Abstract
Background
High-risk breast lesions represent 3–21% of all breast lesions and are non-obligate precursors of malignancy. Various studies have evaluated the value of DCE-MRI including DWI and ADC mapping in the detection of high-risk breast lesions, differentiating them from malignant lesions and predicting upgrade risk after surgical excision reducing misdiagnosis and overtreatment. This study is a retrospective review of all image-guided breast biopsy procedures performed in 2021 at our institution, identified 68 patients with histopathologically proven high-risk breast and available MRI examinations with no concurrent ipsilateral malignancy. Image analysis of MRI examinations included morphological criteria, enhancement pattern, dynamic curves, DWI and ADC mapping assessment. Since our knowledge of high-risk breast lesions is still growing, this study is important to evaluate the merits of DCE-MRI in the assessment of high-risk breast lesions, to allow optimization of treatment, better limit it to those women at risk, and avoid overtreatment in women at low risk.
Results
The mean ADC value of high-risk breast lesions was not significantly different from that of malignant breast lesions (p value = 0.015). Non-mass enhancement and type III enhancing curve proved to be significant indicators of high-risk breast lesions upgrade in surgical pathology. Cut-off average ADC value for differentiating upgraded high-risk lesions from non-upgraded high-risk lesions proved 1.24 mm2/sec with sensitivity and specificity of 94 & 100%, respectively.
Discussion
Management of high-risk breast lesions is important in the screening setting, as they are non-obligate precursors of malignancy, and also function as risk indicators. Frequency and upgrade rates of high-risk lesions detected by MRI provide a reference for clinical management. DCE-MRI has a high negative predictive value in predicting the upgrade risk of high-risk lesions. In this study, non-mass enhancement and type III curve were proven to be indicators of high-risk lesion upgrade. Limitations of the study included small number of patients and limited follow-up period.
Conclusions
The use of multi-parametric DCE-MRI including DWI and ADC mapping provides complementary information to detect high-risk breast lesions and predict their upgrade rate.
Similar content being viewed by others
Background
High-risk breast lesions, also known as B3 lesions, are composed of different pathologies with differing risks of associated malignancy [1, 2]. They include lobular neoplasia, radial scar/complex sclerosing lesions, papillary lesions, and atypical intraductal epithelial proliferation [3–5]].
Widespread screening programs and new imaging techniques significantly increased diagnosis of those lesions in the last few decades [6]. They represent 3–21% of all breast lesions and carry a low risk of associated malignancy ranging from 0.2 to 5% [2, 7].
They are non-obligate precursors of malignancy [7]. Up to 35% of those lesions are upgraded at surgical pathology to DCIS or carcinoma [3, 8].
The diagnosis of high-risk lesions with atypia increases the risk of developing breast cancer in the same or the contralateral breast 4–10 times, so they are considered risk indicators [9].
High-risk breast lesions appear with different imaging features, ranging from masses to architectural distortions or microcalcifications [6, 7].
MRI provides the highest sensitivity for the detection of breast cancer [10]. Various studies have evaluated the MRI features of high-risk breast lesions, and they reported that there is no reliable MRI feature alone that can accurately diagnose high-risk lesions and predict their upgrading risk [11].
The approach to B3 lesions must take on a multidisciplinary approach. It has undergone significant changes in the last decade, still no clear consensus on how they should be managed [3], Tailored management assessing the individual risk of patients has been favored to reduce overtreatment and spare patients from unnecessary anxiety or high costs associated with surgical excision [6].
According to recent guidelines, high-risk lesions are managed with imaging follow-up, surgical or vacuum-assisted excision [8,9,10, 12].
Our knowledge of B3 lesions is still growing, it is important to collect and evaluate this information, to allow optimization of treatment, better limit it to those women at risk, and avoid overtreatment in women at low risk [13].
Methods
Patients
Our institutional review board approved this retrospective study.
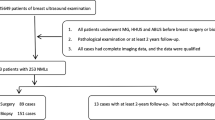
A retrospective review of the database of all 5928 sonographically and stereotactically guided breast biopsy procedures performed in 2021 at our institution identified 85 (1.2%) patients with high-risk breast lesions.
