Abstract
Background
Hepatocellular carcinoma (HCC) is one of the most common causes of cancer-related deaths worldwide in both men and women. Early-stage HCCs are treated either by curative surgical resection and/or by locoregional interventions, such as radiofrequency ablation or trans-arterial chemoembolization. Functional imaging as diffusion-weighted magnetic resonance imaging (DW MRI) and metabolic imaging with 18F-positron emission tomography/computed tomography (18F-FDG PET/CT) has been used for assessment of treatment response. This retrospective study was conducted on 29 patients known to have HCC with locoregional therapeutic interventions and referred for radiological follow-up searching for residual/recurrence neoplasia or metastatic deposits. The study aimed to assess the benefits of using the metabolic parameters of 18F-FDG PET/CT in the follow-up of HCC patients after therapeutic interventions in comparison with DW MRI.
Results
Regarding qualitative assessment of residual active viable HCC by PET/CT and DW MRI, the sensitivity, specificity, PPV, NPP and accuracy were 77.3%, 91.7%, 94.4%, 68.8%, 82.4% and 95.5%, 75%, 87.5%, 90%, 88.2%, respectively. The optimal cutoff point of the SUVmax to differentiate viable from non-viable HCC was 3.4 (AUC = 0.898), with sensitivity, specificity, PPV and NPV of 77.27%, 100.0%, 100.0% and 66.7%, respectively. The optimal ADC cutoff value for discrimination between viable and non-viable HCC was 1247 mm2/s (AUC = 0.976) with sensitivity, specificity, PPV and NPV of 90.48%, 100.0%, 100.0% and 83.3%, respectively. New hepatic lesions were found in 38.2% of patients by DW MRI, while detected only in 26.5% of patients by PET/CT. The PET/CT revealed extrahepatic metastasis in 44.1% of patients, while detected only in 8.8% of patients by DW MRI.
Conclusions
DWI was more sensitive than PET/CT for detecting tumor residual and hepatic recurrence compared to PET/CT which was much better in detecting distant metastases.
Similar content being viewed by others
Background
Hepatocellular carcinoma (HCC) is the sixth most common malignant tumor and the third most common cause of cancer-related deaths in males and females worldwide [1, 2]. High incidence and mortality for HCCs in many regions around the world are largely attributed to dissemination of hepatitis C virus infection [3]. Advances in therapeutic options make curative treatment possible for almost a third of patients if the cancer was detected in its early stage [1]. Early diagnosis, appropriate evaluation of tumor characteristics, accurate staging and proper assessment of treatment response are essential for treating patients with HCC [4]. Early-stage HCC can be treated by surgical resection and/or locoregional interventions, such as radiofrequency ablation (RFA) or trans-arterial chemoembolization (TACE). Liver transplantation is an alternative option in unresectable HCC that has not metastasized [4]. Locoregional therapies (LRT) can be used to downstage patients prior to liver transplantation. Thus, the evaluation of tumor response after LRT is essential in directing management in HCC [2]. In a high proportion of cases the disease recurs after attempts at curative therapy [3]. Conventional anatomic imaging, such as computed tomography (CT) and magnetic resonance imaging (MRI), often provide equivocal results when used for assessment of treatment response with difficulty in identifying whether residual masses consist of viable tumor tissue or represent post-treatment changes using these methods [5]. A diffusion-weighted image (DWI) is a type of MRI that reflects tissue cellularity. An apparent diffusion coefficient (ADC) is a quantitative value that is calculated from a DWI that correlated with the pathological grades of tumors (high-grade tumors tended to be associated with low ADCs) [4]. Metabolic imaging with 18F-FDG PET/CT has been a very successful modality for detection, staging and restaging various cancers with excellent sensitivity and specificity in certain cancers [6,7,8]. PET/CT is a unique combination of the cross-sectional anatomic information provided by CT and the metabolic information provided by PET, which is acquired during a single examination [9,10,11]. 18F-FDG PET detects an increase in metabolic rate and therefore can be a sensitive tool for the diagnosis and follow-up of metastatic liver tumors [5]. 18F-FDG PET/CT provides a detailed comparison of morphologic post-treatment changes and metabolic activity, which are useful for treatment monitoring and for detection, with high accuracy, of viable tumor following RFA and TACE for HCC [5]. It could guide the optimal treatment (combined strategy) in those patients with HCCs with a high standardized uptake value (SUV) ratio by providing a more aggressive locoregional therapy or a concomitant treatment using another modality, in order to improve survival rates [3]. Detection of extrahepatic FDG-avid metastases originating from HCC has also been reported; especially in cases of less-differentiated HCC, metastases appear to be more FDG avid [12]. So, this study aimed to assess the benefits of using the metabolic parameters of 18F-FDG PET/CT in the follow-up of HCC patients after therapeutic interventions, in comparison with diffusion MRI.
