Abstract
Background
During pancreaticoduodenectomy proper dissection of local vessels is required. Normal coeliac and hepatic arterial anatomy can be found in only 50–70% of individuals. Good knowledge about aberrant vascular anatomies is necessary to avoid unnecessary complications.
Case presentation
An elderly gentleman presented to us with history of jaundice. Periampullary carcinoma with abnormal right and left hepatic artery morphology was discovered after a contrast enhanced computerized tomography scan.
Conclusion
Despite the anomalous origin and anterior course of replaced right hepatic artery, Classical pancreatoduodenectomy with preservation of replaced right hepatic artery and regional lymphadenectomy with no major intra and post-operative problems was conducted by superior mesenteric artery first approach. Prior to major hepato-pancreatobiliary surgery, a thorough examination of a contrast enhanced computerized tomography scan is required to understand vascular anatomy, recognize anomalous vessels, and understand their significance. Nevertheless, if the abnormal vessel anatomy like replaced right hepatic artery are identified during surgery, a careful dissection of the anomalous vessel is essential to identify all vascular relationships and avoid irreversible injury.
Similar content being viewed by others
Background
The standard surgical procedure for the management of malignant diseases of the peri-ampullary region (head of the pancreas, ampulla of Vater, distal common bile duct, and second part of the duodenum), extremely selective cases of chronic pancreatitis, and severe trauma to the pancreatic head and duodenum is pancreaticoduodenectomy (PD) [1, 2]. It is a complex and highly challenging operation for surgeons. Vascular anomalies are a rule rather than exception during this surgery. The coeliac trunk usually emerges anteriorly from the abdominal aorta at the twelfth thoracic vertebrae. Then it runs anteriorly or somewhat anterolaterally in the lesser sac eventually splitting into three branches near the pancreas upper border: the left gastric artery (LGA), the splenic artery (SA), and the common hepatic artery (CHA) [3]. Only 55–79% of patients have this normal vascular anatomy, whereas the remaining cases have vascular alterations which are called as “anomalous/aberrant vascular anatomy” and these alterations may contribute to the surgical difficulties during PD [4,5,6]. The course of these vascular anomalies varied according to their origin. Some vascular abnormalities (e.g., replaced right hepatic artery (RRHA)) may demand change in surgical approach and may also interfere with resection and reconstruction of the digestive tract during pancreaticoduodenectomy whereas others (e.g., replaced left hepatic artery (RLHA), accessory hepatic artery (AHA)) may not [7, 8]. Hepatic artery and its anomalies were described into 10 most common variants by Michels [6] based on anatomy of 200 cadaver livers. Later Hiatt et al. [9] simplified Michel classification by proposing a classification scheme with 6 arterial variants. On the basis of the literature in relation to pancreatic head resection, two distinct CHA/RRHA pathways arising from SMA may be identified. These two different pathways are: (1) The extra-parenchymal route (outside the pancreas head)- CHA/RRHA emerges from SMA and passes posterior to the pancreatic head and then to liver. In this scenario, the dissection of artery and separating it from pancreatic head can be done without much difficulty if course has been identified pre operatively. (2) the intra parenchymal route (within the pancreatic head)-CHA/RRHA emerges from the superior mesenteric artery and travels through the pancreatic head parenchyma then to the liver hilum; it may be difficult to preserve the intra parenchymal component of the CHA/RRHA in this situation (when saving of CHA/RRHA is impossible it is necessary to divide and reconstruct the vessel by anastomosing to gastroduodenal artery (GDA)/Aorta/ LGA) [10]. Failing to recognize such vascular abnormalities before surgery may raise the risk of iatrogenic vascular damage. The presence of above anatomical variations may increase the risk of complications through direct (bleeding due to iatrogenic intraoperative vessel injury) or indirect (postoperative ischemia of tissues and anastomotic leakage) mechanisms. Preoperative contrast-enhanced computerized tomography (CECT) scans are useful in guiding the surgeon throughout the PD surgery and reducing the risk of intra/postoperative complications [11]. In this paper, we present a very rare case of replaced right hepatic artery arising from the superior mesenteric artery coursing anterior to pancreatic head associated with replaced left hepatic artery originating from the left gastric artery.