All patients underwent an initial sonomammographic assessment where all high-risk lesions were classified as BI-RADS 4.
Patient management discussed in multidisciplinary meetings where clinical and imaging findings were evaluated, and a consensus decision was taken to perform a breast dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) and histopathological assessment.
Out of the 85 patients with histopathologically proven high-risk breast lesions, 12 patients were excluded due to poof of concurrent malignancy in the same lesions, and 5 patients were excluded due to incomplete clinical data and/or incomplete or unavailable MRI assessment.
Data collection of 68 patients included: histology of the biopsy, clinical information regarding age at diagnosis, oral contraceptive usage history, personal history of breast cancer, and presence of concomitant breast cancer.
Patients with concurrent ipsilateral (different lesion) or contralateral breast cancer weren’t excluded.
ADC values of randomly selected 150 benign and 150 malignant lesions control group were assessed in the same method.
Imaging guided biopsies
Patients were referred to either ultrasound or stereotactic trucut or vacuum-assisted biopsy (VAB) by trained breast imagers.
Stereotactic biopsy procedures were performed on a dedicated digital stereotactic table (GE) with the patient sitting. Sampling of the lesions was performed using a vacuum-assisted EnCore Enspire Breast Biopsy System (BARD) with 10-gauge needles. Biopsy specimens from patients with calcifications were routinely examined by specimen mammography.
Ultrasound-guided biopsy procedures were performed using high-resolution ultrasound machines.
Trucut biopsies were performed on a dedicated GE, Toshiba, or Siemens Ultrasound device with the patient lying supine using a 14-gauge needle.
A clip was left to mark the site of biopsy in all cases of stereotactic guided biopsies and in cases of US-guided biopsies in which the lesion size was < 5 mm or when the lesion was difficultly seen after the biopsy. The sample volume sufficiency was confirmed by the pathologist.
Out of the 68 patients, 45 patients underwent stereotactic guided biopsy and 23 patients underwent ultrasound-guided biopsy.
The outcome of each B3 lesion was determined using the subsequent surgical excision and/or follow-up as a gold standard.
Surgical open biopsy was carried out by breast-conserving surgery in 57 out of 68 patients (83.8%) when:
-
Needle biopsy is not concordant with imaging: pathology does not explain radiographic findings.
-
Needle biopsy findings show atypia or Lobular carcinoma in situ (LCIS) and there is a concern for missed cancer in the adjacent tissue.
Surgical excisional biopsy was omitted in cases with concordance between imaging with histological findings, with no atypia, or because of the patient’s choice, who decided against surgical excision or biopsy performing only follow-up.
MRI examination
Breast DCE-MRI was performed before biopsy or 7–10 days after biopsy.
In premenopausal patients, examinations were performed in the second week of the menstrual cycle.
Patients underwent DCE-MRI with a 1.5-T Philips using a dedicated phased array breast coil.
An axial three-dimensional T1-weighted gradient-echo sequence was used for the dynamic study. Contrast material (0.1 mmol/kg body weight, gadoterate meglumine (Gd-DOTA; Dotarem, Guerbet) was injected intravenously as a bolus at 2 mL/s, with a saline flush after injection.
Images were acquired before and 2, 4, 6, and 8 min after contrast medium administration. Post-processing of the images included subtraction and maximum intensity projection images.
Imaging analysis
Image analysis was retrospectively performed by a radiologist with 12 years of experience in breast imaging, including breast MRI interpretation. The reader was blinded to the lesion’s definitive histopathological examination reports.
Presence of post-biopsy changes including hematoma, seroma, needle tract, and susceptibility artifacts by metal clips and/or thin enhancement along the needle tract or at the periphery of hematoma/seroma was considered not pathological. Any other enhancements at the level of the biopsy site, clearly distinct from the background parenchymal enhancement, were suspicious (BI-RADS 4/5).
Morphologic criteria assessed in cases with mass enhancement (ME) were shape (oval, round, irregular), margin (circumscribed, not circumscribed), and internal enhancement pattern (homogeneous, heterogeneous, rim enhancement).
