Abstract
Background
Full-field digital mammography (FFDM) is the primary screening method for breast cancer, yet the number of cancers that can be missed with mammography is considerable, notably in female with dense breast. In this study, we compared the diagnostic yield and the clinical significance of FFDM for breast cancer detection in female with dense breasts versus its performance when complemented by automated breast ultrasound (ABUS).
Results
This retrospective study was performed during the period between January 2022 and December 2022 including 500 females with dense breast (ACR C&D), who underwent screening using FFDM and ABUS. The images were retrospectively interpreted, and statistical assessments were done comparing the FFDM results alone and after complemented with ABUS. Significance was considered at a p value less than 0.05. The use of FFDM with supplemental ABUS has reduced the numbers of recall and showed improved breast cancer detection with increased positive predictive value (from 74.5 to 83.5%). In comparison, using FFDM alone and associated with ABUS, there was moderate agreement with a kappa test of 0.51; p < 0.001.
Conclusion
ABUS can be a useful and powerful diagnostic imaging tool when adjunct to FFDM for screening of dense breast. In this study, ABUS showed less false-negative results and improved the sensitivity of cancer detection.
Similar content being viewed by others
Background
In spite of the advancement in identifying of breast cancer-related risk factors and genetic markers, approximately 70% of women developing breast cancer show no major predictors [1]. Therefore, the prime strategy for breast cancer early diagnosis and reducing its mortality is the screening [2, 3].
Mammography is considered the primary breast cancer screening method [4, 5] that has significantly reducing breast cancer mortality. Yet in dense breast mammographic screening sensitivity is reduced to 30–48% [6]. The odds for interval cancers diagnosis in dense breasts was about 17.6-fold higher compared with fatty breasts [6,7,8,9].
Mammography is a summation of images, resulting in overlying of all breast tissues in each view that may obscuring masses, which subsequently reduce the mammography performance in dense breasts [10]. Posterior cancer locating far at the retromammary space can also be missed by mammography [11].
That is why The American Cancer Society (ACS) has recently recommended screening MRI for young females with high risk for developing breast cancer [12, 13]. MRI improves the sensitivity of early cancer detection; however, it shows the disadvantages of being costly and risks of its contrast media [14, 15]. Also, breast cancer screening using MRI has shown lower sensitivity and higher rate of false-positive results compared to mammography, which requiring further follow-up and/or biopsy [16,17,18].
MRI also showed lower specificity (82%) compared to mammography (94%) as it increases the recall rate four times compared to mammography and about 70% of these recalls proved to be negative for cancer [19, 20].
Ultrasound is a valuable supplemental tool to mammography due to its wide availability and relatively low cost, and it is well-tolerated by patients [20, 21]. It is also used for scanning all breast parenchyma till the chest wall without tissue overlap. Early studies showed promising results using complementary high-resolution ultrasound for screening [22,23,24,25,26,27,28], but handheld imaging always requires time and expertise for small mass detection, which has discouraged its widespread use [29].
The FDA (Food and Drug Administration) approved automated breast ultrasound as a complementary tool for breast cancer screening in 2012. It shows the advantage of creation of standardized image sets obtained by less experienced personnel and also allows more efficient, time-saving interpretation by physicians. Previous studies also supported the usage of 3D AWBU for breast cancer screening [29,30,31,32].
This study aimed to determine whether there is improvement of the screening mammography diagnostic accuracy when complemented by AWBU.
Methods
Study design and population
From January 2022 to December 2022, women with BI-ACR density D or C (heterogeneously or extremely radiographically dense breasts) that underwent ABUS examinations at presentation for routine mammography were retrieved from the database. The examinations included were acquired for screening purposes.
A consent was approved by the academic research department of the hospital. It specifically clarified that ABUS is a procedure used as a complement for mammography and not replacing it.
FFDM was performed combined with DBT as a routine protocol for our department for women with dense breast (ACR C&D). ABUS and HHUS (handheld ultrasound) were performed independently in the same session.
Breast density was classified by two radiologists based on Breast Imaging-Reporting and Data System (BI-RADS) and classified into: almost fatty (A); have scattered fibroglandular densities (B); heterogeneously dense (C) or be extremely dense (D).
A total of 500 mammograms with ACR C&D and AWBU examinations are enrolled in the study. Patients with BIRDS 3 (requiring short-term follow-up) are excluded from the study.
