Abstract
Background
There are some limitations using the different sequences of clinical cardiac magnetic resonance (cardiac MR) in detection of edema in patients presenting with acute myocardial injury. The purpose of this study is to evaluate the myocardial segmental agreement between the different edema sequences: T2 mapping and turbo inversion recovery magnitude (TIRM) in detection of acute myocardial edema.
Results
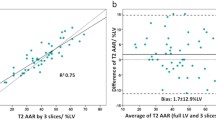
Thirty-seven patients presented with acute infarction were sent to cardiac MR to assess myocardial edema. All cardiac MR studies were scanned using cine, TIRM, and late gadolinium enhancement (LGE) in short axis views (SAX). Position of the T2 mapping slices were copied from the TIRM. The left ventricle (LV) was divided into apical, mid, and basal segments per visualization of the papillary muscles. Edema mass was assessed separately in each segment as well as the total edema mass in both the TIRM and T2 mapping. Twenty-four patients of whom 12.5% had multi-territorial coronary lesions and edema were assessed. Myocardial edema was not assessed in thirteen patients (35%) due to significant intra myocardial hemorrhage (T2 mapping < 60 ms). No statistical significance was found between the TIRM and the T2 mapping neither in the total amount of edema (p = 0.79), nor in the LV basal, mid, and apical segments’ edema (p = 0.69, 0.5, and 0.8 respectively). The upper and lower limits of agreements were tested between the TIRM and the T2 mapping of total edema mass, basal segments, mid, and apical ventricular segments were = 18 and − 7.7 g, 11.3 and − 5.1 g, 12.3 and − 5.2 g, and 15.5 and − 7.8 g respectively.
Conclusion
This study supports the proof of the principle that there is no statistical significant difference per myocardial segments between the T2 mapping and routine edema’s sequences. Larger studies are recommended to assess the impact in clinical outcome.
Similar content being viewed by others
Background
Direct or indirect mapping of biomarkers is a rapidly emerging technique of molecular magnetic resonance imaging (MRI) [1].
In principle, new MRI techniques involved in molecular imaging depend on either measuring or describing signal changes during its fading from a radiofrequency pulse, diffusion mechanism of contrast materials, or using MRI in describing the resonance frequency (RF) of a certain metabolite instead of spatial characterization [1, 2].
Initially, T2 turbo spin echoes (T2 TSE) were used in cardiac magnetic resonance (cardiac MR) to describe areas of myocardial edema subjectively to view areas of high signals. Those areas of high signals represented myocardial edema. Various techniques were later used for edema detection. Among those techniques is using the water sensitive sequences like the turbo inversion recovery magnitude (TIRM) sequences either by measuring the ratio between signals in the myocardium to the nearest skeletal muscles or in relation to a signal behavior from a remote unaffected myocardium [3,4,5,6].
However, an affected skeletal muscle or very frequently multi cardiac segment involvement may induce errors in assessment of myocardial edema using the known T2 TSE, short Tau inversion recovery (STIR), or TIRM sequences.
In addition, the lateral wall of the left ventricle (LV) represented a challenge due to cardiac motion in the TIRM sequences. Also, bright signal of incomplete proton saturation at the myocardium blood interface is another limitation of the T2 and TIRM sequences which sometime is confusing.
Moreover, areas of intra myocardial hemorrhage (IMH) are challengeable to be detected using those routine edema sequences. IMH areas are diagnosed when hypo intense signals in the core of an area of micro vascular obstruction (MVO) exists [7]. Despite the aforementioned points, cardiac MR is considered to be an excellent non-invasive method to assess edema, which is a marker of acute events whether an ischemic event or an inflammatory process [3,4,5,6].
On the other hand, the recently developed T2 mapping is used as a map of myocardial edema. Echoes of myocardial signals below 60 ms are considered normal. A signal in the myocardium above 60 ms reflects area of myocardial edema. The signals are measured per segment, irrespective to the nearest skeletal muscle signals, or remote myocardium signals overcoming the previously described limitations in the T2 TSE or TIRM [8]. Also, areas of reduced T2 mapping signals measured in the core of an MVO segment in an acute event were considered a marker of IMH.
