Accessory RHA (aRHA) (type 6) was found in only 1.3% of the study population. Preoperative identification and proper planning for the management of an aberrant RHA in pancreaticoduodenectomy are of utmost importance due to complications like hepatic ischemia, biliary strictures, or leakage of the bilioenteric anastomosis following an intraoperative injury [21]. Utmost precaution should be taken during Kocher maneuver, and retro pancreatic dissections as excessive retraction of the pancreatic head can injure the aberrant RHA. The aRHA/rRHA usually courses posteriorly to the cystic duct and gallbladder and can sometimes run parallel and medial to the common hepatic duct. During laparoscopic cholecystectomy, such an aberrant RHA might be encountered during dissection of the inferior border of the calot’s triangle with a risk of inadvertent injury [22].
Apart from the usual variants described in Michel’s classification, we also observed some rare miscellaneous variations of the hepatic artery. The prevalence of these miscellaneous variants is 1.8%. The rare celiacomesenteric trunk (CMT) variant was observed in 2 (0.36%) cases, in which both the celiac trunk and superior mesenteric artery arised from a single origin. In both the patients, it was of Morita’s type 1 or the classical CMT variant where all the three branches of celiac trunk and SMA have a common trunk [23]. The rich collateralization between a normal celiac trunk and SMA ensures proper blood flow and avoid visceral ischemia in cases interruption of circulation in either of the circuits. Thus, when there is a CMT with a single origin from aorta, any thrombotic event can be lethal due to full stoppage of the splanchnic arterial supply and inability of a small inferior mesenteric artery to provide collateral supply to so many organs [24]. Other rare variants noted were rRHA from the celiac trunk and aorta in 4 (0.73%) and 1 (0.18%) cases, respectively, aRHA from the celiac trunk [1 (0.18%)], rRHA from celiac trunk with aLHA [1(0.18%)], and rRHA from celiac trunk with CHA from LGA [1(0.18%)]. Though the Michel’s type 10 anomaly (CHA from LGA) has not been detected in our study, an associated variant of along with rRHA from celiac trunk was detected, as mentioned earlier.
Portal vein
The conventional normal portal vein (PV) anatomy refers to the PV running superiorly and right towards the hepatic hilum dividing into a larger right portal vein (RPV) and a relatively smaller left portal vein (LPV). Then the RPV, usually after a short course, enters the right liver and divides into right anterior portal vein (RAPV) and right posterior portal vein (RPPV). The RAPV supplies the segment 5 and 7, and the RPPV gives branches to segments 6 and 7. The LPV has a longer course than the RPV and runs laterally, supplying the segments 2 and 3 and then turning anteriorly to give branches to segment 4. The prevalence of this normal configuration of the portal vein in our study was 81.1%. Various studies have found a prevalence of 65–80% for this normal PV branching anatomy [10, 25,26,27]. The anomalous branching variants of the PV were seen in 18.9% of our study population. Trifurcation of the portal vein was the most common variation portal vein anomaly in our study, accounting for about 16.1%. The next most common type was in which the RPPV is the first branch of the main portal vein, and LPV is the terminal branch, arising after origin of RAPV, also called as the Z type anatomy. Nine (0.17%) patients in our study population had this variant. This variant is further subclassified into 2 types in the Nakamura classification — type 3 (where the RAPV-LPV branching is extrahepatic) and type 4 (where the RAPV-LPV branching is more distal intrahepatic). In our study, the type 3 variant was seen only in 1.1% and type 4 in 0.6%, which is less when compared to what was found in other studies. We did not encounter a Nakamura type 5 variant in this study. Additionally, we have detected uncommon portal vein variants in 6 cases. Out of these six variants, trifurcation of the right portal vein is seen in 3 (0.5%) cases, the early separate origin of the segment 7 branch from the RPV in two cases (0.4%), and non-bifurcation of the RPV with small segmental branches to the right lobe in 1 (0.4%). An early separate origin of the segment 7 branch of the RPV has been described as one of the major variants in most studies with a wide range of prevalence quoted — 0.1–6% [3, 13, 25, 26, 28]. We did not encounter a segment 6 branch arising as a separate branch of RPV in our study population. Variations in the left portal vein are very rare and were not encountered in this study population.