Image acquisition
Mammography was obtained for each breast in the mediolateral oblique and craniocaudal views using GE machine.
The ABUS was performed with an Invenia System 2.0. Images were obtained by a trained radiographer, to ensure the examination was done correctly. The patients lie in the supine position raising the arm up.
Anteroposterior, medial and lateral views are obtained; additional superior and inferior views were acquired in large breasts.
HHUS was performed by two radiologists using a GE LOGIQ 9 machine.
Image review
Independent and sequential interpretation of all examinations was made by two radiologists for 15 years and get a specific training on the Invenia 2.0 ABUS system.
AWBU system is a computer-aided unit used to perform and record whole breast ultrasound images (SonoCine, Reno, NV). These images are acquired by multi-frequency transducers with at least 7–12 mHz range. A computer-guided mechanical arm is attached to the transducer and images acquired with 7–10 mm overlap to ensure whole breast scan. The surface of the transducers measured about 5.2 cm in more than 95% of the studies so the width of the rows was about 4.2–4.5 cm without the overlap, and for each breast, the number of rows varied from 4 to 7. The scans were performed by trained ultrasound technologists that maintain skin vertical orientation and good contact pressure. Speed and position of the transducer are controlled by a mechanical arm. The images are demonstrated immediately on the monitor of the ABUS system with about 150 to 300 images displayed per row and then stored. Imaging time for each participant was about 10–20 min with an additional 5–10 min for patient preparation.
A cine images are created by ABUS software containing about 2000–5000 images based on the size of the breast (average: 3000 images). The interpretation and lesions detection are enhanced by reviewing the cine images.
Using spatial registration recorded as images acquired any point on an image can be described as a distance from the nipple in a specific radius. Then, image revision is performed using a high-resolution monitor to optimize image review allowing image size compression, three-dimensional reconstruction and adjustment of brightness, contrast and the speed of review.
Breast Imaging-Reporting & Data System (BI-RADS) assigns one of six assessment categories (0: incomplete, needs additional assessment; 1: negative; 2: benign findings; 3: probably benign; 4: suspicious for malignancy; 5: highly suggestive of malignancy) [33, 34], which was used for image interpretation.
Reference standard
Our reference standard was histopathological result for the lesions that required biopsy and the typical benign features of other non-biopsied lesions based on the BI-RADS calcifications (cysts, intramammary lymph node).
Statistical analysis
Collection, tabulation and analysis of data were performed by the use of SPSS (Statistical Package for Social Science) version 20.0 on IBM compatible computer (SPSS Inc., Chicago, IL, USA).
Categorical data were described as number and percentage and compared by Z test and Chi-square test accordingly. The agreement in the results of the two imaging modalities was assessed using Kappa agreement analysis (0.01–0.2: slight agreement, 0.21–0.4: fair agreement, 0.41–0.6: moderate agreement, 0.61–0.8: substantial agreement and 0.81–0.99: almost perfect agreement). Positive predictive value was calculated for each modality (as the ratio of patients truly diagnosed as positive to all those who had positive test results). There is significance when p value < 0.05.
Results
Study population
Five hundred women with dense breast (ACR category C&D) were included in this study with an average age of 48.45 ± 7.06 and ranged from 40 to 60 years.
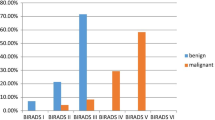
The total number of recalls (BI-RADS 0 + 4 + 5) based on FFDM mammography alone was 202 (40.4%), which had been reduced to 131 (26.2%) after the supplementation of ABUS. The number of cases with BI-RADS 0 deferrers as shown in Table 1. Using ABUS images as a complement to FFDMs, only 10 patients needed additional imaging evaluation. These cases show non-specific thickened skin complex with edema caused by systemic illness with diffuse body anasarca, which totally regress after 1-month follow-up (Table 2).
While the recommended biopsies (BI-RADS 4 + 5) were 141 based on FFDM alone and reduced to 121 suspicious lesions with 111 (22.2%) patients had been confirmed malignant breast lesions (Figs. 1, 2, 3, 4, and 5) by ultrasound-guided biopsy (invasive ductal carcinomas, no special type; forty luminal A subtypes, and seventy-one luminal B subtypes).