Among the frequently seen artifacts during conventional edema sequences are the flow artifacts, motion artifacts which lead to increased noise and signal loss along the lateral wall of the left ventricle. Those images’ artifacts are much less visualized in T2 mapping per left ventricular segments compared to conventional edema sequences [9].
However, T2 mapping sequences are still under validation because its acquisition depends on three saturation images [9] and the comparison between T2 mapping and TRIM sequences is still scarce in literature.
Therefore, we aimed to compare between both sequences (T2 mapping versus TRIM) in edema detection in acute myocardial injury. Moreover, we aimed to compare between both sequences to depict edema per myocardial segment in the different cardiac segments in order to assess the sensitivity of the T2 mapping per segments.
Methods
A written informed consent was obtained from all patients. Our study complies with the Declaration of Helsinki, and the protocol was approved by the Institutional Research Ethics Committee.
Inclusion criteria
Consecutive patients (Killip class I-II) [10, 11] with acute ST segment elevation myocardial infarction (STEMI) who underwent primary percutaneous cardiac intervention (PCI) was sent to cardiac MR to assess the myocardial salvage index within 24‑48 h. Only patients with coronary artery lesion of 20 mm (thrombus burden 4‑5) were included.
Exclusion criteria
-
1.
Subjects who has any MRI contraindication.
-
2.
Critically ill patients (The New York Heart Association (NYHA)) class was decided by the patients’ cardiologist, and hence excluding the critically ill subjects.
-
3.
Subjects with prior myocardial infarction.
Full clinical examination was performed with special emphasis on cardiovascular risk factors and Killip class. Both total time of pain to device and door to device were calculated in minutes.
Body surface area (BSA) was obtained automatically by entering the patient’s data (weight and height) during the cardiac MR study.
A standard cardiac 1.5 Tesla MR-scanner [Aera, Siemens], and a body coil cardiac 48-channel coil were used for all patients.
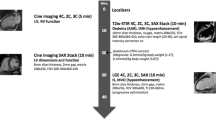
Three orthogonal localizers were obtained in order to plan the different cardiac views. The steady-state free precession [SSFP] sequence was used to assess the ventricular functions and volumes. Two chamber (2ch), four chamber (4ch), three chamber (3ch) cines were used for accurate planning of the short axis (SAX) views. Functions, volumes, and myocardial mass were assessed from the cine SAX slices. Slice thickness: 8 mm, zero gap, and 25 cardiac phases. Delineation of cardiac endo contours was traced manually using the dedicated software [Argus, Siemens®]. Trabeculae and papillary muscles were excluded. The end systole and end diastole were independently identified for the RV and LV.
Through plane 2D phase contrast (2D PC) blood flow was measured in the aorta. Through plane in the aorta was planned using the 3 chamber views and the localizers for intra validation of the ventricular SV. Imaging parameters were free breathing mode (acquisition times: around 1 min), retrospective ECG gating, the velocity encoding (VENC) set to 200 cm/s, slice thickness: 8 mm, TR: 47 ms, and TE: 2.6 ms. Thirty magnitude and velocity-mapped images were obtained per cardiac cycle. Post processing was done by manual contour using the same workstation used for volume assessment.
The left ventricle (LV) was divided into apical, mid, and basal segments per visualization of the papillary muscles [12]. Myocardial edema was assessed in the 16 myocardial segments using pre-contrast TIRM images (TE 52 ms, TR 700 ms, TI 300 ms, acceleration factor × 2, slice thickness (8 mm per slice), and zero mm gap. Slices’ location was copied from the cine SAX slices. A region of interest (ROI) of at least 12 pixels was drawn in the myocardial segment of the culprit artery without contamination from the blood pool or outside the epi-border. Another ROI is drawn in a myocardial segment remote from the culprit artery territory.
