Abstract
Background
Ménière’s disease is a chronic condition of the inner ear that causes vertigo, tinnitus and hearing loss. Its diagnosis relies on clinical criteria that are subjective and pure-tone audiometry results that are not specific. Its pathological substrate is endolymphatic hydrops. Its imaging was recently made possible by the late-enhanced 3D FLAIR sequence. This technique was primarily tested on 3 T. Our objective was to prove its feasibility using a 1.5 T magnet.
Methods
We conducted a prospective study including 30 patients who fulfilled the Bárány society criteria for Ménière’s disease. We performed the late-enhanced 3D FLAIR sequence on all patients. We used it to look for and grade endolymphatic hydrops in the utricle, the saccule and the cochlear canal using the Kahn method.
Results
We found endolymphatic hydrops in all of the 30 patients who fulfilled the diagnostic criteria for Ménière’s disease. We had no false positives and only one false negative with a patient presenting with bilateral disease clinically but having endolymphatic hydrops only on one side. Thus, our correspondence rate between clinical and imaging findings was 97%.
Conclusions
It is possible to diagnose endolymphatic hydrops with the late-enhanced 3D FLAIR sequence using a 1.5 T MRI machine. Since Ménière’s disease diagnosis is sometimes tricky, imaging endolymphatic hydrops can aid in the diagnosis when the clinical picture is incomplete. It also helps guide invasive treatment plans.
Feasibility at 1.5 T ensures broader access to the late-enhanced 3D FLAIR sequence. Beyond the scope of Ménière’s disease, this sequence offers the possibility to better understand pressure-related inner ear diseases.
Similar content being viewed by others
Background
Ménière’s disease (MD) is a chronic condition of the inner ear that manifests itself through a recurring clinical triad of tinnitus, vertigo and hearing loss [1]. Its diagnosis relies mainly on clinical criteria the latest of which are the Bárány society criteria which also include pure-tone audiometry (PTA) results [2]. These criteria lack specificity and the clinical findings tend to be subjective.
It has been established since the last century that the pathological substrate for MD is endolymphatic hydrops (EH) which is the enlargement of the endolymphatic spaces within the inner ear [3].
Visualization of EH was made possible in the beginning of this century, using MRI. Most published studies in this field used late-enhanced 3D FLAIR sequences both after intratympanic and intravenous gadolinium administration [4, 5]. And most of these studies were performed on 3 T MR systems [6].
To the best of our knowledge, there were only three published studies where a 1.5 T MRI machine was used. The first study used the phase-sensitive invasion recovery (PS-IR) sequence 24 h after intratympanic gadolinium injection [7]. The second used a hybrid sequence called “3D HYDROPS” with heavily T2-weighted imaging acquired 4 h after intravenous gadolinium administration [8]. The third used the 3D FLAIR sequence 4 h after intravenous gadolinium administration [9].
Currently, 71% of MRI systems in the world have 1.5 T magnets [10], and these figures are even higher in developing countries.
The aim of our study was to show the feasibility of EH imaging techniques on a 1.5 T machine by adapting a sequence that was used on a 3 T scanner.
Methods
We conducted a prospective study, from February 2022 to December 2022. It included 30 patients who fulfilled the Bárány society criteria for MD.
Clinical and audiometric evaluation of patients was performed by an ENT doctor with a 10-year experience working with patients who suffered from vertigo.
Of the 30 patients who suffered from Ménière’s disease, 28 had a “definite” disease and 2 had a “probable” disease. Two patients had bilateral disease, and the other 28 had a unilateral disease.
All imaging was performed on a 1.5 T MRI system, SIGNA Artist (GE).
We used a 3D FSE (CUBE) FLAIR sequence with the following parameters: FOV = 180 mm; section thickness = 0.8 mm; TR = 8000 ms; TE = 142 ms; TI = 2067 ms; voxel size = 0.7*0.7*0.7; scan time = 9 min 20 s.
The main challenge at 1.5 T was the low signal-to-noise ratio (SNR). To improve it, we opted for the use of a double dose of gadolinium that was administered intravenously. We further improved SNR by using the flex coil which reduced the distance between the coil and the temporal bone. We also used a fixed flip angle for that end.
Scanning was performed 4 h after the intravenous administration of a double dose (0.2 mmol/kg) of gadoteric acid (Dotarem). This dose was administrated once, as a single injection.