Case presentation
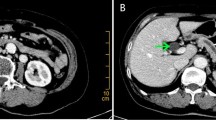
A 68-year-old gentleman who is a known diabetic and hypertensive was admitted to our hospital with complaints of painless progressive jaundice, loss of weight and loss of appetite. Laboratory tests revealed elevation of bilirubin (2.8 mg/dl) and mild elevation of Serum tumor marker CEA (6.4 ng/mL) and normal CA 19-9 (65 U/mL). Triphasic CECT whole abdomen was done. Axial 0.5 mm thin section CT scans was performed through the abdomen with intravenous nonionic contrast of 100 ml and negative oral contrast (water). A plain scan was followed by an arterial, portal (35 s post injection) and late venous phase (70 s post injection) scans. After a study of the axial section, thorough reconstructions were performed in the 3D volume rendered, multiplanar and curved planes and then studied. On CT scan he was found to have periampullary tumor of size 2.5 cm with anomalous vascular anatomy. It also showed RRHA arising from SMA and RLHA arising from LGA. Proper hepatic artery (PHA) continued as segment 4 artery. This RRHA originated from SMA just below neck of pancreas travelled anterior to pancreatic head then coursing cranially between first part of duodenum and Common bile duct (CBD), then it travelled along anterior wall of CBD to reach the hilum (Figs. 1, 2, 3). The tumor was deemed resectable by the multidisciplinary board in the absence of vascular involvement and distant metastases, and the patient was scheduled for surgery. After a protracted Kocher's maneuver and meticulous dissection of the peripancreatic and retroperitoneal area using the "artery first" approach, a palpable mass 2 × 2 cm in the periampullary region was discovered intraoperatively, and suspected hepatic artery abnormalities were verified. This anomalous right hepatic artery originated from SMA just below neck of pancreas traversed anterior to pancreatic head giving rise to inferior pancreaticoduodenal artery, then coursing on superior border of pancreas between first part of duodenum and CBD giving Gastro-duodenal artery (GDA) as branch and then continued anterior to CBD to supply right lobe of liver (Figs. 4, 5, 6). Using meticulous dissection, we successfully dissected RRHA away from pancreatic head, looped and spared it. The RRHA was completely dissected along its course and GDA was ligated and divided. RRHA was dissected along its course upto hilum separating it from CBD and preserving it. Simultaneously, we detected an RLHA arising from the LGA. After securing and confirming RRHA pancreatic neck transaction was done. Classical pancreaticoduodenectomy with lymphadenectomy was performed. Patient had chylous output through drain, with raised triglyceride levels of drain fluid, on post operative day 5 which was managed conservatively. Patient was discharged from the hospital on 14th postoperative day. Pathologic examination of the operative specimen showed a moderately differentiated periampullary adenocarcinoma of stage T2N0M0 with negative margin (R0).
Discussion
Whipple surgery is the standard curative surgery for periampullary malignancy globally [12]. Despite the technical advancements, pancreaticoduodenectomy (Whipple surgery) remains a complex operation with significant morbidity and mortality rates even at experienced centers [13]. To make matters even more difficult, vascular anatomy is hindered by a great deal of variation. Michels [6] classified these hepatic arterial anomalies into 10 types and a modified classification was given by Hiatt et al. [9]. In the literature, there is some ambiguity on when to use the terminology 'aberrant’, ‘replaced’, and 'accessory’. In 11–21% of patients, the right hepatic artery (RHA) can be replaced (taking a path other than the normal course) [3, 14]. Most common anomaly is Michel's Type III i.e., arising from SMA [3, 14]. In 5% of cases, an accessory RHA (a blood supply other than the main artery) arises from the superior mesenteric artery, celiac trunk, or aorta [3, 14]. Both forementioned situations are classified as atypical RHA anatomy, which is referred to as aberrant RHA anatomy. The following are the most typically described anatomical variants of the hepatic artery: (1) an anomalous RHA from the SMA (10–21%); (2) replaced left hepatic artery (LHA) from the LGA (4–10%); (3) replaced RHA and LHA; (4) an accessory RHA and/or LHA (18%); (5) replaced CHA from the SMA or aorta (0.4–4.5%); or (6) quadrifurcation of hepatic artery [15]. Intricate embryological development is to blame for this abnormal vascular structure.