Methods
Patients
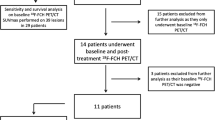
This is a retrospective study, conducted from January 2017 to September 2021 on 32 patients known to have HCC on top of liver cirrhosis presented for radiological follow-up after therapeutic interventions to search for residual/recurrent neoplasia or metastasis. The study received the approval of the institutional ethical committee, and written or informed consents from all patients were taken after explaining the aim of the study.
Inclusion criteria
Patients under regular follow-up post-therapy and patients with elevated tumor marker (AFP) post-therapy, other imaging modalities findings are not conclusive, patients with portal vein thrombosis (for assessment of the type of thrombosis, benign or malignant), and patients planned to perform hepatic transplantation to exclude extrahepatic metastatic disease.
Exclusion criteria
Patients known to have contraindications for MRI (e.g., metal implants as cochlear implants, implanted magnetic device, cardiac defibrillators and pacemakers or claustrophobia), patients with bad general condition needing life support and patients with severe hepato-renal disease, high serum creatinine > 2 mg/dl, patients known to have severe allergy to contrast material. Also, patients with blood glucose level > 200 mg/dl at time of the study and first trimester pregnancy.
Patient preparation
All patients were asked to fast at least 6 h prior to scan with good hydration. All metallic items were removed including pants with zipper, bra, belts, bracelets, etc., and the patients were given gown to wear. Serum glucose was routinely measured prior to 18F-FDG injection, and it should be below 200 mg/dl (including diabetic patients who were advised to properly control their blood glucose level before examination). An intravenous (I.V) cannula was inserted in the patient’s arm for administration of 18F-FDG. The patients were instructed to avoid any kind of strenuous activity prior to the examination (for a minimum of 24 h) and following injection of the radiotracer to avoid physiologic muscle uptake of FDG. To reduce brown fat uptake, a controlled temperature (warm) environment was provided for patients before 18F-FDG injection and low carbohydrate, high fat, protein-permitted diet before the examination. Before MRI scanning, detailed explanation of imaging procedure, including practicing of breath holds, was done.
Technique of 18F-FDG PET/CT scan
The radioactive tracer (18F-FDG) was injected intravenously in a dose of 0.1 mCi/kg body weight. All patients were kept in a warm temperature quiet room and asked to rest without vigorous activity keeping their movements, including talking, at an absolute minimum and void just before imaging. Scanning by a hybrid PET/CT scanner (GE Discovery and Philips Gemini TF (Time-of-Flight) PET/CT machines) was performed 60 min after injection. The patient was positioned supine on the table with a comfortable head fixation position and their arms raised above their heads. We performed low-dose non-enhanced CT scan first and then whole-body PET scan. Triphasic contrast-enhanced CT scan was then performed. The whole study took approximately 20–30 min. Typical whole-body PET/CT scanning began from the skull base and extended caudally to the level of the mid-thighs, the arterial phase CT covering the region of the abdomen from the base of the lungs down to the iliac bones, the venous phase covering from the skull bases down to the mid-thighs and the delayed phase covering the same region as the arterial phase. The total length of CT coverage was an integral number of bed positions scanned during acquisition of PET data. (Approximately 6–7 bed positions are planned in 3D acquisition mode for scanning the entire patient with 3–5-min acquisition at each bed position.) The study was performed with the patient breathing quietly. The scanning parameters for low-dose attenuation correction CT were 120 kV, 100MA, collimator width of (64 × 0.625 mm), pitch of 0.8, gantry rotation time of 0.5 s, and field of view of 50 cm. The scanning parameters for high-dose diagnostic CT were 120 kV, 300MA, collimator width of (64 × 0.625 mm), pitch of 0.8, gantry rotation time of 0.5 s, and field of view of 50 cm. The helical data were retrospectively reconstructed at 1-mm interval. The patient was injected about 100 ml of non-ionic iodinated contrast material using dual syringe Medrad (Stellant) automated injector with injection rate about 2.5 ml/sec; then, liver was scanned in arterial (15–30 s scanning delay), portal (60–90 s scanning delay) and equilibrium/delayed (2–5 min scanning delay) phases. Hundreds of trans-axial PET and CT images were transferred to a dedicated workstation to be reconstructed and then reformatted into coronal and sagittal images to facilitate image interpretation. For each of these sets of PET and CT images, corresponding “fusion” images, combining the two types of data, also were generated.