Areas of myocardial edema were considered in case of culprit artery segments’ signals exceeded the remote myocardium’s signals in addition to twice its standard deviation [12, 13]. Mass of the myocardial edema was assessed using the dedicated software for assessment of myocardial salvage index, where mass of myocardial edema was calculated using a third contour. Contours were drawn manually at the boundary between the blood pool and the endo border of the myocardium as well as the epi border of the myocardium.
Early gadolinium enhancement images: Phase sensitive inversion recovery images (PSIR) were acquired 1‑2 min after contrast injection. Slice location is copied from the TIRM images covering the left ventricle. Sequence was performed using breath hold imaging. The nulling timing point (TI) was determined by the MRI sequence with a thickness of 8 mm covering the entire left ventricle.
Late gadolinium enhancement (LGE) images were performed 10 min after contrast administration. Slice location is copied from the early PSIR short axis covering the left ventricle. Both sequences used was the PSIR (breath hold mode) and the inversion recovery (IR) sequence using free-breathing imaging and multiple signal average imaging. The nulling timing point (TI) was determined by the CMR member attending the study with a thickness of 8 mm covering the entire left ventricle. Infarcted volume was assessed manually as previously described [14].
Pre-contrast T2 maps slices acquired in the same position of the TIRM and covering the entire left ventricle using a T2-prepared single shot SSFP sequence. Logarithmic transformation of the signal intensity of corresponding pixels in the 3 images, was then fitted to derive the T2 value of each pixel. ROIs were drawn in 16 myocardial segments. Edema mass was assessed using same tool for assessment of myocardial salvage index (Segment CMR-Medviso).
Echoes of myocardial signals below 60 ms are considered normal, a signal in the myocardium above 60 ms reflects area of myocardial edema [8].
MVO and IMH
Areas of MVO were identified by cardiac MR as areas of dark signals during the early PSIR and continued persistently dark yet decreasing partially in size during the LGE images.
Areas of MVO which showed either (1) marked reduction of the T2 mapping signals despite the acute event or (2) hypo intense core in the TIRM were diagnosed to have IMH within [7, 15]. Areas of MVO with myocardial edema and no hypointense core were considered to have no IMH within.
Statistics
The non-parametric tests due to abnormal distribution of data were used in order to describe the variables. The p value (Mann Whitney calculators) was used to describe the statistical significance difference between the means of the variables (a value > 0.05 is considered of no statistical significance). Bland Altman analysis was used to describe the upper and lower limits of agreement between variables.
Results
Thirty-seven patients with acute STEMI were studied. Median body surface area of all patients was 1.8 m2. Thirty (81%) were male patients and seven female patients. Twelve (32%) patients had diabetes mellitus (DM). Nine (24%) had controlled hypertension. All female patients were non-smokers. Seventeen (56%) of the male patients were current smokers.
Average length of coronary artery lesions was 29 mm. Three patients received coronary bare metal stents and 35 had drug eluting stents. All patients were discharged from the hospital in good condition. One case had LV thrombus and received anticoagulant.
Edema mass was not measured in thirteen patients (35%) due to the presence of large areas of MVO with significant IMH within. All the thirteen cases showed no myocardial edema in the T2 mapping (signals < 60 ms) along the territory of the culprit artery and hypo intense core in the TIRM. The LGE finding was matching the catheter in all the 13 cases. The LGE was transmural in all those 13 cases along their culprit artery territory. Median time pain to device was 370 min, and median of 65 min door to device time.
Twenty-four (65%) cases of acute STEMI patients had myocardial edema, small MVO area, and/or no significant IMH. They included three females and 22 males.
The LGE finding was matching the catheter in all the 24 cases. The LGE was transmural in all the 24 cases along the culprit artery territory (Fig. 1).
On catheter and intervention basis, those 24 cases with acute MI were as follow:
-
Sixteen cases (67%) of MI along the territory of the left anterior descending (LAD).
