Abstract
Background
The discrimination between multiple sclerosis (MS) and other white matter lesions in adults is still problematic as multiple diseases have similar clinical and radiological findings, especially at the disease onset. This case–control study aimed to evaluate the efficacy of diffusion tensor imaging (DTI) and brain morphometry in the differentiation between adult MS and its magnetic resonance imaging (MRI) mimics.
Methods
Fifty-eight patients (49 women and 9 men; mean age 35.74 ± 11.86 years, range 18–50 years) with clinically suspected MS and age- and sex-matched fifteen healthy subjects were included. All study subjects underwent MRI using 3D FLAIR, 3D T1 MPRAGE, axial T2WI fast spin echo, and DTI sequences. The included patients were classified into MS and mimics groups according to MC Donald’s criteria 2017. Image post-processing using the region of interest (ROI) analysis for DTI parameters and automated voxel brain morphometry and segmentation was done for all study groups. Statistical comparison between the two patient groups and between them and the control group was performed regarding DTI indices (fractional anisotropy [FA], mean diffusivity [MD], and relative anisotropy [RA]) and brain morphometry parameters (including white matter volume (WMV), grey matter volume (GMV), cerebrospinal fluid volume (CSFV), T2 lesion load and deep grey matter volume).
Results
A statistically significant difference was observed between the MD, FA, and RA values of the patients and control groups. The MD values were significantly higher in the MS than in its mimics with a cut-off value of > 1.058 × 10−3 mm2/s (p < 0.001). Compared to the mimics group, the MS patients showed significantly lower WMV (33.31 ± 4.40 versus 35.71 ± 4.58; p = 0.047) and higher CSFV (16.38 ± 5.93 versus 13.06 ± 3.09; p = 0.012) with no significant difference regarding the GMV.
Conclusions
MD analysis of DTI, WMV, and CSFV are useful quantitative measurements in confirming the diagnosis of MS and differentiating it from its imaging mimics.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Multiple sclerosis (MS) is the most frequent chronic demyelinating disease of the central nervous system in young adults leading to a long-term disability [1]. MRI plays a crucial role in MS diagnosis by demonstrating the dissemination in space and time of white matter lesions (WMLs), as well as helping to rule out alternative diagnoses [2].
Although T2WI hyperintensities are characteristic of MS, they are not a specific finding that may result from a wide spectrum of white matter pathologies ranging from cerebrovascular disease and migraine to neuromyelitis optica spectrum disorders (NMOSD) and rarer conditions [3].
Advanced MRI techniques, including diffusion-weighted imaging (DWI), have demonstrated high specificity and sensitivity in detecting white matter pathological tissue damage and play an important role in highlighting brain microstructural damage which is not visible in conventional sequences [4].
Diffusion tensor imaging (DTI) has been widely used in the evaluation of MS patients as it enables noninvasive evaluation of white matter when myelin and/or axon integrity is disrupted [5]. From the DTI, it is possible to derive some parameters that are quantitative and invariant to the choice of reference frame, including the mean diffusivity (MD), which measures the average molecular motion independent of any tissue directionality, and the fractional anisotropy (FA), which reflects the prevalence of diffusivity along one spatial direction [6, 7]. DTI can identify abnormal values of MRI indices in specific white-matter tracts that may underlie clinical disability in MS [8]. Demyelinating plaques destroy white matter fibers which are manifested by increased diffusivity of water molecules in DTI as demonstrated by measuring indices including MD and FA [9].
Brain atrophy is a characteristic feature of multiple sclerosis (MS) that occurs throughout the disease course [10]. As brain volume loss in MS patients about 0.7–1% per year, highly sensitive techniques are needed to quantify those small changes [11]. Manual based morphometry was used for decades to assess the brain atrophy. Automated voxel-based morphometry is widely used nowadays in assessment of neurodegenerative diseases as it is highly reproducible method [12]. Both white matter volume and grey matter volume were affected early in the disease course, regardless of their phenotype [10, 11]. Nevertheless, grey matter damage may occur before white matter atrophy and can be perceived independently of the white matter lesions [12, 13].
Our objective was to evaluate the efficacy of DTI and voxel-based brain morphometry in the differentiation between adult MS and its MRI mimics.