Technique of DW MRI
The MRI studies were done using 1.5 T MR machines (MR Systems Achieva release 3.2.3.4 2016-6-27 SRN: 35073 and GE HEALTH CARE 1.5-T MRI scanner, USA). The patient is positioned supine on the MRI table. All patients were subjected to MRI study with the basic sequences including T1, T2, and diffusion study with ADC map. The study took about 10–20 min: Axial T1 WIs (with TR 550 ms and TE 24 ms) and axial and coronal T2 WIs (with TR 7300 ms and TE 115 ms). All these sequences are single-shot spin echo with flip angle 90°. Slice thickness = 5 mm; spacing = 1 mm; acquisition matrix = 256 × 224; number of averages = 1; acquisition type = 2D. Axial diffusion-weighted images were acquired with EP technique; images obtained with b = 0, b = 200 and b = 800 were included in the evaluation and in the comparison with the other sequences. TR = 2300 ms, TE = 63 ms, EPI factor = 80, slice thickness = 5 mm, gap = 1 mm, flip angle = 90, acceleration factor = 2, FOV 32–44 cm, number of signal averages (NSA) = 2, acquisition time 39 s, half scan factor = 2, bandwidth = 250 kHz, acquisition matrix 192 × 160, reconstruction matrix = 256 × 256.
Image interpretation
The FDG PET/CT images were evaluated by two radiologists of 12 and 8 years of experience in nuclear imaging, while the MRI images were evaluated blindly and independently by 2 radiologists of 15 and 10 years of experience in abdominal imaging.
The findings were correlated with the findings derived from triphasic contrast-enhanced CT images according to the American Association for the Study of Liver Diseases (AASLD) and LI-RADS v2018 lexicon [13, 14]: LR-TR non-viable for a non-enhancing lesion. LR-TR viable for residual or recurrent tissue within or along the treated HCC or de novo lesions associated with one or more of the following features: 1—arterial phase hyper-enhancement (APHE), 2—washout appearance, 3—enhancement similar to pretreated HCC. Also, the findings were correlated with alpha-fetoprotein (AFP) serum level and histopathologic results (specially in cases with negative CT and elevated AFP).
The main followed lesion was assessed qualitatively in the PET/CT images according to the 18F-FDG accumulation within the main followed lesion or operative bed in surgically treated lesions and any de novo lesions in comparison with the surrounding normal liver tissue, whether visually increased or not. Adequate intervention is considered when the intervention bed appears completely photopenic with no detectable FDG uptake seen within. Recurrent and residual disease defined when the intervention bed margin shows one or more nodular/focal areas of increased FDG uptake, not to be mistaken with reactive hyperemia that appears as uniform low-grade metabolic activity. Satellite/new lesions were defined as the presence of single or multiple hepatic nodules demonstrating focal FDG uptake higher than the surrounding liver parenchyma. Also, metastasis sites were defined by correlating the tracer abnormally high uptake sites and the underlying pathology within the diagnostic body contrast CT images.
Quantitative assessment in the PET/CT images of the suspicious lesions is done by identifying areas of pathologically increased FDG uptake avoiding physiologic uptake. Standardized uptake value (SUV) was independently measured by using region of interest (ROI) drawn on the area of maximal metabolic activity on every axial slice of tumor-related increased FDG uptake; SUVmax was defined as the highest pixel value related to the neoplasm burden in each study.
Qualitative evaluation of the suspected lesions whether the main followed hepatic lesion, new hepatic lesions or suspected metastasis was done by DW-MRI as follows: Malignant lesions were defined as lesions with visually restricted diffusion, measured as increased signal intensity in the DWI, and corresponding decreased signal intensity in the ADC map in comparison with the surrounding normal liver tissue. Benign lesions were defined as lesions with visually facilitated diffusion, has no increased signal intensity in the DWI in comparison with the surrounding normal liver tissue, either similar to the normal liver intensity or decreased and also no decreased signal intensity in the ADC map in comparison with the surrounding normal liver tissue.
While qualitative DWI (signal intensity) was used to predict the nature of the lesion (benign or malignant), quantification of water diffusion was done by obtaining DWIs with multiple b values, which is referred to the ADC value. It is measured in the ADC map by drawing a spherical volume of interest (VOI) on the suspected area to be measured. The area with the more diffusion restriction will show bright signal on DWI and a lower ADC value than that of the area with the less diffusion restriction. The apparent diffusion coefficient (ADC) value was applied for the main followed lesion only, and the mean ADC value was documented as mm2/s.
Two cases were treated surgically with clear operative bed and no evidence of local recurrence/residual neoplasia within the surrounding normal hepatic tissue. In these two cases, no quantitative assessment was done by either the PET/CT SUV, or the DW MRI ADC value.
Statistical analysis
Results were tabulated and statistically analyzed. All tests were two-sided and were performed at the 5% level of significance by using SPSS for Windows, version 18.0 (SPSS Inc., Chicago, IL). Descriptive statistics included median (IQR), mean (x) and standard deviation (SD) and analytic statistics included chi-square test (χ2), Student's t test, Mann–Whitney test (U), Spearman correlation and ROC curve analysis (cutoff values, sensitivity and specificity). P value < 0.05 was considered statistically significant.
