Abstract
Background
To determine the sensitivity, specificity, positive predictive value, negative predictive value, and diagnostic accuracy of thoracic ultrasound (TUS) in patients with moderate to high clinical suspicion of pulmonary embolism (PE). Twenty-five patients with moderate or high clinical suspicion of PE were enrolled in a prospective study. The patients’ ages were 20 to 50 years (mean age = 36 years). They were evaluated by TUS and standard contrast-enhanced CT pulmonary angiography (CTPA).
Results
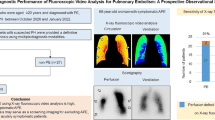
In comparison to and in correlation with CTPA, TUS was found true positive in 12 patients (48%), false positive in one patient (4%), true negative in eight patients (32%), and false negative in four patients (8%), with an overall sensitivity (75%), specificity (89%), positive predictive value (92%), negative predictive value (67%), and accuracy (80%).
Conclusion
TUS with its high specificity and diagnostic accuracy is a noninvasive, widely available, cost-effective method which can be rapidly performed. A negative TUS study cannot rule out PE with certainty, but positive TUS findings with moderate/high suspicion for PE may prove a valuable bedside tool in the diagnosis of PE especially for critically ill and immobile patients, facilitating their immediate treatment.
Similar content being viewed by others
Background
Pulmonary embolism (PE) is one of the major cardiovascular emergencies, and it may present as a fatal complication of an extremity venous thrombosis; nevertheless, its early diagnosis is considered crucial for initiation of an immediate treatment; however, its probabilistic diagnostic approach necessitates a correlative combination of the clinical, laboratory, and the imaging data for starting a specific treatment algorithm or accordingly stopping it [1].
Computed tomography pulmonary angiography (CTPA) had added a great diagnostic value in cases with suspected PE, but its use may be limited in certain medical conditions including renal impairment and hypersensitivity to iodinated contrast media, and in the pregnant patients. Thus, an alternative diagnostic modality should be employed in order to overcome these limitations [1].
Traditionally, the thoracic ultrasound was mainly used for detection of pleural effusion and may be used for effusion guided tapping; currently, it had gained popularity in the evaluation of various pleural, pulmonary, and cardiac diseases with a special concern in the emergency settings [2].
Thanks to its availability, low cost, noninvasive nature, and lacking the hazardous exposure to ionizing radiation or contrast media, the ultrasound had gained superiority as compared to the CT in these aspects; moreover, its portability had facilitated its use in ICU and in the critically ill patients as a simple and a bedside test that could be rapidly performed [3].
Thoracic ultrasound could suggest the diagnosis of PE by the presence of one or more typical pleural-based hypoechoic lesions that could be associated with a pleural effusion or not [3].
Aim of work
To determine the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and diagnostic accuracy of TUS—compared to and in correlation with the CTPA—in patients with moderate to high clinical suspicion of PE.
The protocol was reviewed and approved by the local ethics committee. All patients had given their written consents.
Subjects and methods
Subjects
Between February 2018 and September 2018, 25 patients with moderate or high clinical suspicion of PE were enrolled in this prospective study. The patients’ ages were ranging from 20 to 50 years (mean age = 36 years).
Inclusion criteria
Patients who had a moderate or high clinical suspicion of PE and those who had risk factors for PE as history of venous thromboembolism, lower extremity fracture, malignancy, obesity, congestive heart failure, postpartum period, and operations as well as those who had a hypercoagulable status.
Exclusion criteria
Patients who had relative contraindications to CTPA like an adverse reaction following a previous administration of contrast media, patients with renal impairment and high creatinine levels, and the pregnant females were excluded from the study.
Methods
Patients were subjected to:
-
1)
History taking
A detailed history was obtained; every patient had to answer several questions including the following:
-
a.
Whether there was a chest pain, dyspnea, hemoptysis or not
-
b.
History of any risk factor for PE
-
c.
History of any previous imaging studies or any relevant medical history
-
a.
-
2)
Examination
All the patients were subjected to thoracic ultrasound followed by multi-slice CT pulmonary angiography.
-
A.
Thoracic ultrasound
Thoracic ultrasound was performed with a General Electric (GE) Logic P6 device using the linear probe of (7.5–12 MHz frequency) for pleural and peripheral lung lesions and the low-frequency (convex) probe (4–8 MHz) for the thick chest walls, deep lesions, and detection of any pleural effusions in the costophrenic recesses.
The patients were examined in a sitting or semi-sitting position, the arms were raised, and the hands were placed on the back of the head in order to open the intercostal spaces and to rotate the scapula outward.