Methods
Study design and populations
This case–control study was performed prospectively as one institution study on patients referred to the Diagnostic Radiology department from the neurology and psychiatry department of our hospitals. The study was conducted from May 2019 to November 2021 and was approved by our institutional Ethical Review Board. Informed written consent was obtained from all the study subjects. The study was approved by the clinical trials.gov protocol and registration and results system numbered ID: NCT03608605.
Patients from 18 to 50 years old with neurological manifestations suspicious for MS were included in the study. Age below 18 (pediatric age group) and above 50 years (to avoid age-related white matter changes) was from the study exclusion criteria. Patients with an established diagnosis of MS prior to study conduction were not included in the study. Patients with any absolute or relative contraindications to MRI were also excluded. The clinical history as well as the findings of the neurological examination and the results of the relevant investigations was obtained for each patient. According to their final clinical diagnosis, the patients were divided into two groups: MS and MS mimics. If the patient met the MC Donald's criteria 2017 [14], he or she was clinically diagnosed with MS; otherwise, they were classified as MS mimics. Fifteen healthy people of matched sex and age enrolled in the study as the control group (Fig. 1). The healthy control did not have any neurological or psychiatric disease.
MRI protocol
All patients and healthy controls underwent MRI using a 1.5-T unit (Siemens, Magnetom Sempra, German) using an eight-channel receive-only head coil. Three-dimensional (3D) fluid inversion recovery (FLAIR) with repetition time (TR) 8000 ms, echo time (TE) 86 ms, slice thickness 4.5 mm, the field of view (FOV) 230 × 230 mm2),3D high-resolution magnetization prepared rapid gradient echo (MPRAGE) T1-weighted image (TR = 220 ms, TE = 2.83 ms, slice thickness 1 mm, FOV 250 × 250 mm2) and axial T2 weighted fast spin echo sequence (TR = 391 ms, TE = 120 ms, slice thickness 4.5 mm, FOV 230 × 230 mm2) were included in the protocol. In addition, diffusion tensor images were obtained as axial diffusion-weighted data using a twice-refocused single-shot echo-planar imaging sequence, with 20 diffusion-weighted directions equally spaced over a hemisphere at b0 and b1000 in a scan time of 5 min 28 s (55 × 2.5 mm slices acquired interleaved, FOV 230 × 230mm2, TR/TE = 3500/105 ms).
Image post-processing
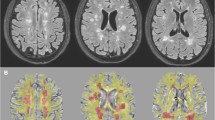
Image analysis was performed blinded to the patient's final diagnosis by two experienced radiologists (20 and 10 year experience in neuroradiology). All MRI images were transferred to the post-processing Siemens workstation, imaging software platform (Syngo via, Siemens Medical Solutions, Erlangen, Germany) for visualization, analysis, and post-processing. All images were corrected for motion and eddy current geometric distortions and non-brain tissues were removed. The DTI indices (fractional anisotropy [FA], mean diffusivity [MD], and relative anisotropy [RA]) values were measured in different slices of B0 and color-coded maps on the axial images (Fig. 2). The DTI images were co-registered automatically with anatomical images (FLAIR and T1WI). In each patient, a single region of interest (ROI) was drawn by both radiologists in the same session on the largest lesion along the corticospinal tract of high signal intensity in FLAIR images (Fig. 2) and isointense signal in T1WI. The ROI size was kept constant (3–4 mm2) in all patients to obtain the same number of voxels and decrease the variation in the DTI parameters and minimize partial volume effects [15]. DTI indices of the MS group were compared to those of the mimics group.
A 31-year-old female with a clinical suspicion of MS. a Sagittal FLAIR image shows multiple deep white matter hyperintense lesions at the right frontal and parietal lobes (arrows). b Axial MD map shows hyperintense lesion at the right posterior parietal region (red ROI). c Axial FA map with 3mm2 ROI drawn on the same lesion (red ROI). d Quantitative map for the same lesion. The FA was 0.176, and the RA was 0.146. MD was denoted by ADC 1.313 × 10−3 mm2/s. The patient was diagnosed as MS
For the control group, The ROI was placed at the corticospinal tract in healthy subjects and had the same size. The same processing and DTI indices were calculated. DTI parameters of the patients and the healthy controls were also compared.