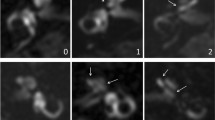
Imaging analysis was performed by two radiologists, one senior with a 10-year experience in head and neck imaging and one junior, a fourth-year resident. Both were blinded to the clinically affected side. Images were evaluated using the software Advantage WorkStation 4.2 and the “Reformat” protocol. We evaluated endolymphatic spaces in the vestibule and the cochlea using the Kahn method [11]. In the vestibule, utricular hydrops is defined by the protrusion of the utricle in the semi-circular canal. There are two grades of utricular hydrops. Grade I is when there are still perilymphatic spaces around the protruded utricle (Fig. 1). Grade II is when these spaces are no longer visible; hydrops is considered severe (Fig. 2). Likewise, an enlarged saccule but still limited by perilymphatic spaces is considered grade I saccular hydrops (Fig. 1), whereas the enlargement of the saccule that leads to the complete absence of visualization of adjacent perilymphatic spaces is considered grade II saccular hydrops (Fig. 2). The enlargement of the cochlear canal defines cochlear hydrops and there are no grades (Fig. 3).
Axial images form the 3D-FLAIR sequence of one patient, male, 64 years old, who had definite MD on the left side. The imaging shows endolymphatic hydrops in both the utricle (thin arrow) and the saccule (arrowhead) on the left, whereas the utricle (thick arrow) and saccule (empty arrowhead) on the right are not dilated. Perilymph is still visible around the dilated saccule and the protruded part in the semi-circular canal of the utricle. This is grade 1 both utricular and saccular endolymphatic hydrops
Axial images from the 3D FLAIR late-enhanced sequence from a patient, female, 59 years old, with a definite left-sided MD. Perilymphatic spaces are no longer visible around the dilated saccule (arrowhead) and the protruded utricle in the semi-circular canal (thin arrow). This is grade 2 endolymphatic hydrops in both the utricle and the saccule. In contrast, the right saccule (empty arrowhead) is non-dilated, and the right utricle (thick arrow) does not protrude into the semi-circular canal: no hydrops
Late-enhanced 3D FLAIR sequence of a patient, male, 48 years old, with a right-sided definite MD. Dilated cochlear canal appears like nodular black dots in the cochlea (thick arrows) that are much more prominent than a normal cochlear canal (thin arrow). Notice the dilated saccule with a preserved perilymphatic space surrounding it (arrowhead) on the right side: saccular hydrops grade 1. The left saccule is not dilated (empty arrowhead)
We obtained approval from the ethics committee of our hospital under the reference number CEBM.EPS.AR/10 IM/2022. All patients were informed of the experimental nature of the imaging being done and signed consent forms.
Results
All but one clinically affected ear showed endolymphatic hydrops in the vestibule or the cochlea, or both (Figs. 1, 2 and 3).
The only false negative was a patient who presented with a clinical bilateral disease but showed hydrops only on one side on imaging.
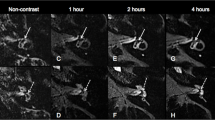
One of our patients, patient number 8, was initially diagnosed as having a unilateral definite disease on the left side. In his case, imaging showed endolymphatic hydrops in the saccule contralateral to the clinically affected ear (Fig. 4). This was initially considered to be a false positive. But during the follow-up, the bilaterality of the disease was clinically confirmed. In fact, two weeks after the MRI was performed, he presented with tinnitus in the right ear and pure-tone audiometry confirmed neurosensorial hearing loss in that same ear. He was thus correctly diagnosed as having bilateral Ménière’s disease. Therefore, we were able to diagnose bilateral disease on imaging prior to its clinical manifestation in this patient and we had no false positives.
Axial images from the late-enhanced 3D FLAIR sequence of patient N°8, male, 46 years old. Patient initially presented with unilateral left-sided MD but two weeks after imaging, presented with symptoms on the right side. Bilaterality is already visible on this image with: on the left side, a protruded utricle in the semi-circular canal (thin arrow) with no perilymph around it (grade 2), a dilated saccule (arrowhead) with no visible perilymph around it (grade 2), a dilated cochlear canal (thick arrow). On the right side, the saccule is dilated (empty arrowhead), but there is still perilymph around it (grade 1). The utricle (double arrow) and the cochlear canal (empty arrow) appear normal
Thus, the final correspondence rate between clinical findings and imaging findings was 97% with only one false negative out of 33 clinically affected ears.
Clinical and demographic data as well as endolymphatic hydrops grading are summarized in Table 1.
Discussion
Visualization of endolymphatic hydrops (EH) in vivo is one of the most clinically significant advances in head and neck imaging of the past decade. EH has been known for quite some time and was constantly found in autopsies performed in patients who had MD when they were alive [3]. The MRI techniques that allowed the imaging of EH were primarily tested on 3 T MR systems [4]. Recently, a few reports were published on the feasibility of these techniques at 1.5 T and that was the aim of our study: to prove that it is possible to visualize EH using the late-enhanced 3D FLAIR sequence on a 1.5 T MR system [8, 9].
We were able to visualize endolymphatic spaces and their enlargement in patients with MD using the late-enhanced 3D FLAIR sequence on a 1.5 T MR system.