Female patient of 54y old. Dense breast (ACR class c) with an irregular speculated dense mass was seen at the right upper inner quadrant on mammography (arrows A, B). On ABUS (C), an irregular hypoechoic mass with posterior shadowing was seen at 1 O`clock which was seen on HHUS (D). This was confirmed invasive ductal carcinoma
Female patient of 35y old of high-risk breast ca. heterogeneous dense breast (ACR class d) showed two irregular speculated dense mass that were seen at the left upper inner quadrant and the deep central breast on mammography (arrows A, B). On ABUS (C, D), two irregular hypoechoic mass with posterior shadowing was seen at 2 O`clock & 6 O`clock, which was seen on HHUS (E–G). This was confirmed multifocal invasive ductal carcinoma
Female patient of 40y old. Dense breast (ACR class c) with lobulated dense mass was seen at the right upper inner quadrant on mammography (arrows A, B). On ABUS (C, D), lobulated hypoechoic mass with posterior shadowing was seen at 2 O`clock, which was seen on HHUS (E, F). This was confirmed invasive ductal carcinoma
Female patient of 57y old. Dense breast (ACR class c) with irregular speculated dense mass with pleomorphic microcalcifications was seen at the right upper outer quadrant on mammography (arrows A, B) with rounded axillary lymph node. On ABUS (C, D), a large irregular hypoechoic mass with posterior shadowing was seen at 9 O`clock (c) with pathological rounded axillary lymph node showing loss of normal architecture (D), HHUS (E, F) showed the mass with high vascularity and pathological axillary lymph node. This was confirmed invasive ductal carcinoma with malignant lymph nodes
Female patient of 51y old. Heterogeneously dense breast (ACR class d) with large lobulated dense mass was seen involving the whole left breast on mammography (A, B). On ABUS (C, D), a large complex mass with cystic and soft tissue components and thick septation. HHUS (E, F) was done. This was confirmed phyllodes tumor
Table 3 demonstrates the diagnostic performance of FFDM and FFDM + ABUS in the form of positive predictive value.
Table 4 shows moderate agreement between FFDM and FFDM complemented with ABUS.
Discussion
Screening mammography for breast cancer detection consumes much time and considers a challenging task owing to the large numbers of examinations and low cancer yield [5]. Females with dense breast show the greatest percentage of female with intermediate risk and show up to 20% risk to develop cancer. The use of an additional supplemental imaging modality is currently recommended for screening of dense breast to reduce the number of occult cancer that can be missed by mammography [6]. Complementary ultrasound has showed a substantial improvement in the diagnosis of cancer breast, which were missed on mammography in female having dense breasts with or with increased risk for cancer breast [11, 13, 14].
Our study compared FFDM diagnostic performance for screening when used alone and its performance if combined with ABUS in female with dense breast. Our results clarify that the implantation of ABUS with FFDM significantly improved the readers’ ability to identify true-negative and true-positive cancers in dense breasts.
Friedewald et al. [10] found that supplementing FFDM with ABUS improved the rate of breast cancer detection from 2.9/1000 to 4.1/1000 along the screened patients without significant modification in the diagnosis of cases with ductal carcinoma in situ (DCIS), signifying that the use of supplemental ABUS may show more clinical significance in breast cancer detection.
Our study showed that the use of mammography combined with ABUS increased positive predictive value for cancer detection from 74.5 to 83.5%. This is similar to the results of another earlier study adding handheld ultrasound as complementary for mammography (sensitivity 87.8%) [23].
However, the use of AWBU has some advantages compared to the handheld ultrasound: (1) it is reproducible, including the whole breasts; (2) it has higher image definition, sharpness and contrast; (3) it provides smaller images for revision by the use of a high-resolution 2,000-line reading monitor 3D; (4) it optimizes the reading environment by allowing delayed interpretation at monitor-based computer stations with non-real-time review. Other previous studies [11, 35] showed higher sensitivity (about 95%) similar to the MRI sensitivity, but with high difference in cost.
Waldherr et al. [13] described comparable sensitivity of FFDM + ABUS (91.9%), but a higher specificity of 90.5% (our study showed 71.97% specificity). A prospective study including 7292 asymptomatic female by Houssami et al. [12] with aged over 48 years and an average risk showed 85% sensitivity and 97% specificity. Another study done on 113 females showed different results by identifying 119 breast lesions with the three readers (with 8–14 years’ experience); average sensitivity and specificity were 97.3 and 44.7%, respectively [16].