Then, an automated MRI brain volumetry online system (https://volbrain.upv.es) using compressed 3D-T1WI and 3D FLAIR sequence in Neuroimaging Informatics Technology Initiative (NIfTI) format (converted using MRIcron program) was done for all patients in this study and uploaded to volbrain online system to calculate intracranial brain volume including grey matter “GMV”, white matter “WMV” and cerebrospinal fluid volume “CSFV”. All the volumes were presented in absolute value (measured in cm3) and in relative value (measured concerning the intracranial volume). The online system also calculated volumes of different subcortical structures (Figs. 3e, 4e). Labeled colored graphical maps of the above-mentioned anatomical structures were also provided for each case (Figs. 3d, 4d). A statistical comparison between the relative value of GMV, WMV, and CSFV in the two studied groups and the healthy control group was done.
A 28-year-old female with clinically suspected MS. a axial FLAIR image shows bilateral multiple deep white matter hyperintense lesions in the frontal and parietal lobes (arrows). b FA map with ROI placed on the right frontal corticospinal lesion (arrow). c Corresponding MD map shows a right frontal lesion (arrow). d Volumetric report representing automated colored graphical maps of the different intracranial structures in axial, coronal and sagittal planes. e Volumetry brain report for estimated brain structures volumes revealed that WMV was 36.95%, GMV was 51.80%, and CSFV was 11.60%. The diagnosis was MS
A 45-year-old female presented with headache and numbness in the face. a Sagittal FLAIR image showing left frontal deep white matter hyperintense lesion (arrow). b Color-coded map with left frontal lesion causing fiber disruption (arrow). c Corresponding FA map of the detected lesion. d Volumetry report representing quantitative assessment of intracranial structures in different planes. e Volumetry brain report showing WMV was 31.35%, GMV was 51.68%, and CSFV was16.97%.The patient was diagnosed as cerebral small vessel disease
Statistical analysis
All statistical calculations were performed using the computer program SPSS ((IBM-SPSS) version 26.0 software) for Microsoft Windows. Data were statistically described in terms of mean ± standard deviation (± SD), median and range, or frequencies (number of cases) and percentages when appropriate. Comparison of numerical variables between the study groups was done using the Student t-test for independent samples. For comparing categorical data, the Chi-square test was performed.
The independent t-test was used to compare the FA, MD, and RA values in the patients and controls. Analysis of variance (ANOVA) test and paired t-test were performed to compare the FA, MD, and RA values of the three groups.
Receiver operating characteristic (ROC curve) was generated using MedCalc Statistical Software version 18.11.3 (MedCalc Software bvba, Ostend, Belgium; https://www.medcalc.org; 2018) for the studied diagnostic markers, to determine the efficacies (sensitivity, specificity, and area under the curve (AUC)) of these different parameters. The Youden index was employed to identify the optimal cut-off value. The probability of significance (P value): P value < 0.05 was considered significant.
Results
Out of 58 patients (49 women and 9 men; mean age 35.74 ± 11.86 years, range 18–50 years), thirty-one (53.4%) patients with mean age 33.97 ± 10.75 were diagnosed as MS while the remaining 27 (46.6%) patients were diagnosed as MS mimics with a mean age 37.78 ± 12.92 (p = 0.226). The distribution of patients with MS mimics is shown in (Table 1).
One-way ANOVA test showed a high statistically significant difference between all DTI parameters of the MS patients and those of the control group. Moreover, the MD values were significantly different between all groups where the MS group demonstrated the highest mean value (Table 2).
ROC curve analysis was done for the DTI parameters (RA, FA, and MD) to assess their diagnostic ability in the differentiation between the different groups of the study. Multiple ROC curves were performed for the discrimination of MS and controls (Table 3; Fig. 1a), MS mimics and controls (Table 4; Fig. 1b), and finally for the discrimination of MS and mimics patients (Table 5; Fig. 1c), which show lower sensitivity with nearly the same specificity and high statistically significant difference.
The maximal AUC value for the differentiation between MS and MS mimics (Table 5) is 0.502 for FA identified via the Youden Index with a specificity of 37% and a sensitivity of 96.8%. Regarding the same concern, MD showed a statistically significant AUC value of 0.845, with a cut-off point of > 1.058 × 10−3mm2/s according to the Youden Index with a specificity of 87.1% and a sensitivity of 77.8%, p value < 0.001. All other metrics (RA, FA) showed no significant difference.