We here report a rare case of combination of RRHA originating from SMA passing anterior to head of pancreas and RLHA from LGA. RRHA after originating from SMA passes anterior to pancreas head and then anterior to CBD (Figs. 7 and 8). This course of anterior passage of RRHA is very rare. There are numerous anatomical variants (1.4–3%) that cannot be described using Michels and Hiatt’s classifications [16]. Using CT scan and digital subtraction angiography, Song et al. [3] presented the biggest dataset of celiac and hepatic artery variations in 5002 individuals. They described suprapancreatic, infrapancreatic, trans-pancreatic, pre superior mesenteric vein, and retro superior mesenteric vein, courses of CHA/RRHA [3]. Jah et al. [10], after observing 135 patients, proposed three different anatomical courses of RRHA in relation to the pancreatic head. Type 1 has a posterior route with respect to pancreatic head, Type 2 has intraparenchymal course and type 3 have a deeper route in the superior mesenteric vein (SMV) groove [10]. However, none of the anomalies described followed the same prepancreatic path as described in our case. The importance of this artery stems from the possibility of mistaking it for GDA, which comes from CHA, and ligating it while doing dissection, resulting in a reduction in blood flow to the liver and biliary tract. At this juncture, a clamp test would at the very least alert us to a problem. Furthermore, because this artery starts from the SMA near the pancreas neck and travels anteriorly, it might be damaged during neck transection or uncinate dissection. As a result, this case was set up for SMA first, with careful dissection of the artery from the pancreatic head using scissor and bipolar cautery. A non-artery first approach might have cause difficulty in recognizing the proper course of vessel and result in vascular damage at various points during the surgery, which would go unnoticed intra-operatively. Because hepatic artery primarily supplies the biliary system, maintaining arterial circulation to the liver during pancreaticoduodenectomy is critical. Damage to these can lead to bile leaks or biliomas, which can cause significant morbidity in the post-operative period. If arterial variations were discovered during the procedure, rather than prior to it, it could impact the outcome of pancreaticoduodenectomy and raise the rate of surgical complications such as pancreatic fistula, haemorrhage, hepatic ischemia or failure, and bile leakage [17]. It is possible to avoid morbidity and mortality by being aware of arterial aberrance and modifying surgical strategies accordingly.
Angiography can provide important details about aberrant vessels, but it does not provide details about relationship between the paths of the arteries and the pancreatic parenchyma, which is important information during presurgical planning. Hence contrast enhanced computed tomography with angiography becomes necessary in preoperative planning [11]. In major hepatobiliary and pancreatic surgery, various strategies have been described to manage an aberrant RHA: (1) resection versus preservation/reconstruction depending on whether the aberrant RHA is accessory or replaced, as well as depending on course of the artery in relation to pancreatic head and depending on whether the tumor infiltrates the vessel; (2) preoperative embolization; (3) temporary clamping and confirmation collateral circulation before ligation if discovered intraoperatively; and (4) neoadjuvant chemotherapy and reassessment [15, 18, 19]. In situations of early detection and proper therapy, an aberrant RHA appears to have no significant influence on the postoperative course and oncological results following PD [19, 20]. To minimize needless problems during and after PD surgery, a multidetector CT scan with reconstruction forms important part of preoperative evaluation and any aberrant vessels and there course should be noted and surgery planning should be done accordingly. Following that, the surgical strategy and intraoperative procedure are determined by the found vascular anatomical differences. If hepatic artery abnormalities are discovered during surgery, intraoperative treatment options include-ligation, dissection, and traction away from the dissection site, division, and anastomosis [15].
Conclusion
The most common aberration in the hepatic arterial tree is an aberrant right hepatic artery. A careful examination of contrast enhanced CT scan is essential prior to major hepato-pancreatobiliary surgery, to grasp vascular anatomy, locate anomalous vessels, and understand their relevance to avoid unintended adverse effects. Vascular anomaly may also determine surgery strategy, as in this case, the SMA first approach. Careful preoperative contrast enhanced CT scan examination and meticulous surgical technique, increases the possibility of preserving an aberrant hepatic artery and avoiding morbidity post operatively.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AHA:
-
Accessory hepatic artery
- CA 19-9:
-
Carbohydrate antigen 19-9
- CEA:
-
Carcinoembryonic antigen