Additional analytic data were performed by Rafferty et al. [11], showing that the use of complementary ABUS improved the detection rate of breast cancer in female with heterogeneously or extremely dense breasts and reducing the rate of recall.
Our study reported similar results and clarified that complementary ABUS significantly reduced the recall rate from 40.4 to 26.2%. The interpreted BI-RADS 0 also was significantly reduced from 60 by the use of FFDM alone to only 10 by the use of supplemental ABUS. Twenty of them were scars for previous benign lesion excisions, 40 cases with apparent focal asymmetries and small lesion with slight angulated borders that are demonstrated as simple cysts by ABUS.
Our findings are also comparable to previous studies [14, 16, 19] that using complementary screening ultrasound that resulted in the diagnosis of additional cancers that were missed by the use of mammography alone. Through our study, 10 cases with small lesions (range 4–8 mm) were missed along the FFDM, which appeared normal and reported as BI-RADS 1 while breast scanning using ABUS detects small specious lesions upgrading the diagnosis to BI-RADS 4 (Fig. 6) and 6 of these cases proved by invasive ductal carcinoma by histopathology.
Female patient of 44y old. Dense breast (ACR class c) with normal mammography (arrows A, B). On ABUS (C–E), there was focal retroareolar dilated duct with echogenic content (C) and scattered anechoic cysts (D, E). HHUS (F, G) showed the dilated duct (F) and anechoic cyst (G). MRI (H) showed small enhancing intraductal lesion (arrow). This was confirmed intraductal papilloma
Previous study [26, 31] similarly showed that the supplemental ABUS could lead to detection of additional small cancers that were not be detected by DBT (not seen even retrospectively), but unlike our study they showed that supplemental ABUS was associated with increased recall rate.
Other studies [26, 28] used the ABUS as an individual screening modality and compared it to mammography and also showed increased the recalls number for performing additional imaging or handheld ultrasound, which considered one of the drawbacks of this technology. This can be explained by the higher detection rate of cancer when using supplemental ABUS compared with mammography and by the availability of comparison of ABUS examinations for only 31% of the studies. Almost all mammograms also had previous studies for comparison. These studies also showed similar PPV of the recommended biopsy based on ABUS compared with mammography findings so although the ABUS can increase the recall rate, it does not increase the rate of false-positive biopsies.
The number of false-positive cases after the use of FFDM + ABUS in our study was 10 cases of BI-RADS 4 (which are proved by ultrasound-guided biopsies as 3 cases focal adenosis, 5 cases atypical fibroadenomas and 2 cases granulomatous mastitis).
Previous study [19] showed substantial agreement of HHUS compared to ABUS (k = 0.76 ± 0.14) for lesions characterization (regarding: outline, border, parallel or not, echotexture, acoustic features, calcifications and associated features). Lesions orientation was the most concordant ones (parallel or not parallel to the skin, k = 0.61 ± 0.23), while the presence of posterior shadowing was the most discordant features (k = 0.35 ± 0.26). Golatta et al. [27] also showed that a study performed on 913 women had a good agreement comparing the two ultrasound methods, k = 0.31 (95% CI [0.27; 0.35]).
Our results reported 83.5% PPV and that was significantly higher than the 41% PPV yielded by the ACRIN Trial [32] for the biopsy prompted by the handheld ultrasound, and the 63% PPV showed by the breast cancer surveillance consortium (BCSC) report [34] for the ABUS. This may be because BI-RADS 3 lesions that need long-term follow-up were initially excluded from our study and the final interpretation for woman with suspicious findings was based on the mammography complemented by ABUS and these women were treated as recall patients. The recalled female that were not assigned for biopsy was interpreted as BI-RADS 1 (negative) or 2 (benign findings).
ABUS was well accepted by participants and easily used in the breast imaging. Effective screening is more beneficial for female with high breast cancer risk and does not matching the criteria of ACS for annual MRI than mammography alone. Because of the easy use and low-cost AWBU, it become a good alternative modality to MRI for female with dense breasts or has risk factors like personal or family breast cancer history. Compared to mammography, less breast compression, lack of ionizing radiation exposure and no need to contrast medium make ABUS well tolerated by the participants.