Moreover, there was a significant difference between white matter volume and CSFV in both groups (Table 6).
Testing of the deep grey matter volume changes (including the thalamus, caudate, putamen, and hippocampus) between MS and MS mimics group show that there is the significant statistical difference for the thalamic volume between MS and MS mimics with p = 0.028 (Table 7).
Discussion
Conventional MRI sequences are largely nonspecific, and only provide a limited view of the complex morphological changes associated with MS. Diffusion-weighted imaging (DWI), tractography, and volumetry are advanced techniques having a complementary roles to that of conventional sequences by revealing more-specific information on microstructural tissue changes which can be used also to differentiate MS from other pathologies [4].
The current study evaluated different white matter lesions using combined conventional and advanced MRI sequences in 58 patients having symptoms suspected by the physician to be MS. Then, the patients were classified as MS and MS mimics after completing their investigations. The results of the MRI were compared to those of 15 healthy control subjects.
The current understanding is that the RA is derived from a ratio between the anisotropic portion of the diffusion tensor and the isotopic portion and it associate with myelin integrity. Decreased FA has been interpreted to report a decrease in axonal density, whereas the increased MD is associated with axonal and myelin loss [5]. By demonstrating increased MD and decreased FA in MS lesions in comparison to mimics and controls, our results are in agreement with the study conducted by Kato et al. [16] who found that the ROI analysis of FA showed decreased values in diseases that cause neurodegeneration, including MS. In accordance with our study, they also concluded that the MS group had significantly higher AD, MD, and RD in WM lesions than the NMOSD group and that all DTI indices (except FA) differed significantly between the relapsing remitting MS and NMOSD groups. Similar results were reported by El-Sourgy et al. [9] who found a statistically significant reduction of FA (Fiber directionality/axonal loss) and an increase of MD (amount of water diffusion/myelin loss) in all MS patients compared to the healthy control.
As regard MS mimics patients, especially those diagnosed with cerebrovascular small vessel disease, Caunca et al. [17] reported that greater WMH in vascular disease is associated with lower FA suggesting white matter integrity loss.
Our study hypothesized that quantitative DTI parameters may aid in differentiation between MS and its mimics, and to our knowledge, this is the first study to provide cut-off values by comparing DTI parameters between MS patients and MS mimics. The present results showed that the MD values of MS lesions were significantly higher than those of their mimics with a high sensitivity (87.1%) and a cut-off value > 1.058 × 10−3 mm2/s for differentiating both groups with AUC of about 0.845. The cut-off value for RA and FA shows no significant results. This implies that the measurement of MD in suspicious brain lesions can add significance in differentiating MS from its mimics at the time of diagnosis.
In the present study, a comparison of GMV, WMV, and CSFV between the MS group and the mimics group, revealed significantly higher WMV with significantly lower CSFV in MS patients (p value = 0.047 and 0.012; respectively). When interpreting these measures, it should be noted that brain atrophy is not specific to the pathological events underlying it, additionally, in the early stage of the disease, atrophy may not be easily detectable, but neuroaxonal loss has already started [13].
In our study, volumetric assessment of bilateral thalamus, caudate, putamen, and hippocampus were automatically calculated and compared between MS and mimics patients. We found a significantly lower thalamic volume in MS patients (p-value 0.028) with no significant difference between both groups regarding putamen, caudate, and hippocampal volumes (p value = 0.331, 0.175, and 0.241; respectively).
Solomon et al. [18] observed significant results in differentiating thalamic volume proportion in relation to the intracranial volume between MS and non-MS groups. Those data suggest that the thalamocortical tract disruption by any white matter disorders other than MS is not associated with the same degree of thalamic volume loss seen in MS patients. The pathologic processes in SVD occurring at the site of white matter lesions are distinct from MS, and these processes, as well as direct thalamic injury in MS, may be responsible for the volumetric differences we observed [19].
Early cross-sectional studies [11, 20] conducted on patients with MS indicated that both white matter and grey matter loss occurred early in the disease course, regardless of disease phenotype. Other studies concluded that grey matter damage start before white matter atrophy and can take place independently of white matter lesions [13, 21]. Moreover, two preceding studies; one based on the estimation of cortical thickness [22], and the other was a meta-analysis of voxel-based morphometry [23]; revealed statistically significant associations between the endpoints of disability and the degree of grey matter atrophy. In their recent study Eshaghi et al. [24] reported that gray matter atrophy occurs bilaterally and predominantly affects the cingulate, pre-central, and/or post-central gyri, the thalami, and basal ganglia.