We used the latest method for positive diagnosis and grading of endolymphatic hydrops to have the best possible accuracy.
Our false negative ratio was 1 in 33 clinically affected ears, so 3%. It is less than results on 3 T machine where false negative ratios are about 10% [9]. But this discrepancy can be explained by a smaller sample size.
In the other published studies about endolymphatic hydrops imaging on 1.5 T machines, Grieve et al. [7], using a 2D sequence and intratympanic gadolinium administration, had a false negative ratio of 15%. Naganawa et al. [8], using a hybrid 3D sequence and intravenous administration of a single dose of gadolinium, had no false negatives in their series. Kenis et al. [9] had the closest protocol to the one we used: a 3D FLAIR sequence and a double dose of gadolinium administrated intravenously, had a 11% false negative ratio. Table 2 offers a summary of the protocols of the three previously published studies on endolymphatic hydrops imaging at 1.5 T as well as our protocol.
In all three studies previously published on 1.5 T hydrops imaging, there was not an instance reported like patient N°8 where initial clinical evaluation concluded to a unilateral disease and imaging made the diagnosis of bilaterality prior to the clinical symptoms appearing on the opposite side. In previously published studies, endolymphatic hydrops in contralateral ears was found among 10% [12] to 22% [13] of cases. It should be noted that in both instances, clinical follow-up and eventual confirmation of bilaterality was not indicated. In clinical studies, bilaterality occurred in 35% of MD cases in a 10-year follow-up [12]. Detecting bilaterality early and pre-clinically can be very useful since a bilateral disease contradicts the use of radical treatments like gentamycin injections, labyrinthectomy and vestibular neurectomy [14].
We found imaging quality on 1.5 T to be comparable to the results obtained using 3 T magnets. The main difference is in the cochlear hydrops. Considering that in the pathophysiology of Ménière’s disease, endolymphatic hydrops starts in the cochlea and that both in our study and in other studies where 1.5 T MR systems were used, vestibular hydrops was found more often than cochlear hydrops, it might be possible that minimal cochlear hydrops is better seen on 3 T imaging. This needs to be confronted to the results of wider studies both in terms of number of patients and of grading methods used.
Our use of a double dose of gadolinium was inspired by our experience with cardiac MR on 1.5 T systems. It is expected that imaging at 1.5 T has lower signal-to-noise ratio than 3 T. But, in the study published by Naganawa et al. [8], a single dose was used. It should be noted that the sequence was also different, it was a hybrid sequence, and not the more conventional 3D FLAIR FSE used more widely on 3 T systems. Kenis et al. [9], who used the same sequence as our study, also administrated a double dose of gadolinium. With the emerging findings on both gadolinium retention in the brain and its toxicity, studies comparing perilymphatic enhancement after both a single and a double dose of gadolinium on 1.5 T are needed in order to confirm the reliability of the administration of a single dose.
One of the main limits of our study is that we did not directly compare the results obtained on a 1.5 T MR system and a 3 T MR system for the same patients.
Our 1.5 T 3D FLAIR sequence takes about 9 min which is similar to scanning time on 3 T. It is also considerably shorter than the times recorded in the previous studies on 1.5 T, where scanning time ranged from 14 to 45 min (Table 2).
Our findings will have a positive impact on both patient care and workflow management. This will enable centers where only 1.5 T MR systems are available to perform this imaging technique that can have a tangible impact on both diagnosis and treatment plans. And in cases where both 1.5 T and 3 T are available, it can be used to ease the workload on the 3 T scanner.