In cases with non-mass enhancement (NME), morphologic criteria assessed were distribution (focal, linear, segmental, regional, diffuse) and internal enhancement pattern (homogenous, heterogeneous, clumped, clustered ring) according to the American College of Radiology BI-RADS MRI criteria.
While evaluating the kinetic curves, the ROIs (Regions of interest) were placed in the most enhancing solid part of the lesion and kinetic curves were constructed. Kinetic curves were categorized into 3 types that were obtained from DCE-MRI: type 1 (persistently rising curve), type 2 (plateau curve), and type 3 (washout curve).
For Apparent diffusion coefficient (ADC), the ROIs were placed within the lesion. Calculations were made based on mean ADC maps of the circular sampling ROI, with care taken to perform measurements in solid areas rather than necrotic/cystic areas and visual artifacts. We manually placed 3 circular ROIs within the lesion after referring to DCE-MRI for verification of the lesion boundaries on the ADC map. We calculated the average of the ADC values for all 3 ROIs within the lesion.
ADC values of randomly selected 150 benign and 150 malignant lesions control group were assessed in the same method.
Statistical analysis
Data analysis was performed with commercially available software (IBM SPSS Statistics for Windows version 24.0.2).
Data were summarized using mean, standard deviation, median, minimum and maximum in quantitative data and using frequency and percentage for categorical data.
The ADC values of the 3 groups of lesions (benign, high risk, and malignant) were compared, using Mann–Whitney U test. The determination of the optimal ADC threshold value for differentiating high-risk breast lesions from benign and malignant lesions and for differentiating upgraded and non-upgraded high-risk breast lesions was performed by receiver operating characteristic (ROC) curve. Sensitivity, specificity, and likelihood ratios were calculated at different cut-off values with a 95% confidence interval. P values ≤ 0.05 were considered statistically significant.
Statistical evaluation was performed with the Chi-squared and the Fisher exact test for categorical variables.
Results
The patients’ ages ranged from 34 to 72 years (mean age: 49.6 + 10.6 years), and all patients were females. Clinical characteristics are presented in Table 1.
Family history proved to be a risk factor for developing high-risk breast lesions P < 0.001.
Detailed lesion histopathological diagnoses are summarized in Table 2.
MRI findings of high-risk breast lesions detailed in Table 3
As illustrated in Fig. 1, by comparing ADC values of 150 benign breast changes, 68 high-risk breast lesions and 150 malignant breast lesions, the mean ADC value of high-risk lesions (1.34 ± 0.22 × 10–3 mm2/sec) wasn’t significantly different from those of malignant lesions (1.28 ± 0.13 × 10–3 mm2/sec) (p value = 0.015).
The cut-off average ADC value for detecting high-risk breast lesions from benign breast changes was found to be ≤ 1.51 × 10–3 mm2/sec with 84% specificity and 85.3% sensitivity.
However, the cut-off average ADC value for differentiating high-risk breast lesions from malignant breast lesions proved to be 1.3 mm2/sec with much less sensitivity and specificity 67 and 64.7%, respectively.
After the MDT decision of surgically removing 57 breast lesions, 18 breast lesions were upgraded in surgical pathology into mainly DCIS and invasive breast malignancies, showing the following imaging criteria Table 4.
Non-mass enhancement and type III enhancing curve proved to be significant indicators of high-risk breast lesions upgrade in surgical pathology (P = 0.001 and P = 0.02 respectively) in univariate and multivariate analysis.
High-risk lesions upgraded to malignancy, showed lower mean ADC (1.09 × 10–3 mm2/sec) compared to the non-upgraded lesions (1.37 × 10–3 mm2/sec) (p < 0.001).
Cut-off average ADC value for differentiating upgraded high-risk lesions from non-upgraded high-risk lesions proved to be 1.24 mm2/sec with sensitivity and specificity of 94 & 100%, respectively.