Results
A total of 32 patients were enrolled in this study (22 males and 10 females), with ages ranging from 20 to 77 years (the mean age was 59.47 ± 10.84 years), and a total of 34 treated lesions were studied. Most of the studied lesions were treated by RFA 13 (38.2%) and TACE 13 (38.2%), while 3 (8.8%) lesions were treated by ethanol injection, 3 (8.8%) lesions were treated surgically and only 2 (5.9%) lesions were treated by both RFA and ethanol injection. Findings of triphasic PET/CT based upon LI-RADS v2018 revealed LR-TR viable HCC in 22 (64.7%) lesions and LR-TR non-viable/well-treated HCC in 12 (35.3%) lesions (Table 1).
The qualitative assessment of the PET/CT revealed residual active viable HCC in 18 lesions (52.9%) and non-viable HCC in 16 lesions (47.1%) with sensitivity, specificity, PPV, NPP and accuracy of 77.3%, 91.7%, 94.4, 68.8 and 82.4%, respectively. The DWI qualitative assessment revealed 24 lesions (70.6%) with restricted diffusion and 10 lesions (29.4%) showing no diffusion restriction, with sensitivity, specificity, PPV, NPP and accuracy of 95.5%, 75%, 87.5%, 90% and 88.2%, respectively (Figs. 1, 2, 3).
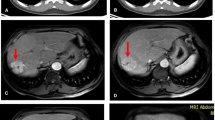
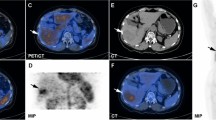
A 66-year-old female patient with a history of segment VII HCC, underwent TACE. TR-LR viable lesion with increased FDG uptake and diffusion restriction, as well as mildly active metastatic pulmonary nodule, with no de novo lesions. MIP image (A) showing focal hepatic right lobar upper segmental increased tracer uptake. Arterial (B) and delayed (C) triphasic CT showing partial lipiodol retention, and residual/recurrent viable tumoral tissue (white arrow) shows APHE and washout. DWI (D) and ADC map (E) shows restricted diffusion in the embolized HCC (white arrow) with mean ADC value measuring 991 mm2/s. Fused PET/CT study shows peripheral increased FDG uptake in the embolized HCC (white arrow) achieving SUVmax of 6.4 (F) and mild FDG uptake in a pulmonary nodule (white arrow) (G)
A 69-year-old female patient with a history of segment VI HCC, had both RFA and ethanol injection. TR-LR viable lesion with increased FDG uptake and diffusion restriction, also had active metastatic cervical lymph node, with two de novo hepatic lesions. MIP image (A) showing focal hepatic right lobar lower segmental increased tracer uptake (horizontal black arrow), two left lobar upper segmental small nodular tracer uptake (vertical blue arrows), and lower neck focal increased nodular uptake. Arterial (B) and portovenous (C) triphasic CT show residual/recurrent viable tumoral tissue (white arrow) shows APHE and washout. DWI (D) and ADC map (E) shows restricted diffusion in the ablated HCC (white arrow) with mean ADC value measuring 949 mm2/s. Fused PET/CT images show increased FDG uptake in the ablated HCC (red circle) achieving SUVmax of 7.1 (F) and focal increased FDG uptake in a right lower deep cervical lymph node (white arrow) (G). De novo two small hepatic left lobar lesions with restricted diffusion are noted at the DW MR axial images (H and I)
A 56-year-old male patient with a history of HCC at segment V, underwent TACE. It was TR-LR non-viable, yet, with increased peripheral FDG uptake in PET/CT and diffusion restriction in DWI. No definite de novo lesions and also no detected metastatic lesions. MIP image (A) showing focal hepatic right lobar lower segmental increased tracer uptake (black arrow). Arterial (B) and portovenous (C) triphasic CT of the lesion showing traces of lipiodol retention, with no evidence of enhancing residual/recurrent viable tumoral tissue (white arrow). DWI (D) and ADC map (E) of the lesion shows restricted diffusion bright signal within the embolized HCC (white arrow); however, the mean ADC value was elevated measuring 2205 mm2/s. Fused PET/CT images show peripheral increased FDG uptake in the embolized HCC (white arrow), achieving SUVmax of 3.4 (F and G) identical to our cutoff value
Regarding the quantitative assessment, the mean value of the SUVmax for the residual viable HCC was significantly higher than the non-viable treated HCC (P < 0.001), while the mean ADC value of the viable HCC was significantly lower than the non-viable HCC (P < 0.001).