-
Three (12.5%) cases of LCx MI.
-
Three (12.5%) cases of MI along the right coronary artery (RCA).
-
Two (8%) cases had combination of coronary artery lesions (Figs. 2, 3) one case LCx and LAD MI, and LAD with RCA MI.
-
Median time pain to device was 395 min, and median of 48 min door to device time.
-
According to MRI assessment of cardiac functions: median ejection fraction of LV (LVEF%) = 42% and median ejection fraction of RV (RVEF%) = 55%.
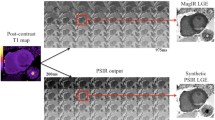
Late gadolinium enhancement (LGE) short axis views (first column) show transmural infarction along the territorial supply of the culprit artery, and dark core signals representing areas of microvascular obstruction in two different patients. The corresponding turbo inversion magnitude (TIRM) images short axis views (middle column) show myocardial edema as well as hypointense core within. T2 mapping images (third column) show dropped signal (< 60 ms) of the corresponding hypointense cores representing areas of intra myocardial hemorrhage
Table 1 shows the mass of myocardial edema was assessed in the 16 segments in the 24 subjects in both sequences (16 × 38 = 608 segments per sequence, i.e., 400 × 2 = 1216). Segment seventeen was excluded.
The p value between the two sequences T2 mapping and TIRM (Table 2) in measuring the edema mass in the myocardium was as follow:
-
Total amount of myocardial edema p = 0.7.
-
Basal segments’ edema p = 0.6.
-
Mid segments’ edema p = 0.5.
-
Apical segments’ edema p = 0.8.
The upper and lower limits of agreements were tested between the TIRM and the T2 mapping of the total edema mass as well as of the different LV levels:
-
1.
Total edema mass: Upper (UL) and lower limits (LL) of agreement were = 17 and − 7.7 g respectively.
-
2.
Basal segments: Upper (UL) and lower limits (LL) of agreement were = 11.3 and − 5.1 g respectively.
-
3.
Mid ventricular segments: Upper (UL) and lower limits (LL) of agreement were = 12.3 and − 5.2 g respectively.
-
4.
Apical segments: Upper (UL) and lower limits (LL) of agreement were = 15.5 and − 7.8 g respectively.
Average total LV mass was 125 g. Average LV myocardial edema mass using the TIRM sequence was as follow: total edema mass, basal, mid, and apical segments’ edema mass (29.3 ± 33, 6 ± 12, 10.7 ± 11.9, and 13 ± 14 g, respectively).
T2 mapping’s average LV myocardial edema mass; total edema mass, basal, mid and apical segments’ were 28 ± 30, 5 ± 8.8, 8.8 ± 10.9, and 14 ± 13 g respectively.
Discussion
This study showed that there is no statistical significant difference between the means of myocardial edema between both the T2 mapping and the routinely used TIRM sequences. The study revealed that both have similar sensitivity in all apical, mid ventricular, and basal level segments.
The advance in the development of the T2 maps promoted less signal variation to cardiac motion as well as it overcame the high signal artifact in dark blood sequences from stagnant blood at the sub-endocardial borders [16]. In our study, the upper and lower limits of agreement showed good agreement in the basal level more than in the apical or mid ventricular segments.
Among the explanations may include the mis-registration error between images in the T2 maps are more at the highly contractile mid and apical segments, or due respiratory movements. All T2 mapping images in our study performed during breath hold.
Our study confirms the results of Giri et al. [16] about the consistency of the T2 mapping in measuring myocardial edema; however, our study was performed in human subjects with acute injury compared to pigs in their study. He analyzed the T2 variability between the myocardial segments and concluded no statistically significant differences among the twelve basal and mid-ventricular segments and a possibility of overestimation of T2 in the four apical segments.
In addition, in our study, two (8%) cases had combination of coronary artery lesions. Depiction of the edema along the culprit artery was challengeable. Subsequently measuring the salvage index in those three patients with the conventional edema sequences TRIM solely was very confusing. T2 mapping helped in delineating the edema per each segment. Also, it confirms the limitations of the available conventional edema sequences and emphasize the role of T2 mapping in routine cardiac MR.