Discussion
Breast cancer remains the second leading cause of cancer-related death [14,15,16]. During the evaluation of concerning mammographic findings, several non-malignant breast lesions, Atypia, and carcinoma in situ may be identified on needle biopsy [17].
Management of lesions with uncertain malignant potential (B3) by histological examination is important in the screening setting, where the primary objectives are early detection and treatment of breast cancer. To avoid unnecessary surgery for benign disease, data are needed that can apply to clinical practice to increase the precision of nonoperative diagnosis [17].
They are non-obligate precursors of malignancy, and they can develop into high-grade lesions, predominantly ductal carcinoma in situ (DCIS), and less frequently low-grade invasive tumors [2, 7]. They also function as risk indicators, and diagnosis of lesions with atypia increases the risk of developing breast cancer in any location of the same or the contralateral breast [9].
Those patients’ risk of future breast cancer varies depending on the type of atypia or LCIS and other factors including family history, childbearing history, breast density, and lifestyle [17].
The risk is higher in younger women, as the risk decreases with progressive reduction of sexual hormones serum concentrations, which have a positive effect on the proliferative trend of breast lesions [18, 19]. The mean age of this population was 49.6 years, very close to the results of Cedolini et al. with the mean age of patients with B3 lesions in their study (49.02 years) [19].
The mean age of patients with breast cancer in our institute was 59 years. Therefore, we can deduce that borderline lesions affected premenopausal patients (Fig. 2). This agrees with Mohrmann et al. who stated that age and postmenopausal status were other significant risk factors for a malignant upgrade of a B3 lesion (p = 0.015) [20].
Case 1: 45 years old with strong family history and pathologically proven left breast IDC showing multiple bilateral complex cystic lesions on sonomammographic assessment (not illustrated on images). DCE-MRI: STIR (A), post-contrast dynamic sequences (B) with time–intensity curves (C), and ADC mapping (D) revealed bilateral cystic lesions with intralesional soft tissue components showing mass enhancement (B) On plotting the time–intensity curve, the lesions revealed type II (plateau curve) (C). The mass showed facilitated diffusion and ADC value of 1.28 (D). Given the patient’s history of contralateral breast cancer, U/S guided VAB-assisted excision of bilateral breast lesions (E & F) revealed intraductal papilloma, UDH, and ADH. Surgical excision revealed low-grade DCIS
The association with a family history of breast cancer remains controversial [18]. In this study population, family history was proven to be a high risk of development of B3 lesions.
In the literature, many studies revealed an increased breast cancer risk among women assuming exogenous hormones, as contraceptives during their fertile age or as substitutive hormonal therapy after menopause [19]. In the present population, the association between B3 lesions and the use of oral contraceptives wasn’t statistically significant.
The most commonly detected B3 lesion in the population was papillary breast lesions, which is in agreement with Mohrmann et al., while in other studies Bianchi et al. and Rakha et al, ADH was not the most commonly detected B3 lesion [20,21,22].
Studies have shown that performing MRI and a core needle biopsy or vacuum-assisted breast biopsy (VABB) of patients diagnosed with high-risk lesions can benefit the patient by reducing the need for surgery [4, 23].
Multiparametric dynamic contrast-enhanced MRI yields information about enhancement of the lesion by using kinetic curves and DWI (Diffusion-weighted imaging), with significant specificity differentiating benign lesions from malignancy [11, 24, 25].
Different studies are investigating MRI role in predicting malignancy in patients with a biopsy diagnosis of high-risk lesions and distinguishing high-risk lesions that require surgery, from those that can be managed with follow-up [6, 18, 26].
DCE-MRI has a high NPV (negative predictive value) in predicting the upgrade risk of high-risk lesions. The negative predictive value of non-enhancing B3 lesions (concerning the presence of malignancy at excision) was 98%. In the absence of suspicious enhancement, the risk of upgrade is low and surgical excision could be avoided [18, 26]. It can reduce misdiagnosis and overtreatment [11, 13]. Furthermore, considering only microcalcifications, MRI appears to be a valuable tool to exclude malignancy in BI-RADS 4 microcalcifications [6].