The optimal cutoff point of the SUVmax to differentiate viable from non-viable HCC was 3.4 (AUC = 0.898), which revealed sensitivity, specificity, PPV and NPV of 77.27%, 100.0%, 100.0% and 66.7%, respectively. On the other hand, the optimal ADC cutoff value for discrimination between viable and non-viable HCC was 1247 mm2/s (AUC = 0.976) with sensitivity, specificity, PPV and NPV of 90.48%, 100.0%, 100.0% and 83.3%, respectively (Fig. 4, Table 2).
New hepatic lesions were found on 13 patients (38.2%), all of them were detected by DW MRI, while detected only in 9 patients (26.5%) by PET/CT (Figs. 2, 5).
A 57-year-old male patient with a history HCC underwent right lobectomy, had TR-LR viable recurrent lesion at segment IV, with two active focal lesions having diffusion restriction at segments IV and III, as well as active metastatic pulmonary nodules. Arterial (A), venous (B) and delayed (C) triphasic CT show recurrent HCC at segment IV (white arrow) shows APHE and washout. DWI (D) and ADC map (E) shows two focal lesions of restricted diffusion in segment IV (white vertical arrow) with mean ADC value measuring 798 mm2/s, and also at segment III (red horizontal arrow). Fused PET/CT (F) and PET (G) images show increased FDG uptake in both lesions, achieving up to 7.7 SUVmax. Fused PET/CT (H) image revealed active right lung metastatic nodules (white arrows)
The PET/CT revealed extrahepatic metastatic lesions in 15 (44.1%) patients (Figs. 1, 2, 5), while DW MRI revealed extrahepatic metastatic lesions only in 3 (8.8%) of them.
Discussion
Hepatocellular carcinoma is one of the most common malignant tumors worldwide. Early detection and treatment of recurrent HCC after surgical and locoregional interventional managements are important for patient survival [1]. Assessment of tumor response after various therapeutic interventions is important to determine whether the tumor is completely eradicated or needs additional treatment [15]. Diffusion-weighted imaging represents a promising noninvasive diagnostic tool for the evaluation of HCC treatment responses to locoregional therapies. ADC value changes have been shown to occur early after treatment and correlate well with tumor necrosis [5]. PET/CT is a unique combination of the cross-sectional anatomic data provided by CT and the metabolic data provided by PET. It has the advantage of local therapy assessment as well as detection of extrahepatic spread of HCC which is crucial for planning of liver transplantation [16].
Our study showed that qualitative method using PET/CT and DW MRI in assessment of HCC patients after therapeutic interventions had a sensitivity of 74.60% and 81.30%, specificity of 57.54% and 69.20%, PPV of 87.5% and 94.1%, NPV of 90.0% and 68.58% as well as accuracy of 88.2% and 82.4%, respectively. For the PET/CT, similar results were also noted by Kim et al. and Song et al.; they revealed a sensitivity of 87.5% and 89.3%, specificity of 71.4% and 65.7% and accuracy of 80% and 80.2%, respectively [17, 18]. For the DW MRI, Ebeed et al. revealed 82.3% sensitivity and 73.9% specificity and Saleh et al. revealed a lower sensitivity of 52.6% and higher specificity of 90.5% [19, 20].
In this study, the range of SUVmax of the lesions with residual/recurrent activity was 2.20–17.20 with median (IQR) 5.55 (3.6–7.9), while the range of ADC value of the same lesions was 690–1310 with mean 1055.71 ± 164.83. In the same line, the study by Hetta W and Atyia H found that the median value of tumor SUVmax in positive cases was 6.6 (ranged from 1.4 to 24), most of them were poorly differentiated HCCs, yet a single case measured about 1.4 SUVmax (well-differentiated HCC type) [21]. In the study done by Ahn et al., the median value of tumor SUVmax was 4.3 (ranged from 2.0 to 11.6) [22]. Also, a study done by Song et al., over 83 patients with HCC to investigate the correlation of 18F-FDG PET/CT with clinical features and the prediction of treatment response, found that SUVmax ranged from 1.5 to 20.8, with a cutoff value of 4.0 [23]. The study by Abduljaleel et al. revealed that the mean ADC value of ablated zones was significantly decreased in patients with residual lesion than in patients without residual lesion [5]. The study by Yu et al. reported that the mean ADC value for necrotic lesions was 1.16 and 1.24 × 10−3 mm2/sec as detected by reader 1 and 2, respectively [24].
Our study showed that SUVmax value of PET/CT and ADC mean value of MRI in assessment of HCC patients after therapeutic interventions had a sensitivity of 77.27%, and 90.48%, specificity of 100.0% and 100.0%, PPV of 100.0% and 100.0%, NPV of 66.7% and 83.3% with a cutoff value > 3.4 and > 1247, at AUC of 0.898 and 0.976, respectively. In the same line, the study by Ahmed et al. found that the sensitivity, specificity, PPV, NPV and accuracy of SUVmax were 85.24%, 75%, 87.24%, 75% and 82%, respectively [25]. These results are comparable to many studies as Song et al., who reported that SUVmax sensitivity, specificity and accuracy for detection of viable HCC after TACE were 89.29%, 65.71% and 80.22%, respectively in detection of local viable tumoral tissue following TACE [23]. Also, a higher ADC mean cutoff value was reported by Ebeed et al. who revealed a sensitivity of 76.5% and specificity of 65.2% at a cutoff value of 1380 mm2/s [19].