- CBD:
-
Common bile duct
- CHA:
-
Common hepatic artery
- CT:
-
Computerized tomography
- CECT:
-
Contrast-enhanced computerized tomography
- GDA:
-
Gastroduodenal artery
- HPB:
-
Hepato Pancreato Biliary
- LGA:
-
Left gastric artery
- LHA:
-
Left hepatic artery
- PD:
-
Pancreaticoduodenectomy
- PHA:
-
Proper hepatic artery
- RLHA:
-
Replaced left hepatic artery
- RRHA:
-
Replaced right hepatic artery
- RHA:
-
Right hepatic artery
- SA:
-
Splenic artery
- SMA:
-
Superior mesenteric artery
- SMV:
-
Superior mesenteric vein
References
Scaife CL, Hewitt KC, Mone MC, Hansen HJ, Nelson ET, Mulvihill SJ (2014) Comparison of intraoperative versus delayed enteral feeding tube placement in patients undergoing a Whipple procedure. HPB 16(1):62–69
Leichtle SW, Kaoutzanis C, Mouawad NJ, Welch KB, Lampman R, Hoshal VL et al (2013) Classic Whippleversus pylorus-preserving pancreaticoduodenectomy in the ACS NSQIP. J Surg Res 183(1):170–176
Song SY, Chung JW, Yin YH, Jae HJ, Kim HC, Jeon UB et al (2010) Celiac axis and common hepatic artery variations in 5002 patients: Systematic analysis with spiral CT and DSA. Radiology 255:278–288
Koops A, Wojciechowski B, Broering DC, Adam G, Krupski-Berdien G (2004) Anatomic variations of the hepatic arteries in 604 selective celiac and superior mesenteric angiographies. Surg Radiol Anat 26(3):239–244
Covey AM, Brody LA, Maluccio MA, Getrajdman GI, Brown KT (2002) Variant hepatic arterial anatomy revisited: digital subtraction angiography performed in 600 patients. Radiology 224(2):542–547
Michels NA (1966) Newer anatomy of the liver and its variant blood supply and collateral circulation. Am J Surg 112(3):337–347
Shukla PJ, Barreto SG, Kulkarni A et al (2010) Vascular anomalies encountered during pancreatoduodenectomy: do they influence outcomes? Ann Surg Oncol 17:186–193
Sakorafas GH, Friess H, Balsiger BM et al (2001) Problems of reconstruction during pancreatoduodenectomy. Dig Surg 18:363–369
Hiatt JR, Gabbay J, Busuttil RW (1994) Surgical anatomy of the hepatic arteries in 1000 cases. Ann Surg 220:50–52
Jah A, Jamieson N, Huguet E et al (2009) The implications of the presence of an aberrant right hepatic artery in patients undergoing a pancreaticoduodenectomy. Surg Today 39:669–674
Aghalarov I, Lutz T, Uhl W, Belyaev O (2021) Preserving a rare type of variant right hepatic artery combines surgical radicality and intact liver perfusion during pancreatectomy. Visc Med 37:219–221
Kamisawa T, Wood LD, Itoi T, Takaori K (2016) Pancreatic cancer. Lancet 388:73–85
Sánchez-Velázquez P, Muller X, Malleo G, Park JS, Hwang HK, Napoli N et al (2019) Benchmarks in pancreatic surgery: a novel tool for unbiased outcome comparisons. Ann Surg 270:211–218
Panagouli E, Venieratos D, Lolis E, Skandalakis P (2013) Variations in the anatomy of the celiac trunk: a systematic review and clinical implications. Ann Anat 195:501–511
Pallisera A, Morales R, Ramia JM (2014) Tricks and tips in pancreatoduodenectomy. World J Gastrointest Oncol 6:344–350
Sakamoto Y, Fujikawa T, Tanaka A (2017) Successful radical resection of pancreatic head carcinoma in a patient with replaced right hepatic artery originating from posterior inferior pancreaticoduodenal artery: a case report. Surg Case Rep 3(1):78
Chamberlain RS, El-Sedfy A, Rajkumar D (2011) Aberrant hepatic arterial anatomy and the Whipple procedure: lessons learned. Am Surg 77:517–526
El Amrani M, Pruvot FR, Truant S (2016) Management of the right hepatic artery in pancreaticoduodenectomy: a systematic review. J Gastrointest Oncol 7(2):298–305
Trofin AM, Vlad N, Zabara M, Rusu-Andriesi D, Bradea C, Vornicu A et al (2016) Pancreaticoduodenectomy in patients with hepatic artery anatomic variants: tailoring, perioperative care and surgical outcomes. Rev Med Chir Soc Med Nat Iasi 120(4):874–879
Németh K, Deshpande R, Máthé Z, Szuák A, Kiss M, Korom C et al (2015) Extrahepatic arteries of the human liver—anatomical variants and surgical relevancies. Transpl Int 28(10):1216–1226
Acknowledgements
I thank Ms. Greeshma S, and Mrs Askhaya P , Research Assistants from the DME Office, Apollo Mains Hospitals for their expertise and help in proofreading the manuscript and uploading it in the journal.
Funding
There was no funding for this study.
Author information
Authors and Affiliations
Contributions
R., T.G.B., R.P., S.K.S.: Study design, Conceptualization methodology supervision and interpretation. R., T.G.B., S.K.S.: Study coordination. R.: Drafted the work. All authors constitute the surgery team. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The patient gave his written informed consent to publish the case and the images.
Consent for publication
Institutional Consent was obtained for the study.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Raghavendra, D., Balachandar, T.G., Prabhakaran, R. et al. Replaced right hepatic artery passing anterior to pancreas: a rare and challenging anatomical variant during pancreaticoduodenectomy. Egypt J Radiol Nucl Med 53, 45 (2022). https://doi.org/10.1186/s43055-022-00721-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-022-00721-3