Frequency and upgrade rates of high-risk lesions detected by MRI provide a reference for clinical management [11].
Eighteen out of 57 (31.5 %) of the cases histologically classified as B3 preoperatively proved malignant in the final histological analysis. Thus, the results are much higher than the previously published data by Houssami et al., Decker et al. and Mohrmann et al. where 14.1% of the cases histologically classified as B3 preoperatively proved malignant in the final histological analysis [20, 27, 28].
Previous studies reported that there are no specific MRI characteristics that can predict high-risk lesions that can upgrade to malignant lesions after surgery [29]. However, in recent years, there have been some promising findings regarding high-risk lesions [11].
In this study, non-mass enhancement and type III curve were proven to be indicators of high-risk lesion upgrade where 83.3 % of upgraded surgical pathology revealed non-mass enhancement and 77.8 % revealed type III enhancing curve on earlier MRI studies (Figs. 3 and 4). This may be explained by the fact that most of the high-risk lesions reported by were upgraded to DCIS, which usually shows NME
Case 2: A 52-year-old female patient presented with left Breast UIQ asymmetry warranting further investigation (not illustrated on images). DCE-MRI: STIR (A), post-contrast dynamic sequences (B) with time–intensity curves (C), and ADC mapping (D) revealed Left breast UIQ non-mass enhancement extending to the retro-areolar region showing type II (plateau curve) on plotting time–intensity curve (C) and facilitated diffusion with ADC value of 1.35 (D). Ultrasound-guided VAB-assisted biopsy histopathological results revealed intraductal papilloma UDH and focal sclerosing adenosis with no malignant cells. MDT advised follow-up
Case 3: 35 years old with a long history of OCP use. Sonomammographic assessment revealed right breast UOQ asymmetry with underlying parenchymal distortion on U/S (not illustrated on images). DCE-MRI: STIR (A), post-contrast dynamic sequences (B) with time–intensity curves (C), and ADC mapping (D) revealed right breast UOQ non-mass enhancement showing type III (washout) curve on plotting time–intensity curve (C) and ADC value of 1.28 (D). Histopathological results of U/S guided trucut biopsy revealed ADH and apocrine metaplasia. Surgical excision after wire localization histopathological results revealed ADH with DCIS
Londero et al. also reported that the upgrade risk was significantly elevated in high-risk lesions with NME than in those with ME [30]. However, Verheyden et al. and Li et al. stated that mass enhancement (ME) on MRI is an important feature that indicates the upgrade of high-risk lesions [11, 31]. And Cheeney et al. showed no difference between mass and non-mass enhancement in upgrade Rate [32].
DWI and ADC are potentially useful in predicting the upgrade risk of high-risk lesions [11, 31]. The ADC of upgraded high-risk lesions (1.09 x10−3 mm2/sec) is lower than that of non-upgraded lesions (1.37 x10-3 mm2/sec) which is in agreement with Cheeney et al. and Li et al. [11, 32].
Cheeney et al. obtained similar ADC values of upgraded and non-upgraded lesions to our study results, 1.09 x10-3 mm2/s & 1.39 x10-3 mm2/s, respectively [32].
Our study adds to promising emerging data of Partridge et al., Mooney et al. and Li et al. on the use of DWI provides complementary information to DCE-MRI to reduce unnecessary biopsies and surgeries [32,33,34] (Figs. 5 and 6).
Case 4: 37 years old presenting with a palpable left breast mass. Sonomammographic assessment revealed focal palpable adenosis (not illustrated in images). DCE-MRI: T1WI (A), STIR (B), post-contrast dynamic sequences (C) with Time–intensity curves (D), and ADC mapping (E) revealed LIQ mass enhancement showing type II plateau curve on plotting time–intensity curve (D) and ADC value of 1.29 (E). U/S guided trucut histopathology revealed sclerosing adenosis, wire localization (F), excision was recommended by MDT, and post-surgical histopathology revealed no malignant cells
Case 5: 44 Years old presenting with a palpable left breast mass. Sonomammographic assessment revealed UOQ focal parenchymal distortion (not illustrated on images). DCE-MRI: STIR (A), post-contrast dynamic sequences (B) with time–intensity curves (C), and ADC mapping (D) revealed UOQ mass enhancement showing type III washout curve on plotting time–intensity curve (C) and ADC value of 1.21 (D) U/S guided trucut histopathology revealed sclerosing adenosis and ADH and apocrine metaplasia. Wire localization and excision was recommended by MDT, and post-surgical histopathology revealed focal DCIS
Limitations
The principal limitations of this study are:
-
The small number of followed-up patients and the retrospective study design.