The specificity and accuracy of quantitative ADC value were higher than the DWI qualitative assessment alone for the detection of residual/recurrent HCC. The present study also showed a significant negative correlation between SUVmax with ADC value. Our results agree with the study by Tyng et al., who found the existence of a significant inverse correlation between SUVmax and ADC values in HCC (high values of SUVmax are associated with low values of ADC) [26]. Also, the study of Regier et al. [27] verified the significant inverse correlation between these two quantitative variables (SUVmax and ADC) in 41 patients. Another study by Nunez et al. [28] observed an inverse significant correlation between the mean SUV and the mean ADC. These results demonstrate that there is a direct relationship between the mobility of water molecules in the tissue evaluated by DW-MRI and glycolytic metabolism evaluated by PET/CT, possibly related to tumor aggressiveness.
On the other hand, the studies by Freihat et al., Min et al. and Surov et al. found that SUVmax was not significantly correlated with the ADC value [29,30,31]. The explanation for the lack of correlation is the fact that both imaging parameters explain different tissue microstructures characteristics. DWI assesses the water molecule motion in the tissue and is affected by the cellularity, proliferation rate and cell count which, in clinical use, is affected by ROI size placement and inter-observer variability, while metabolic activity was independent of tumor size and shape because tumor is segmented by adaptive thresholding [32, 33].
DW MRI was better than FDG PET/CT in detecting new non-treated hepatic lesions. In our study, de novo HCC lesions were found in 13 patients (38.2%) based on triphasic CT LI-RADS criteria, all of them were also diagnosed by MRI, while diagnosed by FDG PET/CT in only 9 patients (26.5%).
FDG PET/CT had a great advantage in detecting extrahepatic metastases; in our study, extrahepatic metastases were detected by FDG PET/CT in 15 patients, three of them only were also seen in the DWI, as some of the metastases were detected within the abdominal LNs and the other metastatic lesions were seen sporadically distributed within the body.
In the present study, 13 lesions (38.2%) were treated by RFA, 13 lesions (38.2%) by TACE (38.2%), 3 lesions (8.8%) had ethanol injection, 3 lesions (8.8%) had surgical management and only 2 lesions (5.9%) were managed by both ethanol injection and RFA. A study by Kim et al. revealed that, during follow-up, disease progression was observed in RFA as well as TACE patients and combination therapy of TACE with RFA is a safe and effective treatment for patients with medium-to-large HCC, with the long-term beneficial effect of retarding tumor progression [34]. According to our results, we found no significant difference in detecting residual or recurrent viable HCC on using different methods of therapeutic interventions (P value > 0.05).
So, we proposed that if a local residual or recurrent tumoral tissue is suspected following therapeutic intervention, the imaging modality of choice should be MRI with basic anatomical and dynamic sequences, and also functional images such as DWIs and ADC; however, if distant metastasis is suspected, high serum levels of alpha-fetoprotein or liver transplantation is intended, then PET/CT is the imaging modality of choice.
Conclusions
18F-FDG PET/CT and DW MRI are effective functional imaging modalities for assessment of HCC patients after therapeutic intervention. DWI was more sensitive than PET/CT for detecting hepatic tumor residual and recurrence, and adding the quantitative assessment using the ADC value increases its accuracy, compared to PET/CT which was much better in detecting distant metastases. Also adding triphasic protocol to the diagnostic contrast whole-body CT of the standard PET/CT protocol will be of great diagnostic benefit with no additional risks nor costs upon the patient.
Availability of data and material
All data generated or analyzed during this study are included in this article.