The effect of IMH on patient prognosis after PCI, as well as IMH timing, whether acute or late after PCI, is currently being discussed in literature [17,18,19]. This led to emerging comparative cardiac MR studies aiming to validate sequences for the detection and quantification of reperfusion hemorrhage in vivo. For instance, Kumar et al. [19] studied the T2*-weighted in comparison with conventional T2 weighted in canines. Our study did not aim to validate T2 mapping in detecting reperfusion IMH. However, it revealed the importance of performing at least three slices of T2 mapping in the different myocardial segments, to increase MRI accuracy in depicting IMH in routine cardiac MR. Thirteen cases (35%) had MVO with IMH. The detected IMH in those cases was significant enough to reduce T2 mapping signals along the culprit artery’s territory [15, 20, 21]. All cases that fulfilled the criteria of IMH by TRIM also showed reduced signals in the T2 mapping sequence denoting the T2 mapping’s high sensitivity. It is important to mention that the number of cases that had IMH in our study does not reflect the prevalence of IMH in acute myocardial infarctions. The inclusion criteria of our study included only patients with heavy burden culprit coronary artery lesions (thrombus burden grades 4‑5). Therefore, many cases had large areas of MVO and contained areas of significant IMH. Other studies may be required to detect its prevalence. However, the study showed that T2 mapping was sensitive to such phenomena. Similarly, T2 mapping may play an important role in stable cases of non-ST elevated ischemia (NSTEMI). Segmental detection of edema of the myocardium may uncover the territory of the culprit artery and hence provide the cardiologist with important information before intervention. In this aspect, the importance of T2 mapping over the TRIM would appear because some of those patients suffer from mural thrombi at complex non-culprit coronary lesions with subsequent micro-infarctions in the corresponding myocardial territories (Fig. 4). A phenomenon which is frequently found in autopsy studies of fatal MI cases [6]. Subsequently, the myocardial edema using cardiac MR in any territory could detect resultant micro-embolization of minute thrombotic material and/or platelets aggregates disintegrating from the ulcerative coronary lesions during an episode of pan-coronary inflammation. A phenomenon if proved, cardiac MR is the modality of choice to be used for detecting myocardial edema.
Left upper panel, ECG (a): ST segment elevation in inferior leads (II, III, aVF) (arrows) with reciprocal depression in leads I and aVL. Coronary angiography (b): showed culprit lesion in the proximal circumflex artery (LCx) (arrow head). c and d 1ry percutaneous coronary intervention (PCI) to the culprit lesion in LCx. Right upper panel A, Left anterior descending coronary artery (LAD) angiography of same patient showed proximal ulcerative lesion (arrow) causing moderate stenosis (50‑60% diameter reduction). b and c Fractional flow reserve (FFR) wire in mid LAD to assess its functional significance. Lower row, late gadolinium enhancement short axis views (a) showed inferior wall acute infarction. The corresponding turbo inversion magnitude (b) was confusing in detection of the myocardial edema, T2 mapping images (c) showed edema (> 60 ms) along the culprit artery territory as well as along the remote LAD myocardium
Recently, T1 mapping, a sequence used to differentiate between types of cardiomyopathies with intracellular or extra cellular deposition of abnormal materials [22,23,24,25,26,27], was used to detect areas of infarction and edema. The native and post contrast T1 mapping were used as a parameter of edema and fibrosis delineating the infarcted myocardium. However, T2 mapping showed higher sensitivity [27].
Diffusion is another coming non-invasive technique to detect isotropic water molecule movement [28]. However, diffusion-weighted images (DWI) are used heavily in the brain, liver, breast, and many other organs to detect ischemia and other pathologies because they are static organs. In the heart, cardiac motion causes signal loss which hinders DWIs potentialities and hence it is still under trials [29,30,31,32].