-
We had a limited follow-up (6 months–1 year) on women who did not undergo surgery, which decreases our ability to conclude future breast cancer development.
-
The high upgrade rate has been due to preselection of cases in the clinicopathological conference since surgery was only recommended for cases with a discrepancy between imaging and histology.
Its strengths are the accuracy of data collection and the reproducibility of both imaging and surgical procedures, which were always performed with the same specialist’s equipment.
Conclusions
In conclusion, radiological pathological correlation is the essence of high-risk breast lesions management, the use of DCE-MRI including DWI and ADC mapping provides complementary information in detecting high-risk breast lesions and understanding their frequency and upgrade rate to develop a lesion-specific tailored therapy approach to balance underestimation and overtreatment.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- ADH:
-
Atypical ductal hyperplasia
- AIDEP:
-
Atypical intraductal epithelial proliferation
- ALH:
-
Atypical lobular hyperplasia
- BI-RADS:
-
Breast imaging reporting and data system
- DCE -MRI:
-
Dynamic contrast-enhanced magnetic resonance imaging
- DWI:
-
Diffusion-weighted imaging
- FEA:
-
Flat epithelial atypia
- LCIS:
-
Lobular carcinoma in situ
- LN:
-
Lobular neoplasia
- ME:
-
Mass enhancement
- NME:
-
Non-mass enhancement
- NPV:
-
Negative predictive value
- ROC:
-
Receiver operating characteristic
- ROIs:
-
Regions of interest
- VAB:
-
Vacuum-assisted biopsy
References
Orsaria P et al (2020) Heterogeneous risk profiles among B3 breast lesions of uncertain malignant potential. Tumori 106(2):115–125. https://doi.org/10.1177/0300891619868301
Catanzariti F et al (2021) High-risk lesions of the breast: concurrent diagnostic tools and management recommendations. Insights Imag 12(1):1–19. https://doi.org/10.1186/s13244-021-01005-6
Grippo C et al (2020) External validation of a risk stratification score for B3 breast lesions detected at ultrasound core needle biopsy. Diagnostics. https://doi.org/10.3390/DIAGNOSTICS10040181
Giuliani M et al (2018) A new risk stratification score for the management of ultrasound-detected B3 breast lesions. Breast J 24(6):965–970. https://doi.org/10.1111/TBJ.13115
Tagliati C et al (2021) Apparent diffusion coefficient values in borderline breast lesions upgraded and not upgraded at definitive histopathological examination after surgical excision. Polish J Radiol 86(1):e255–e261. https://doi.org/10.5114/pjr.2021.105857
Clauser P, Kapetas P, Stöttinger A, Bumberger A, Rudas M, Baltzer PAT (2021) A risk stratification algorithm for lesions of uncertain malignant potential diagnosed by vacuum-assisted breast biopsy (VABB) of mammographic microcalcifications. Eur J Radiol. https://doi.org/10.1016/J.EJRAD.2020.109479
Rageth J et al (2019) Second international consensus conference on lesions of uncertain malignant potential in the breast (B3 lesions). Breast Cancer Res Treat 174(2): 279–296. https://doi.org/10.1007/S10549-018-05071-1
Shaaban AM, Sharma N (2019) Management of B3 lesions—practical issues. Curr Breast Cancer Rep 11(2):83–88. https://doi.org/10.1007/S12609-019-0310-6
Parijatham ST (2018) Diagnosis and management of high-risk breast lesions. J Natl Compr Canc Netw 16(11):1391–1396. https://doi.org/10.6004/JNCCN.2018.7099
Milos RI et al (2020) The Kaiser score reliably excludes malignancy in benign contrast-enhancing lesions classified as BI-RADS 4 on breast MRI high-risk screening exams. Eur Radiol 30(11):6052–6061. https://doi.org/10.1007/S00330-020-06945-Z/FIGURES/6