Abbreviations
- HCC:
-
Hepatocellular carcinoma
- 18F-FDG:
-
18F-Fluorodeoxyglucose
- PET/CT:
-
Positron emission tomography/computed tomography
- MRI:
-
Magnetic resonance imaging
- DWI:
-
Diffusion-weighted images
- RFA:
-
Radiofrequency ablation
- TACE:
-
Trans-arterial chemoembolization
- LRT:
-
Locoregional therapies
- ADC:
-
Apparent diffusion coefficient
- SUV:
-
Standard uptake value
- mg/dL:
-
Milligram per deciliter
- mCi:
-
Millicurie
- Kg:
-
Kilogram
- mL:
-
Milliliter
- mA:
-
Milliampere
- kV:
-
Kilovolt
- s:
-
Second
- mm:
-
Millimeter
- AFP:
-
Alpha-fetoprotein
- NPV:
-
Negative predictive value
- PPV:
-
Positive predictive value
References
Chotipanich C, Kunawudhi A, Promteangtrong C, Tungsuppawattanakit P, Sricharunrat T, Wongsa P (2016) Diagnosis of hepatocellular carcinoma using C11 choline PET/CT: comparison with F18 FDG, contrast enhanced MRI and MDCT. Asian Pac J Cancer Prev 17(7):3569–3573
Gordic S, Corcuera-Solano I, Stueck A, Besa C, Argiriadi P, Guniganti P et al (2017) Evaluation of HCC response to locoregional therapy: validation of MRI-based response criteria versus explant pathology. J Hepatol 67(6):1213–1221
López NO (2015) PET/computed tomography in evaluation of trans arterial chemoembolization. PET Clin 10(4):507–517
Hong CM, Ahn BC, Jang YJ, Jeong SY, Lee SW, Lee J (2017) Prognostic value of metabolic parameters of 18F-FDG PET/CT and apparent diffusion coefficient of MRI in hepatocellular carcinoma. Clin Nucl Med 42(2):95–99
Abduljaleel JK, Hamid HF, Soliman AH (2018) Role of diffusion weighted MR imaging in assessment of hepatocellular carcinoma after radiofrequency ablation. Egypt J Hosp Med 71(5):3229–3231
Sarhan EA, El Gohary MI, El Moneim LA, Ali SA (2020) Role of 18 fluorine-fluorodeoxyglucose positron emission tomography/computed tomography in assessment of neoadjuvant chemotherapy response in breast cancer patients. EJRNM 51:116
Ali SA, Abdelkawi MM, Hussien NM (2019) Delayed post-diuretic 18F-FDG PET/CT: can it help in determination of the best clinical decision for muscle invasive UB cancer patients? EJRNM 50:111n
Tawfik MM, Monib AM, Yassin A, Ali SA (2020) Comparison between RECIST and PERCIST criteria in therapeutic response assessment in cases of lymphoma. EJRNM 51:82
Ali SA, AbdElkhalek YI (2016) Added value of combined 18F-FDG PET/CT for detection of osseous metastases in cancer patients. EJRNM 47(2):453–458
Kapoor V, McCook BM, Torok FS (2004) An introduction to PET/CT imaging. Radiographics 24(2):523–543
Ali SA, AbdElkhalek YI (2016) Value of integrated PET/CT in detection of hepatic metastatic deposits. EJRNM 47(2):459–465
Tsurusaki M, Okada M, Kuroda H, Mastsuki M, Ishii K, Murakami T (2014) Clinical application of 18F-fluorodeoxyglucose positron emission tomography for assessment and evaluation after therapy for malignant hepatic tumor. J Gastroenterol 49:46–56
American College of Radiology. CT/MRI LI-RADS v2018 core. Liver Imaging Reporting and Data System. https://www.acr.org/-/media/ACR/Files/RADS/LI-RADS/LI-RADS-2018-Core.Pdf
Marrero JA, Kulik LM, Sirlin CB et al (2018) Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 68(2):723–750
Mansour MG, Ali SA (2016) Transarterial chemoembolization using drug eluting microspheres in refractory colorectal liver metastases with 18F-FDG PET/CT follow-up to assess therapeutic response. EJRNM 47(4):1467–1472
Ali SA, Amin DH, Abdelkhalek YI (2020) Efficiency of whole-body 18F-FDG PET CT in detecting the cause of rising serum AFP level in post-therapeutic follow-up for HCC patients. Jpn J Radiol 38:472–479
Kim SH, Won KS, Choi BW et al (2012) Usefulness of F-18 FDG PET/CT in the evaluation of early treatment response after interventional therapy for hepatocellular carcinoma. Nucl Med Mol Imaging 46:102–110
Song HJ, Cheng JY, Hu SL, Zhang GY, Fu Y, Zhang YJ (2015) Value of 18F-FDG PET/CT in detecting viable tumor and predicting prognosis of hepatocellular carcinoma after TACE. Clin Radiol 70(2):128–137
Ebeed A, Romeih M, Refat M et al (2017) Role of dynamic contrast enhanced and diffusion weighted MRI in evaluation of hepatocellular carcinoma after chemoembolization. Egypt J Radiol Nucl Med 48:807–815
Saleh TY, Bahig S, Shebrya N et al (2019) Value of dynamic and DWI MRI in evaluation of HCC viability after TACE via LI-RADS v2018 diagnostic algorithm. Egypt J Radiol Nucl Med 50:113
Hetta WM, Atyia HR (2020) Role of PET CT in comparison to triphasic CT in early follow-up of hepatocellular carcinoma after trans arterial chemoembolization. Egypt J Radiol Nuclear Med 51:33