Comparison per segment with the routine edema sequences is scarce in literature [33]. T2 mapping was recently used in measuring the area at risk after acute myocardial infarctions and per cutaneous intervention (PCI). Our recommendations in clinical practice is to include minimum of three T2 mapping slices (apical, basal, and mid ventricular) in routine cases of acute myocardial injuries. This includes cases whether ischemic due to the multi territorial affection or inflammatory.
Different MRI machines and sequences are in use for myocardial T2 mapping. Therefore, cut-off values considerably differ. Moreover, the heterogeneity of myocardium in patients also might have a significant impact on myocardial T2 reference values. In our study, we used a 1.5 Tesla with a reference of 60 ms as a cut-off reference [34].
Limitation
A limited number of patients were included. This is due to the strict inclusion criteria of our study, it included only patients with heavy burden culprit coronary artery lesions (thrombus burden grades 4‑5). In addition, a number of individual assessed segments were more than thousand segments.
Conclusion
This study supports the proof of the principle that there is no statistical significant difference per myocardial segments between the T2 mapping and routine edema’s sequences. Larger studies are recommended to assess the impact in clinical outcome.
Availability of data and materials
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- TIRM:
-
Turbo inversion recovery magnitude
- Cardiac MR:
-
Cardiac magnetic resonance
- LGE:
-
Late gadolinium enhancement
- RF:
-
Resonance frequency
- T2 TSE:
-
Turbo spin echoes
- STIR:
-
Short Tau inversion recovery
- IMH:
-
Intra myocardial hemorrhage
- MVO:
-
Micro vascular obstruction
- STEMI:
-
ST segment elevation myocardial infarction
- BSA:
-
Body surface area
- 2D PC:
-
2D phase contrast
- VENC:
-
Velocity encoding
- PSIR:
-
Phase sensitive inversion recovery images
- LV:
-
Left ventricle
- RV:
-
Right ventricle
- SAX:
-
Short axis
References
Gallagher FA (2010) An introduction to functional and molecular imaging with MRI. Clin Radiol 65:557–566
Hengerer, A., & Grimm, J.Molecular magnetic resonance imaging. Biomedical imaging and intervention journal, 2006;2(2):e8.
Payne AR, Casey M, McClure J, McGeoch R, Murphy A, Woodward R et al (2011) Bright-blood T2-weighted MRI has higher diagnostic accuracy than dark-blood short tau inversion recovery MRI for detection of acute myocardial infarction and for assessment of the ischemic area at risk and myocardial salvage. Circ Cardiovasc Imaging 4:210–219
Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT et al (2009) International consensus group on cardiovascular magnetic resonance in myocarditis. Cardiovascular magnetic resonance in myocarditis: a JACC white paper. J Am Coll Cardiol 53:1475–1487
Dall’Armellina E, Karia N, Lindsay AC, Karamitsos TD, Ferreira V, Robson MD et al (2011) Dynamic changes of edema and late gadolinium enhancement after acute myocardial infarction and their relationship to functional recovery and salvage index. Circ Cardiovasc Imaging 4:228–236
Friedrich MG, Marcotte F (2013) Cardiac magnetic resonance assessment of myocarditis. Circ Cardiovasc Imaging 6:833–839
Wu KC (2012) CMR of microvascular obstruction and hemorrhage in myocardial infarction. J Cardiovasc Magn Reson 14:68
Wassmuth R, Prothmann M, Utz W, Dieringer M, von Knobelsdorff-Brenkenhoff F, Greiser A et al (2013) Variability and homogeneity of cardiovascular magnetic resonance myocardial T2-mapping in volunteers compared to patients with edema. J Cardiovasc Magn Reson 15:27