Li X, Sun K, Chai W, Zhu H, Yan F (2021) Role of breast MRI in predicting histologic upgrade risks in high-risk breast lesions: a review. Eur J Radiol 142:109855. https://doi.org/10.1016/j.ejrad.2021.109855
Pinder SE et al (2018) NHS Breast Screening multidisciplinary working group guidelines for the diagnosis and management of breast lesions of uncertain malignant potential on core biopsy (B3 lesions). Clin Radiol 73(8):682–692. https://doi.org/10.1016/j.crad.2018.04.004
Heywang-Köbrunner SH, Nährig J, Hacker A, Sedlacek S, Höfler H (2010) B3 lesions: radiological assessment and multi-disciplinary aspects. Breast Care (Basel) 5(4):209–217. https://doi.org/10.1159/000319326
Tezcan S, Ozturk FU, Uslu N, Akcay EY (2021) The role of combined diffusion-weighted imaging and dynamic contrast-enhanced MRI for differentiating malignant from benign breast lesions presenting washout curve. Can Assoc Radiol J 72(3):460–469. https://doi.org/10.1177/0846537120907098
Bonfiglio R, Di Pietro ML (2021) The impact of oral contraceptive use on breast cancer risk: state of the art and future perspectives in the era of 4P medicine. Semin Cancer Biol 72:11–18. https://doi.org/10.1016/j.semcancer.2020.10.008
Cao W, Da Chen H, Yu YW, Li N, Chen WQ (2021) Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J (Engl) 134(7):783–791. https://doi.org/10.1097/CM9.0000000000001474
D’Antonio KB, McGuire M, Rupert K (2021) Managing patients with high-risk breast lesions, precursors on the breast neoplasia pathway. J Am Acad Phys Assist 34(5):35–39. https://doi.org/10.1097/01.JAA.0000742948.34484.aa
Bertani V et al (2020) Atypical ductal hyperplasia: breast DCE-MRI can be used to reduce unnecessary open surgical excision. Eur Radiol 30(7):4069–4081. https://doi.org/10.1007/S00330-020-06701-3/TABLES/3
Cedolini C et al (2019) Risk factors for breast cancer development in patients with borderline breast lesions: a retrospective analysis of our outpatient facility. Eur J Gynaecol Oncol 40(4):572–578. https://doi.org/10.12892/ejgo4536.2019
Mohrmann S et al (2021) Malignancy rate and malignancy risk assessment in different lesions of uncertain malignant potential in the breast (B3 Lesions): an analysis of 192 cases from a single institution. Breast Care. https://doi.org/10.1159/000517109
Rakha EA, Lee AHS, Jenkins JA, Murphy AE, Hamilton LJ, Ellis IO (2011) Characterization and outcome of breast needle core biopsy diagnoses of lesions of uncertain malignant potential (B3) in abnormalities detected by mammographic screening. Int J Cancer 129(6):1417–1424. https://doi.org/10.1002/IJC.25801
Bianchi S et al (2011) Positive predictive value for malignancy on surgical excision of breast lesions of uncertain malignant potential (B3) diagnosed by stereotactic vacuum-assisted needle core biopsy (VANCB): a large multi-institutional study in Italy. Breast 20(3):264–270. https://doi.org/10.1016/j.breast.2010.12.003
Nori J, Kaur M, Kornecki A, Meenal JD, Yaffe MJ (2021) Atlas of contrast-enhanced mammography. Springer International Publishing
Mann RM, Cho N, Moy L (2019) Breast MRI: state of the art. Radiology 292(3):520–536. https://doi.org/10.1148/RADIOL.2019182947/ASSET/IMAGES/LARGE/RADIOL.2019182947.TBL4.JPEG
Zhang L, Tang M, Min Z, Lu J, Lei X, Zhang X (2016) Accuracy of combined dynamic contrast-enhanced magnetic resonance imaging and diffusion-weighted imaging for breast cancer detection: a meta-analysis. Acta Radiol 57(6):651–660. https://doi.org/10.1177/0284185115597265
Lucioni M et al (2021) Positive predictive value for malignancy of uncertain malignant potential (B3) breast lesions diagnosed on vacuum-assisted biopsy (VAB): is surgical excision still recommended? Eur Radiol 31(2):920–927. https://doi.org/10.1007/S00330-020-07161-5/TABLES/5