Conclusions
It is possible to diagnose endolymphatic hydrops using the late-enhanced 3D FLAIR sequence on a 1.5 T MR system with reliable results and good imaging quality. In the short run, this will improve patient care by ensuring broader access to this powerful imaging technique. In the long run, it will improve our understanding of the pathophysiology of inner ear pressure diseases by widening the study pool and allowing more research to be done.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Abbreviations
- EH:
-
Endolymphatic hydrops
- FATSAT:
-
Fat saturation
- FLAIR:
-
Fluid attenuation inversion recovery
- FOV:
-
Field of view
- MD:
-
Ménière’s disease
- MR:
-
Magnetic resonance
- MRI:
-
Magnetic resonance imaging
- PS-IR:
-
Phase-sensitive inversion recovery
- PTA:
-
Pure-tone audiometry
- TE:
-
Echo time
- TI:
-
Inversion time
- TR:
-
Repetition time
References
Committee on Hearing and Equilibrium Guidelines for the Diagnosis and Evaluation of Therapy in Meniere’s Disease (1995) Otolaryngol Head Neck Surg 113:181–185. https://doi.org/10.1016/S0194-5998(95)70102-8
Lopez-Escamez JA, Carey J, Chung W-H, Goebel JA, Magnusson M, Mandalà M, Newman-Toker DE, Strupp M, Suzuki M, Trabalzini F, Bisdorff A, Classification Committee of the Barany Society, Japan Society for Equilibrium Research, European Academy of Otology and Neurotology (EAONO), Equilibrium Committee of the American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS), Korean Balance Society (2015) Diagnostic criteria for Menière’s disease. J Vestib Res 25:1–7. https://doi.org/10.3233/VES-150549
Merchant SN, Adams JC, Nadol JBJ (2005) Pathophysiology of Ménière’s syndrome: Are symptoms caused by endolymphatic hydrops? Otol Neurotol 26:74
Nakashima T, Naganawa S, Sugiura M, Teranishi M, Sone M, Hayashi H, Nakata S, Katayama N, Ishida IM (2007) Visualization of endolymphatic hydrops in patients with Meniere’s disease. Laryngoscope 117:415–420
Nakashima T, Naganawa S, Pyykkö I, Gibson WP, Sone M, Nakata S, Teranishi M (2009) Grading of endolymphatic hydrops using magnetic resonance imaging. Acta Otolaryngol 129:5–8
Bernaerts A, De Foer B (2019) Imaging of Ménière disease. Neuroimaging. Clinics 29:19–28
Grieve SM, Obholzer R, Malitz N, Gibson WP, Parker GD (2012) Imaging of endolymphatic hydrops in Meniere’s disease at 1.5 T using phase-sensitive inversion recovery:(1) demonstration of feasibility and (2) overcoming the limitations of variable gadolinium absorption. Eur J Radiol 81:331–338
Naganawa S, Yamazaki M, Kawai H, Bokura K, Sone M, Nakashima T (2013) Visualization of endolymphatic hydrops in Ménière’s disease after intravenous administration of single-dose gadodiamide at 1.5 T. Magn Reson Med Sci 12:137–139
Kenis C, Crins T, Bernaerts A, Casselman J, Foer BD (2022) Diagnosis of Menière’s disease on MRI: feasibility at 1.5 Tesla. Acta Radiol 63:810–813
Magnetic Resonance Imaging (MRI) Equipment Market Size, Share & Industry Analysis, By Strength. https://www.fortunebusinessinsights.com/industry-reports/magnetic-resonance-imaging-mri-equipment-market-100087. Accessed 21 Mar 2023.
Kahn L, Hautefort C, Guichard J-P, Toupet M, Jourdaine C, Vitaux H, Herman P, Kania R, Houdart E, Attyé A, Eliezer M (2020) Relationship between video head impulse test, ocular and cervical vestibular evoked myogenic potentials, and compartmental magnetic resonance imaging classification in menière’s disease. Laryngoscope 130:E444–E452. https://doi.org/10.1002/lary.28362
Huppert D, Strupp M, Brandt T (2010) Long-term course of Menière’s disease revisited. Acta Otolaryngol 130:644–651. https://doi.org/10.3109/00016480903382808
Baráth K, Schuknecht B, Naldi AM, Schrepfer T, Bockisch CJ, Hegemann SCA (2014) Detection and grading of endolymphatic hydrops in Menière disease using MR imaging. AJNR Am J Neuroradiol 35:1387–1392. https://doi.org/10.3174/ajnr.A3856
de Pont LMH, van Steekelenburg JM, Verbist BM, van Buchem MA, Blom HM, Hammer S (2020) State of the art imaging in Menière’s disease. Tips and tricks for protocol and interpretation. Curr Radiol Rep 8:25. https://doi.org/10.1007/s40134-020-00365-z
Acknowledgements
The authors would like to thank the MR technicians at the Radiology Department of La Rabta teaching Hospital for their contribution to this work.
Funding
The authors did not receive any external funding to conduct the research.
Author information
Authors and Affiliations
Contributions
ABL interpreted imaging, collected patient data and imaging data and was a major contributor in the writing of the manuscript. SB interpreted imaging and was a major contributor in the writing of the manuscript. RB performed clinical evaluation and pure-tone audiometry for the patients included in this study and contributed to the writing of the manuscript. SM also participated in both clinical and functional evaluation of patients included in this study and participated in the writing of the manuscript. HM helped solve discrepancies in imaging interpretation and gave major input on the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
We obtained approval from the ethics board of our institution “La Rabta Teaching Hospital” under the reference “CEBM.EPS.HR/10 TM/2022.” All participants signed consent forms in Arabic/French prior to undergoing the imaging.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ben Lakhal, A., Boukriba, S., Bechraoui, R. et al. MR imaging of endolymphatic hydrops in Ménière’s disease: feasibility at 1.5 T. Egypt J Radiol Nucl Med 55, 133 (2024). https://doi.org/10.1186/s43055-024-01309-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-024-01309-9