Determination of the portal venous variants are of significant importance in right lobe donation. Special attention is given to the types 2 and 3 variants resulting in two venous openings, needing complex anastomosis and venous grafting. A type 4 anatomy where there is an intraparenchymal branching of the RAPV from the LPV becomes an absolute contraindication for right lobe donation. The portal vein branching anomalies can also lead to significant difficulties and complications during a portal vein embolization (PVE) or a transjugular intrahepatic portosystemic shunt (TIPS) procedure. During PVE, trifurcation anatomy can cause difficult and unstable catheterization with more risk of migration of embolic materials, thus resulting in nontarget embolization.
Hepatic veins
Hepatic veins were classified based on the classification adopted by Sureka et al. [3], Cheng et al. [10], Soyer et al. [9], and Fang et al. [11]. The RHV is usually the largest vein and usually drains into the IVC separately. In our study, the prevalence of single RHV is found in 77.4% of cases, which is similar to international studies. Single RHV prevalence in our study was less than the study by Sureka et al. [3] and higher than the study published by Anwar et al. [15]. The commonest variant of anomalous RHV in our study population was early branching of RHV (14.5%). The prevalence of early branching RHV ranges from 8.5 to 40.2%. The prevalence of two RHV (7.8%) was similar to other studies. Prevalence of one accessory inferior RHV ranges from 6 to 27%. We detected 16.6% of one accessory inferior RHV, which is similar to the Indian study by Anwar et al. [15]. Two accessory RHVs were noted in 3.5% of our study population, which ranges from 0 to 9% in other similar studies. An accessory inferior right hepatic vein is an important variant to be identified before any liver resection or transplantation. Before transplantation, the size of this accessory inferior vein must be evaluated and its distance from the main right hepatic vein. An accessory inferior vein with > 3–5 mm cross-sectional diameter is considered to be significant as it is likely to drain a significant part of the liver, failing to preserve or re-anastomose, which can cause graft congestion leading to poor transplant outcomes. When the distance between the AIRHV (accessory inferior right hepatic vein) and the RHV is > 4 cm, it becomes difficult to implant both the AIRHV and RHV with a single partially occluding clamp on the IVC [29]. As per the existing literature, the MHV and LHV usually form a short common trunk in 65–85% that drains slightly cranial and left of the RHV [10]. LHV-MHV common trunk was found in 76.3%, and independent drainage was found in 23.7%.
This is the largest study until date from South India, studying all three hepatic vascular anatomical variations in a single study. We have also included schematic diagrams of anatomical variants and the 3-D volumetric images of the rarest variants seen in the study. The limitations of our study would include the study design — being a retrospective study and reviewing CTs from the hospital patient population might add an element of Berksonian bias. We did not look for the celiac artery variants in the study and segmental branching variants of the hepatic veins in this study, which could have added some more information.