Houssami N, Ciatto S, Bilous M, Vezzosi V, Bianchi S (2007) Borderline breast core needle histology: predictive values for malignancy in lesions of uncertain malignant potential (B3). Br J Cancer 96:1253–1257. https://doi.org/10.1038/sj.bjc.6603714
Decker T, Ravensburg O, Korsching E, Tio J and Heindel W (2011) Minimal invasive biopsy results of ‘uncertain malignant potential’ in digital mammography screening: high prevalence but also high predictive value for malignancy bildverarbeitung & IT view project case report traumatology view project. https://doi.org/10.1055/s-0031-1273330
Santucci D, Faiella E, Calabrese A, Favale L, Zobel BB, de Felice C (2019) Our radiological experience on B3 lesions: correlation between mammographic and MRI findings with histologic definitive Result. Clin Breast Cancer 19(5):e643–e653. https://doi.org/10.1016/j.clbc.2019.06.004
Londero V, Zuiani C, Linda A, Girometti R, Bazzocchi M, Sardanelli F (2012) High-risk breast lesions at imaging-guided needle biopsy: usefulness of MRI for treatment decision. Am J Roentgenol. https://doi.org/10.2214/AJR.11.7869
Verheyden C et al (2016) Underestimation rate at MR imaging-guided vacuum-assisted breast biopsy: a multi-institutional retrospective study of 1509 breast biopsies. Radiology 281(3):708–719. https://doi.org/10.1148/RADIOL.2016151947/ASSET/IMAGES/LARGE/RADIOL.2016151947.TBL4.JPEG
Cheeney S, Rahbar H, Dontchos BN, Javid SH, Rendi MH, Partridge SC (2017) Apparent diffusion coefficient values may help predict which MRI-detected high-risk breast lesions will upgrade at surgical excision. J Magn Reson Imag 46(4):1028–1036. https://doi.org/10.1002/jmri.25656
Partridge SC, DeMartini WB, Kurland BF, Eby PR, White SW, Lehman CD (2009) Quantitative diffusion-weighted imaging as an adjunct to conventional breast MRI for improved positive predictive value. Am J Roentgenol 193(6):1716–1722. https://doi.org/10.2214/AJR.08.2139
Mooney KL, Bassett LW, Apple SK (2016) Upgrade rates of high-risk breast lesions diagnosed on core needle biopsy: a single-institution experience and literature review. Mod Pathol 29(12):1471–1484. https://doi.org/10.1038/modpathol.2016.127
Acknowledgements
No acknowledgements.
Funding
This research did not receive specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
SH wrote the manuscript and is responsible for correspondence to journal. AM collected patient data and was responsible for image processing and collection of patient’s images. SA &YA participated in the design of the study and performed the statistical analysis. SH &AM conceived of the study and participated in its design and coordination and helped to draft the manuscript. MF & HA were responsible for the review of the draft from a clinical point of view. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Institutional Review Board (IRB) with ethical committee approval number 202111290036. Informed written consent was taken from all subjects.
Consent for publication
All patients included in this research gave written informed consent to publish the data contained within this study.
Competing interests
No financial or non-financial competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hussein, S.A., EL-Dhurani, S., Abdelnaby, Y. et al. High-risk breast lesions: role of multi-parametric DCE-MRI in detection and histopathological upgrade prediction. Egypt J Radiol Nucl Med 53, 212 (2022). https://doi.org/10.1186/s43055-022-00898-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-022-00898-7