Limitations
There are some limitations affect the interpretation of the results of the current work. First, the small patient cohort for each group analysis might influence the statistical power of our findings. Second, the comparison between MS mimics including variable diseases might affect the results. Third, the use of multiple lesions assessment in each case in quantitative measures of DTI parameters may provide better results than a single lesion assessment. Lastly, ROI method for analysis is operator dependent, and could subject to partial volume artifacts so we used only lesions with a diameter greater than 5 mm that underwent analysis.
Conclusions
In conjunction with good clinical, neurological, and laboratory assessment MRI plays an important role in the diagnosis of MS and ruling out of mimics. Advanced MRI sequences add more sensitivity and specificity in confirming or excluding the diagnosis of MS. Quantitative DTI measurements, particularly MD can discriminate MS from its mimics with good accuracy. Voxel-based brain morphometry may add value to the differentiation of MS from its mimics.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ANOVA:
-
Analysis of variance
- AUC:
-
Area under the curve
- CSFV:
-
Cerebrospinal fluid volume
- DTI:
-
Diffusion tensor imaging
- FA:
-
Fractional anisotropy
- FLAIR:
-
Fluid inversion recovery
- FOV:
-
Field of view
- GMV:
-
Grey matter volume
- HC:
-
Healthy control
- MD:
-
Mean diffusivity
- MPRAGE:
-
Magnetization prepared rapid gradient echo
- MRI:
-
Magnetic resonance image
- MS:
-
Multiple sclerosis
- NIFTI:
-
Neuroimaging Informatics Technology Initiative
- NMOSD:
-
Neuromyelitis optica spectrum disorder
- RA:
-
Radial anisotropy
- ROC curve:
-
Receiver operating characteristic
- ROI:
-
Region of interest
- SVD:
-
Small vessel disease
- TE:
-
Echo time
- TR:
-
Repetition time
- WM:
-
White matter
- WMH:
-
White matter hyperintensity
- WMLs:
-
White matter lesions
- WMV:
-
White matter volume
References
Dekker I, Wattjes MP (2017) Brain and spinal cord MR imaging features in multiple sclerosis and variants. Neuroimaging Clin 27:205–227
Wildner P, Stasiołek M, Matysiak M (2020) Differential diagnosis of multiple sclerosis and other inflammatory CNS diseases. Mult Scler Relat Disord 37:101452. https://doi.org/10.1016/j.msard.2019.101452
Geraldes R, Ciccarelli O, Barkhof F et al (2018) The current role of MRI in differentiating multiple sclerosis from its imaging mimics. Nat Rev Neurol 14:199–213
Enzinger C, Barkhof F, Ciccarelli O et al (2015) Nonconventional MRI and microstructural cerebral changes in multiple sclerosis. Nat Rev Neurol 11:676–686
Sbardella E, Tona F, Petsas N, Pantano P (2013) DTI measurements in multiple sclerosis: evaluation of brain damage and clinical implications. Mult Scler Int 2013:671730. https://doi.org/10.1155/2013/671730
Melhem ER, Mori S, Mukundan G et al (2002) Diffusion tensor MR imaging of the brain and white matter tractography. Am J Roentgenol 178:3–16
Deppe M, Krämer J, Tenberge J et al (2016) Early silent microstructural degeneration and atrophy of the thalamocortical network in multiple sclerosis. Hum Brain Mapp 37:1866–1879
Ozturk A, Smith SA, Gordon-Lipkin EM et al (2010) MRI of the corpus callosum in multiple sclerosis: association with disability. Mult Scler J 16:166–177
El-Sourgy L, Ahmad N, El-Rakhawy M et al (2015) Applications of MR fiber tractography imaging in multiple scleros. Egypt J Radiol Nucl Med 46:449–454
De Stefano N, Silva DG, Barnett MH (2017) Effect of fingolimod on brain volume loss in patients with multiple sclerosis. CNS Drugs 31:289–305
Filippi M, Rocca MA (2011) MR imaging of multiple sclerosis. Radiology 259:659–681
Callaert DV, Ribbens A, Maes F et al (2014) Assessing age-related gray matter decline with voxel-based morphometry depends significantly on segmentation and normalization procedures. Front Aging Neurosci. https://doi.org/10.3389/fnagi.2014.00124
Wang C, Beadnall HN, Hatton SN et al (2016) Automated brain volumetrics in multiple sclerosis: a step closer to clinical application. J Neurol Neurosurg Psychiatry 87:754–757