Our limitation in this study was lacking long-term follow-up; previous studies show about 42% of cancers diagnosed clinically before the time of next screening and are visible retrospectively on previous ABUS scanning. It is postulated that improving the experience of readers can increase cancer detection. In addition, the availability of previous studies for comparison may lead to better sensitivity, fewer recalls and reducing the number of AWBU “missed” cancers.
Conclusions
We concluded that supplemental ABUS to FFDM in female having dense breasts may improve the diagnostic accuracy with detection of more cancers not visible on FFDM even retrospectively.
Availability of data and materials
All data and material are available.
Abbreviations
- FFDM:
-
Full-field digital mammography
- ABUS:
-
Automated breast ultrasound
- ACR:
-
American College of Radiology
- ACS:
-
The American Cancer Society
- AWBU:
-
Automated whole breast ultrasound
- BI-RADS:
-
Breast Imaging-Reporting and Data System
- HHUS:
-
Handheld ultrasound
- DBT:
-
Digital breast tomosynthesis
- MLO:
-
Mediolateral oblique
- CC:
-
Craniocaudal
- FDA:
-
Food and Drug Administration
- PPV:
-
Positive predictive value
- BCSC:
-
Breast Cancer Surveillance Consortium
- DCIS:
-
Ductal carcinoma in situ
- MRI:
-
Magnetic resonance imaging
References
Sardanelli F, Fallenberg EM, Clauser P et al (2017) Mammography: an update of the EUSOBI recommendations on information for women. Insights Imaging 8:11–18
Lip G, Zakharova N, Duffy S et al (2010) Breast density as a predictor of breast cancer risk. Breast Cancer Res 12:P1
Weigert J, Steenbergen S (2012) The connecticut experiment: the role of ultrasound in the screening of women with dense breasts. Breast J 18:517–522
Berg WA, Zhang Z, Lehrer D et al (2012) Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA J Am Med Assoc 307:1394–1404
Sung JS, Dershaw DD (2013) Breast magnetic resonance imaging for screening high-risk women. Magn Reson Imaging Clin N Am 21:509–517
Girometti R, Tomkova L, Cereser L et al (2018) Breast cancer staging: Combined digital breast tomosynthesis and automated breast ultrasound versus magnetic resonance imaging. Eur J Radiol 107:188–195
Rella R, Belli P, Giuliani M et al (2018) Automated breast ultrasonography (ABUS) in the screening and diagnostic setting: indications and practical use. Acad Radiol 25:1457–1470
Brem RF, Tabár L, Duffy SW et al (2015) Assessing improvement in detection of breast cancer with three-dimensional automated breast US in women with dense breast tissue: the somoinsight study. Radiology 274:663–673
Niu L, Bao L, Zhu L et al (2019) Diagnostic performance of automated breast ultrasound in differentiating benign and malignant breast masses in asymptomatic women: a comparison study with handheld ultrasound. J Ultrasound Med 38:2871–2880
Friedewald SM, Rafferty EA, Rose SL et al (2014) Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA J Am Med Assoc 311:2499–2507
Rafferty EA, Durand MA, Conant EF et al (2016) Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA J Am Med Assoc 315:1784–1786
Houssami N, Macaskill P, Bernardi D et al (2014) Breast screening using 2D-mammography or integrating digital breast tomosynthesis (3D-mammography) for single-reading or double-reading—evidence to guide future screening strategies. Eur J Cancer 50:1799–1807
Waldherr C, Cerny P, Altermatt HJ et al (2013) Value of one-view breast tomosynthesis versus two-view mammography in diagnostic workup of women with clinical signs and symptoms and in women recalled from screening. Am J Roentgenol 200:226–231
Hooley RJ, Greenberg KL, Stackhouse RM et al (2012) Screening US in patients with mammographically dense breasts: initial experience with Connecticut Public Act 09–41. Radiology 265:59–69
Zuley ML, Bandos AI, Ganott MA et al (2013) Digital breast tomosynthesis versus supplemental diagnostic mammographic views for evaluation of noncalcified breast lesions. Radiology 266:89–95
Hakim CM, Chough DM, Ganott MA et al (2010) Digital breast tomosynthesis in the diagnostic environment: a subjective side-by-side review. Am J Roentgenol 195:W172–W176
Kim SA, Chang JM, Cho N et al (2015) Characterization of breast lesions: comparison of digital breast tomosynthesis and ultrasonography. Korean J Radiol 16:229–238
Wallis MG, Moa E, Zanca F et al (2012) Two-view and single-view tomosynthesis versus full-field digital mammography: high-resolution X-ray imaging observer study. Radiology 262:788–796