Naßenstein K, Nensa F, Schlosser T, Bruder O, Umutlu L, Lauenstein T et al (2014) Cardiac MRI: T2-mapping versus T2-weighted dark blood TSE imaging for myocardial edema visualization in acute myocardial infarction. Rofo. 186:166–172
Mello BH, Oliveira GB, Ramos RF, Lopes BB, Barros CB, Carvalho Ede O et al (2014) Validation of the Killip-Kimball classification and late mortality after acute myocardial infarction. Arq Bras Cardiol 103:107–117
El-Menyar A, Zubaid M, AlMahmeed W, Sulaiman K, AlNabti A, Singh R et al (2012) Classification in patients with acute coronary syndrome: insight from a multicenter registry. Am J Emerg Med 30:97–103
Oh-Ici D, Ridgway JP, Kuehne T, Berger F, Plein S, Sivananthan M et al (2012) Cardiovascular magnetic resonance of myocardial edema using a short inversion time inversion recovery (STIR) black-blood technique: diagnostic accuracy of visual and semi-quantitative assessment. J Cardiovasc Magn Reson 14:22
Viallon M, Mewton N, Thuny F, Guehring J, O’Donnell T, Stemmer A et al (2012) T2-weighted cardiac MR assessment of the myocardial area-at-risk and salvage area in acute reperfused myocardial infarction: comparison of state-of-the-art dark blood and bright blood T2-weighted sequences. J Magn Reson Imaging 35:328–339
De Waha S, Eitel I, Desch S, Fuernau G, Lurz P, Haznedar D et al (2012) Time-dependency, predictors and clinical impact of infarct transmurality assessed by magnetic resonance imaging in patients with ST-elevation myocardial infarction reperfused by primary coronary percutaneous intervention. Clin Res Cardiol 101:191–200
Hansen ES, Pedersen SF, Pedersen SB, Kjærgaard U, Schmidt NH, Bøtker HE et al (2016) Cardiovascular MR T2-STIR imaging does not discriminate between intramyocardial haemorrhage and microvascular obstruction during the subacute phase of a reperfused myocardial infarction. Open Heart 20:3
Giri S, Chung YC, Merchant A, Mihai G, Rajagopalan S, Raman SV et al (2009) T2 quantification for improved detection of myocardial edema. J Cardiovasc Magn Reson 11:56
Hamirani YS, Wong A, Kramer CM, Salerno M (2014) Effect of microvascular obstruction and intramyocardial hemorrhage by CMR on LV remodeling and outcomes after myocardial infarction: a systematic review and meta-analysis. JACC Cardiovasc Imaging 7:940–952
Brener SJ, Kirtane AJ, Stuckey TD, Witzenbichler B, Rinaldi MJ, Neumann FJ et al (2016) The impact of timing of ischemic and hemorrhagic events on mortality after percutaneous coronary intervention: the ADAPT-DES study. JACC Cardiovasc Interv 9:1450–1457
Kumar A, Green JD, Sykes JM, Ephrat P, Carson JJ, Mitchell AJ, Wisenberg G et al (2011) Detection and quantification of myocardial reperfusion hemorrhage using T2*-weighted CMR. JACC Cardiovasc Imaging 4:1274–1283
Ding S, Li Z, Ge H, Qiao ZQ, Chen YL, Andong AL et al (2015) Impact of early ST-segment changes on cardiac magnetic resonance-verified intramyocardial haemorrhage and microvascular obstruction in ST-elevation myocardial infarction patients. Medicine 94:35
Mangion K, Corcoran D, Carrick D, Berry C (2016) New perspectives on the role of cardiac magnetic resonance imaging to evaluate myocardial salvage and myocardial hemorrhage after acute reperfused ST-elevation myocardial infarction. Expert Rev Cardiovasc Ther 14:843–854
Sado DM, White SK, Piechnik SK, Banypersad SM, Treibel T, Captur G et al (2013) Identification and assessment of Anderson-Fabry disease by cardiovascular magnetic resonance non-contrast myocardial T1 mapping. Circ Cardiovasc Imaging 6:392–398
Fontana M, White SK, Banypersad SM, Sado DM, Maestrini V, Flett AS et al (2012) Comparison of T1 mapping techniques for ECV quantification. Histological validation and reproducibility of ShMOLLI versus multi breath-hold T1 quantification equilibrium contrast CMR. J Cardiovasc Magn Reson 14:88