Thompson AJ, Banwell BL, Barkhof F et al (2018) Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 17:162–173. https://doi.org/10.1016/S1474-4422(17)30470-2
Froeling M, Pullens P, Leemans A (2016) DTI Analysis methods: region of interest analysis. In: Van Hecke W, Emsell L, Sunaert S (eds) Diffusion tensor imaging: a practical handbook. Springer, New York, pp 175–182. https://doi.org/10.1007/978-1-4939-3118-7_9
Kato S, Hagiwara A, Yokoyama K et al (2022) Microstructural white matter abnormalities in multiple sclerosis and neuromyelitis optica spectrum disorders: Evaluation by advanced diffusion imaging. J Neurol Sci 436:120205
Caunca MR, De Leon-Benedetti A, Latour L et al (2019) Neuroimaging of cerebral small vessel disease and age-related cognitive changes. Front Aging Neurosci 11:145
Solomon AJ, Watts R, Dewey BE, Reich DS (2017) MRI evaluation of thalamic volume differentiates MS from common mimics. Neurol Neuroinflammation 4(5):e387
Wardlaw JM, Smith C, Dichgans M (2013) Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol 12:483–497
Quarantelli M, Ciarmiello A, Morra VB et al (2003) Brain tissue volume changes in relapsing-remitting multiple sclerosis: correlation with lesion load. Neuroimage 18:360–366
Roosendaal SD, Bendfeldt K, Vrenken H et al (2011) Grey matter volume in a large cohort of MS patients: relation to MRI parameters and disability. Mult Scler J 17:1098–1106
Narayana PA, Govindarajan KA, Goel P et al (2013) Regional cortical thickness in relapsing remitting multiple sclerosis: a multi-center study. NeuroImage Clin 2:120–131
Lansley J, Mataix-Cols D, Grau M et al (2013) Localized grey matter atrophy in multiple sclerosis: a meta-analysis of voxel-based morphometry studies and associations with functional disability. Neurosci Biobehav Rev 37:819–830
Eshaghi A, Prados F, Brownlee WJ et al (2018) Deep gray matter volume loss drives disability worsening in multiple sclerosis. Ann Neurol 83:210–222
Acknowledgements
The authors thank all the study participants for their patience and support.
Funding
This study was not financially supported by any institute.
Author information
Authors and Affiliations
Contributions
SME, SAS and GSH suggested the idea and contributed to the design and construction of the main outlines of the study methodology to reach a conclusion. SME, AHA, GSH and SAS performed the technical parts of the study and contributed to the interpretation of the results with help from GAS, who referred the cases included in the study. SME performed the numerical calculations and statistical analysis of data. SME, AHA, SAS, GSH and GAS contributed discussion of the results and provided a draft. SMM wrote the manuscript with input from all authors. SME, AHA, SHA and GAS contributed revision of spelling, grammar and intellectual contents of the manuscript. SAS took the responsibility of organizing and supervising the course of the work. All authors agreed to the final version of the manuscript and provided final approval to submission. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study had approval from Assiut University, Egypt, Faculty of Medicine Research Ethics Committee. All patients participated in this study signed an informed written consent for participation and publication ofthe data contained in this research. Ethical considerations: (1) Risk Benefit assessment for all patients were indicated for the examination during this study. (2) Confidentiality: All patients’ data were confidentially kept. (3) The research done by scientifically qualified and trained personnel. (4) The procedures imaging sequences included in this study had been already used in hospitals and centers in and outside Egypt.
Consent for publication
Not applicable.
Competing interests
Authors declare that they had no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elkossi, S.M., Sayed, S.A.EA., Shehata, G.A. et al. Diffusion tensor imaging and voxel-based morphometry in differentiating multiple sclerosis and its mimics. Egypt J Radiol Nucl Med 54, 216 (2023). https://doi.org/10.1186/s43055-023-01155-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-023-01155-1