Conclusion
Variations in the anatomy of hepatic arteries, portal veins, and hepatic veins are common. A good knowledge of the same is necessary especially for a hepatobiliary surgeon or for an interventional radiologist, to plan and avoid complications during a procedure. Preoperative MDCT scan and whenever necessary a volume rendered or a MIP image will precisely help in identifying these variations.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- 3D:
-
Three dimensional
- aLHA:
-
Accessory left hepatic artery
- aRHA:
-
Accessory right hepatic artery
- AIRHV:
-
Accessory inferior right hepatic vein
- CECT:
-
Contrast-enhanced computed tomography
- CHA:
-
Common hepatic artery
- CMT:
-
Celiacomesenteric trunk
- GDA:
-
Gastroduodenal artery
- HPB:
-
Hepatopancreaticobiliary
- IVC:
-
Inferior vena cava
- LGA:
-
Left gastric artery
- LHA:
-
Left hepatic artery
- LHV:
-
Left hepatic vein
- LPV:
-
Left portal vein
- MHV:
-
Middle hepatic vein
- MIP:
-
Maximum intensity projection
- MPR:
-
Multiplanar reconstruction
- PACS:
-
Picture archiving and communication system
- PVE:
-
Portal vein embolization
- RAPV:
-
Right anterior portal vein
- RHA:
-
Right hepatic artery
- RHV:
-
Right hepatic vein
- rLHA:
-
Replaced left hepatic artery
- RPPV:
-
Right posterior portal vein
- RPV:
-
Right portal vein
- rRHA:
-
Replaced right hepatic artery
- aRHA:
-
Accessory right hepatic artery
- SMA:
-
Superior mesenteric artery
- TACE:
-
Transarterial chemoembolization
- TIPS:
-
Transjugular intrahepatic portosystemic shunt
References
Eipel C, Abshagen K, Vollmar B (2010) Regulation of hepatic blood flow: the hepatic arterial buffer response revisited. World J Gastroenterol 16:6046–6057
Reda M, Refaat M, Abdel Aziz SZ (2021) Anatomical variations of the celiac artery detected by multidecteor computated tomography. Benha Med J. https://doi.org/10.21608/bmfj.2021.81963.1431
Sureka B, Sharma N, Khera PS, Garg PK, Yadav T (2019) Hepatic vein variations in 500 patients: surgical and radiological significance. Br J Radiol 92:20190487
Sureka B, Mittal MK, Mittal A, Sinha M, Bhambri NK, Thukral BB (2013) Variations of celiac axis, common hepatic artery and its branches in 600 patients. Indian J Radiol Imaging 23:223–233
Sureka B, Patidar Y, Bansal K, Rajesh S, Agrawal N, Arora A (2015) Portal vein variations in 1000 patients: surgical and radiological importance. Br J Radiol 88:20150326
Michels NA (1966) Newer anatomy of the liver and its variant blood supply and collateral circulation. Am J Surg 112:337–347
Nakamura T, Tanaka K, Kiuchi T et al (2002) Anatomical variations and surgical strategies in right lobe living donor liver transplantation: lessons from 120 cases. Transplantation 73:1896–1903
Cheng YF, Huang TL, Lee TY, Chen TY, Chen CL (1996) Variation of the intrahepatic portal vein; angiographic demonstration and application in living-related hepatic transplantation. Transplant Proc 28:1667–1668
Soyer P, Bluemke DA, Choti MA, Fishman EK (1995) Variations in the intrahepatic portions of the hepatic and portal veins: findings on helical CT scans during arterial portography. AJR Am J Roentgenol 164:103–108
Cheng YF, Huang TL, Chen CL, Chen TY, Huang CC, Ko SF, Lee TY (1996) Variations of the left and middle hepatic veins: application in living related hepatic transplantation. J Clin Ultrasound 24:11–16
Fang C-H, You J-H, Lau WY, Lai ECH, Fan Y-F, Zhong S-Z, Li K-X, Chen Z-X, Su Z-H, Bao S-S (2012) Anatomical variations of hepatic veins: three-dimensional computed tomography scans of 200 subjects. World J Surg 36:120–124
Zaki SM, Abdelmaksoud AHK, Khaled BEA, Abdel Kader IA (2020) Anatomical variations of hepatic artery using the multidetector computed tomography angiography. Folia Morphol (Warsz) 79:247–254
Saba L, Mallarini G (2008) Multidetector row CT angiography in the evaluation of the hepatic artery and its anatomical variants. Clin Radiol 63:312–321
Coco D, Leanza S (2019) Celiac trunk and hepatic artery variants in pancreatic and liver resection anatomy and implications in surgical practice. Open Access Maced J Med Sci 7:2563–2568
Anwar AS, Srikala J, Papalkar AS, Parveez MQ, Sharma A (2020) Study of anatomical variations of hepatic vasculature using multidetector computed tomography angiography. Surg Radiol Anat 42:1449–1457