Shin SU, Chang JM, Bae MS et al (2014) Comparative evaluation of average glandular dose and breast cancer detection between single-view digital breast tomosynthesis (DBT) plus single-view digital mammography (DM) and two-view DM: correlation with breast thickness and density. Eur Radiol 25:1–8
Kim H, Cha JH, Oh H-Y et al (2014) Comparison of conventional and automated breast volume ultrasound in the description and characterization of solid breast masses based on BI-RADS features. Breast Cancer 21:423–428
Skaane P, Bandos AI, Gullien R et al (2013) Prospective trial comparing full-field digital mammography (FFDM) versus combined FFDM and tomosynthesis in a population-based screening program using independent double reading with arbitration. Eur Radiol 23:2061–2071
Svahn T, Houssami N, Sechopoulos I et al (2015) Review of radiation dose estimates in digital breast tomosynthesis relative to those in two-view full-field digital mammography. Breast 24:93–99
Dang PA, Freer P, Humphrey KL et al (2014) Addition of tomosynthesis to conventional digital mammography: effect on image interpretation time of screening examinations. Radiology 270:49–56
Vourtsis A, Berg WA (2019) Breast density implications and supplemental screening. Eur Radiol 29:1762–1777
Kotsianos-Hermle D, Wirth S, Fischer T et al (2009) First clinical use of a standardized three-dimensional ultrasound for breast imaging. Eur J Radiol 71:102–108
Chang JM, Moon WK, Cho N et al (2011) Performance in the detection of benign and malignant masses with 3D automated breast ultrasound (ABUS). Eur J Radiol 78:99–103
Golatta M, Baggs C, Schweitzer-Martin M et al (2014) Evaluation of an automated breast 3D-ultrasound system by comparing it with hand-held ultrasound (HHUS) and mammography. Arch Gynecol Obstet 291:889–895
Tailored BW (2009) Supplemental screening for breast cancer: What now and what next? Am J Roentgenol 192:390–399
Wilczek B, Wilczek HE, Rasouliyan L et al (2016) adding 3D automated breast ultrasound to mammography screening in women with heterogeneously and extremely dense breasts: report from a hospital-based, high-volume, single-center breast cancer screening program. Eur J Radiol 85:1554–1563
Kelly KM, Dean J, Lee S-J et al (2010) Breast cancer detection: Radiologists’ performance using mammography with and without automated whole-breast ultrasound. Eur Radiol 20:2557–2564
Giuliano V, Giuliano C (2013) Improved breast cancer detection in asymptomatic women using 3D-automated breast ultra-sound in mammographically dense breasts. Clin Imaging 37:480–486
Kopans DB (1999) Breast cancer screening with ultrasonography. Lancet 354:2096–2097
Skaane P, Gullien R, Eben EB et al (2015) Interpretation of automated breast ultrasound (ABUS) with and without knowledge of mammography: a reader performance study. Acta Radiol 56:404–412
Weaver DL, Rosenberg RD, Barlow WE et al (2006) Pathologic findings from the breast cancer surveillance consortium: population-based outcomes in women undergoing biopsy after screening mammography. Cancer 106:732–742
Giger ML, Inciardi MF, Edwards A et al (2016) Automated breast ultrasound in breast cancer screening of women with dense breasts: reader study of mammography-negative and mammography-positive cancers. Am J Roentgenol 206:1341–1350
Acknowledgements
We would like to thank all people who helped us in this work including the clinicians and technicians.
Funding
Self-funding.
Author information
Authors and Affiliations
Contributions
WG, RY, MIY, and SO have equal sharing between authors as regarding writing of the manuscript, the collection and analysis of data and revising the final manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the local Ethics Committee (HSC Ethical Committee). All study procedures were performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. No available ethics committee’s reference number. A written consent was taken from all patients prior to the study to be included in our study.
Consent for publication
A written consent was taken from all patients prior to the study for publication.
Competing interests
No competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gouda, W., Yasin, R., Yasin, M.I. et al. Automated breast ultrasound in breast cancer screening of mammographically dense breasts: added values. Egypt J Radiol Nucl Med 55, 86 (2024). https://doi.org/10.1186/s43055-024-01258-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-024-01258-3