Ahn SG, Kim SH, Jeon TJ, Cho HJ, Choi SB, Yun MJ et al (2011) The role of preoperative [18F] fluorodeoxy glucose positron emission tomography in predicting early recurrence after curative resection of hepatocellular carcinomas. J Gastrointest Surg 15(11):2044–2052
Song MJ, Bae SH, Yoo IR, Park CH, Jang JW, Chun HJ et al (2012) Predictive value of 18F-fluorodeoxyglucose PET/CT for trans arterial chemolipiodolization of hepatocellular carcinoma. World J Gastroenterol 18(25):3215
Yu JI, Park HC, Lim DH, Choi Y, Jung SH, Paik SW et al (2014) The role of diffusion-weighted magnetic resonance imaging in the treatment response evaluation of hepatocellular carcinoma patients treated with radiation therapy. Int J Radiat Oncol Biol Phys 89(4):814–821
Ahmed EI-Hassan MS, Abdel-Mutaleb MG (2018) The role of diffusion weighted magnetic resonance imaging and subtraction magnetic resonance imaging in assessing treatment response of hepatocellular carcinoma after transarterial chemoembolization. Egypt J Hosp Med 72(3):4165–4174
Tyng CJ, Guimarães MD, Bitencourt AG, dos Santos LC, Barbosa PN, Zurstrassen CE et al (2018) Correlation of the ADC values assessed by diffusion-weighted MRI and 18 F-FDG PET/CT SUV in patients with HCC. Appl Cancer Res 38(1):1–7
Regier M, Derlin T, Schwarz D, Laqmani A, Henes FO, Groth M (2012) Diffusion weighted MRI and 18F-FDG PET/CT in non-small cell lung cancer (NSCLC): does the apparent diffusion coefficient (ADC) correlate with tracer uptake (SUV)? Eur J Radiol 81(10):2913–2918
Nunez DA, Medina AL, Iglesias MM, Gomez FS, Dave A, Hatzoglou V et al (2017) Multimodality functional imaging using DW-MRI and 18F-FDG-PET/CT during radiation therapy for human papillomavirus negative head and neck squamous cell carcinoma: Meixoeiro Hospital of Vigo Experience. World J Radiol 9(1):17
Freihat O, Tó Z, Pinter T, Kedves A, Sipos D, Cselik Z et al (2020) Association between diffusion weighted-imaging (DWI) and simultaneous 18F-FDG-PET/MRI parameters with a comparison of their diagnostical role in head and neck squamous cell carcinoma (HNSCC). Eur J Radiol 130:109157
Min M, Lee MT, Lin P, Holloway L, Wijesekera D, Gooneratne D et al (2016) Assessment of serial multi-parametric functional MRI (diffusion-weighted imaging and R 2*) with 18F-FDG-PET in patients with head and neck cancer treated with radiation therapy. Br J Radiol 89(1058):20150530
Surov A, Stumpp P, Meyer HJ, Gawlitza M, Höhn AK, Boehm A et al (2016) Simultaneous 18F-FDG-PET/MRI: associations between diffusion, glucose metabolism and histopathological parameters in patients with head and neck squamous cell carcinoma. Oral Oncol 58:14–20
Lambregts DM, Beets GL, Maas M, Curvo-Semedo L, Kessels AG, Thywissen T, Beets-Tan RG (2011) Tumour ADC measurements in rectal cancer: effect of ROI methods on ADC values and interobserver variability. Eur Radiol 21(12):2567–2574
Jeong JH, Cho IH, Chun KA, Kong EJ, Kwon SD, Kim JH (2016) Correlation between apparent diffusion coefficients and standardized uptake values in hybrid 18F-FDG PET/MR: preliminary results in rectal cancer. Nucl Med Mol Imaging 50(2):150–156
Kim JW, Kim JH, Sung KB, Ko HK, Shin JH, Kim PN et al (2014) Transarterial chemoembolization vs. radiofrequency ablation for the treatment of single hepatocellular carcinoma 2 cm or smaller. Off J Am Coll Gastroenterol 109(8):1234–1240
Acknowledgements
Not applicable.
Funding
This work has not received any funding.
Author information
Authors and Affiliations
Contributions
IT carried out the PET/CT studies and collected the data. SA, MH and HA participated in the design of the study. IT performed the statistical analysis, and SA drafted the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the Research Ethics Committee (REC) of Ain Shams University, Faculty of Medicine (FMASU M D 212/2018), and written informed consent was obtained from all patients to participate in the study.
Consent for publication
Written informed consent was obtained from all patients for publication of the study.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nashi, I.T., Morsy, H.A., Shalaby, M.H. et al. Role of 18F-FDG PET/CT in assessment of HCC patients after therapeutic interventions compared to DW MRI. Egypt J Radiol Nucl Med 53, 186 (2022). https://doi.org/10.1186/s43055-022-00867-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-022-00867-0