Thangarajah A, Parthasarathy R (2016) Celiac axis, common hepatic and hepatic artery variants as evidenced on MDCT angiography in South Indian population. J Clin Diagn Res 10:TC01–TC05
Koops A, Wojciechowski B, Broering DC, Adam G, Krupski-Berdien G (2004) Anatomic variations of the hepatic arteries in 604 selective celiac and superior mesenteric angiographies. Surg Radiol Anat 26:239–244
Cirocchi R, D’Andrea V, Amato B, Renzi C, Henry BM, Tomaszewski KA, Gioia S, Lancia M, Artico M, Randolph J (2020) Aberrant left hepatic arteries arising from left gastric arteries and their clinical importance. The Surgeon 18:100–112
Okano S, Sawai K, Taniguchi H, Takahashi T (1993) Aberrant left hepatic artery arising from the left gastric artery and liver function after radical gastrectomy for gastric cancer. World J Surg 17:70–73 discussion 74
Yamamoto M, Zaima M, Yamamoto H, Harada H, Kawamura J, Yamada M, Yazawa T, Kawasoe J (2017) Liver necrosis shortly after pancreaticoduodenectomy with resection of the replaced left hepatic artery. World J Surg Oncol 15:77
Rammohan A, Palaniappan R, Pitchaimuthu A, Rajendran K, Perumal SK, Balaraman K, Ramasamy R, Sathyanesan J, Govindan M (2014) Implications of the presence of an aberrant right hepatic artery in patients undergoing pancreaticoduodenectomy. World J Gastrointest Surg 6:9–13
Polguj M, Podgórski M, Hogendorf P, Topol M (2014) Variations of the hepatobiliary vasculature including coexistence of accessory right hepatic artery with unusually arising double cystic arteries: case report and literature review. Anat Sci Int 89:195–198
Morita M (1935) Reports and conception of three anomalous cases on the area of the celiac and superior mesenteric arteries. Igaku kenkyu (Acta Med) 9:1993–2006
Boukoucha M, Yahmadi A, Znaidi H, Ben Khelifa R, Daghfous A (2020) Spontaneous celiacomesenteric trunk dissection: case report. Int J Surg Case Rep 71:128–131
Covey AM, Brody LA, Getrajdman GI, Sofocleous CT, Brown KT (2004) Incidence, patterns, and clinical relevance of variant portal vein anatomy. Am J Roentgenol 183:1055–1064
Koç Z, Oğuzkurt L, Ulusan S (2007) Portal vein variations: clinical implications and frequencies in routine abdominal multidetector CT. Diagn Interv Radiol 13:75–80
Atasoy C, Ozyürek E (2006) Prevalence and types of main and right portal vein branching variations on MDCT. AJR Am J Roentgenol 187:676–681
Sharma V, Chauhan RS, Sood RG, Makhaik S, Negi K, Chawla K, Diwan Y, Partap A, Rana S, Gupta A (2017) Study of the normal anatomy and variations of portal vein in North Indian population: a MDCT study. Eur J Anatomy 21:13–18
Erbay N, Raptopoulos V, Pomfret EA, Kamel IR, Kruskal JB (2003) Living donor liver transplantation in adults: vascular variants important in surgical planning for donors and recipients. AJR Am J Roentgenol 181:109–114
Acknowledgements
Not applicable.
Funding
The authors received no funding for this study.
Author information
Authors and Affiliations
Contributions
Concept and design, SD and PR. Data acquisition and analysis and manuscript preparation, PR and SD. Critical revision and finalizing of the manuscript, SD, KA, KN, and VPNR. All authors contributed to the conceptualization and design of the case report. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Institutional Ethics Committee of Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India (JIP/IEC/2022/027). The institutional ethics committee waived the requirement of obtaining consent for this study, as the study was a retrospective observational study involving de-identified patient data.
Consent for publication
The institutional ethics committee waived the requirement of obtaining consent for this study, as the study was a retrospective observational study involving de-identified patient data.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rajapriyan, P., Dutta, S., Nagarajan, K. et al. Prevalence of hepatic vascular anomalies in consecutive contrast-enhanced computed tomography images — a retrospective observational study. Egypt Liver Journal 12, 65 (2022). https://doi.org/10.1186/s43066-022-00225-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43066-022-00225-9