Liu S, Han J, Nacif MS, Jones J, Kawel N, Kellman P et al (2012) Diffuse myocardial fibrosis evaluation using cardiac magnetic resonance T1 mapping: sample size considerations for clinical trials. J Cardiovasc Magn Reson 14:90
Puntmann VO, Voigt T, Chen Z, Mayr M, Karim R, Rhode K et al (2013) Native T1 mapping in differentiation of normal myocardium from diffuse disease in hypertrophic and dilated cardiomyopathy. J Am Coll Cardiol Img 6:475–484
White SK, Sado DM, Flett AS, Moon JC (2012) Characterizing the myocardial interstitial space: the clinical relevance of non-invasive imaging. Heart 98:773–779
Dall’Armellina E, Piechnik SK, Ferreira VM, Si QL, Robson MD, Francis JM et al (2012) Cardiovascular magnetic resonance by non contrast T1-mapping allows assessment of severity of injury in acute myocardial infarction. J Cardiovasc Magn Reson 14:15
Van Everdingen KJ, van der Grond J, Kappelle LJ, Ramos LM, Mali WP (1998) Diffusion-weighted magnetic resonance imaging in acute stroke. Stroke. 29:1783–1790
Laissy JP, Gaxotte V, Ironde-Laissy E, Klein I, Ribet A, Bendriss A et al (2013) Cardiac diffusion-weighted MR imaging in recent, subacute, and chronic myocardial infarction: a pilot study. J Magn Reson Imaging 38:1377–1387
Dou J, Reese TG, Tseng WY, Wedeen VJ (2002) Cardiac diffusion MRI without motion effects. Magn Reson Med 48:105–114
Mekkaoui C, Reese TG, Jackowski MP, Bhat H, Sosnovik DE (2015) Diffusion MRI in the heart. NMR Biomed 20:e3426
Moulin K, Viallon M, Romero W, Chazot A, Mewton N, Isaaz K, Croisille P (2020) MRI of reperfused acute myocardial infarction edema: ADC quantification versus T1 and T2 mapping. Radiology 295(3):542–549
Wiesmueller M, Wuest W, Heiss R, Treutlein C, Uder M, May MS (2020) Cardiac T2 mapping: robustness and homogeneity of standardized in-line analysis. J Cardiovasc Magn Reson 22:39
Bönner F, Janzarik N, Jacoby C, Spieker M, Schnackenburg B, Range F et al (2015) Myocardial T2 mapping reveals age- and sex-related differences in volunteers. J Cardiovasc Magn Reson 17:9
Acknowledgements
We would like to thank Ms. Randa Elkady who provided professional writing services and language revision of this manuscript.
Funding
Not applicable (no funding was provided). This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors have read and approved the manuscript. AK, MM, AE, ND, WE, and NB collected, analyzed, and interpreted the patient MRI. AK, MH, MH, WE, and NB reviewed patients’ data and the manuscript. AK and MH designed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Institutional ethical clearance of the protocol was obtained from the Institutional Research Ethics Committee, Aswan Heart Centre, Egypt, although this study is a retrospective study.
Written consent was obtained from patients or their authorized representatives.
All procedures contributing to this work comply with the ethical standards of the Helsinki Declaration of 1975, as revised in 2008.
Consent for publication
All patients included in this research gave written informed consent to publish the data contained within this study. If the patient was less than 16 years old, deceased, or unconscious when consent for publication was requested, written informed consent for the publication of this data was given by their parent or legal guardian.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kharabish, A., Hosny, M., Hassan, M. et al. Assessment of segmental agreement of T2 mapping versus triple inversion recovery in detection of acute myocardial edema. Egypt J Radiol Nucl Med 52, 94 (2021). https://doi.org/10.1186/s43055-021-00476-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-021-00476-3