All examinations were performed systematically by a single operator through the intercostal spaces in six vertical lines which were the paravertebral, midscapular, posterior axillary, mid-axillary, anterior axillary, and the midclavicular lines.
We started from the midclavicular line by identification of the pleura which often appeared as a fine echogenic line between the two adjacent anechoic ribs.
The examinations were performed while the patients were taking a deep breath and holding it in order to widen their intercostal spaces, thus making the detection of the lesions more feasible.
Identification of a pleural-based, wedge-shaped or rounded hypoechoic infarct area that may show central hyperechoic bronchioles with or without pleural effusion was considered as positive findings for pulmonary embolism.
-
B.
MDCT pulmonary angiography
A standard contrast-enhanced multi-detector CT examination was performed by using a 16-section (Siemens) CT scanner.
The patients were examined in the supine position. The field of view was adjusted to obtain a complete anatomical imaging of the chest, and 0.5-mm sections of the entire chest were acquired. The rotation time was 0.5 s, the tube current was 300–350 mAs, and the tube voltage was 120 kV. The acquisitions were performed during a single breath-hold that lasted for 10–12 s (Table 1) [4].
Table 1 The computed tomography angiography (CTPA) for pulmonary embolism protocol [4] A total of 80–100 mL of the contrast agent (ultravest) was injected intravenous at a rate of 4.0 mL/s. The scanning had started when the main pulmonary artery lighted up. A further reconstruction in the mediastinal window and MIP was performed, and axial thin slice acquisition was used to create the multiplanar reconstruction and the volume rendered images of a high spatial resolution.
The diagnosis of pulmonary embolism was confirmed by the presence of any of the followings:
-
Intraluminal filling defects that showed a sharp interface with the intravenous (I.V.) contrast material
-
Complete arterial occlusion with non-opacified lumina
-
A central arterial filling defect within a distended artery that may be surrounded by an opacifying contrast material.
-
-
A.
-
3)
Statistical methods and data analysis
Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS version 17) software. The results were expressed as mean or frequencies. The data were collected, analyzed, and tabulated. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and diagnostic value of the TUS in the diagnosis PE were calculated using the standard definitions.
Results
Fourteen patients came presenting with hemoptysis (nine males and five females), 25 presented with dyspnea (fifteen males and ten females), and 21 presented with chest pain (12 males and nine females).
Associated medical conditions were DVT in 13 patients (52%), vascular disorders (Behcet disease) was seen in 11 patients (44%), post fracture status was present in three patients (12%), postoperative status was existing in three patients (12%), postpartum status was present in two patients (8%), and two patients had morbid obesity (8%).
Out of the 25 patients, 16 patients were finally diagnosed as PE by CTPA. Anatomical location of the thromboemboli in CTPA is shown in Table 2.
Thirty-four parenchymal lesions were detected by CTPA, and the characteristics of the lesions are shown in Table 3.
TUS gave positive results suggestive of PE in 13 patients (52%), where at least one typical sub-pleural hypoechoic lesion was detected.
TUS demonstrated a total of 17 lesions in 13 patients with a mean of 1.3 lesion/patient; the lesion characteristics regarding the distribution, shape, and size are shown in Table 4. Central echo was found in 2 lesions (12%).
So in comparison to and in correlation with the CTPA, TUS was found true positive in 12 patients (48%), false positive in one patient (4%), true negative in eight patients (32%), and false negative in four patients (8%) with an overall sensitivity (75%), a specificity (89%), a positive predictive value (92%), a negative predictive value (67%), and an accuracy (80%) (charts in Figs. 1 and 2).
Discussion
Pulmonary embolism is one of the serious cardiovascular emergencies that are frequently seen in the clinical setting. However, its diagnosis is still probabilistic and is considered as a challenging diagnosis in many cases, thus an imaging confirmation is usually needed for this purpose. Many diagnostic modalities had been implemented in the diagnosis of PE, and each of them had its own benefits and limitations including the gold standard CTPA [5].
Early detection and treatment of PE is potentially lifesaving, and this necessitates the presence of a simple, non-invasive, and an accurate imaging modality that could be rapidly performed for immobile and critically ill patients as a bedside test. TUS may serve in this regard and had been recently used for this purpose; moreover, it avoids the hazardous exposure to radiation and contrast media that could be met with the use of the CTPA [3].
Many studies had tested the validity of the use of the TUS in the diagnosis of PE, but few of them had compared its results with those of the multi-slice CTPA [3].
In the present study, we investigated the validity of TUS—compared to and correlated with the multi-slice CTPA—in the diagnosis of the clinically suspected cases with PE, and its sensitivity and specificity were assessed.
The criteria to diagnose PE with TUS applied in this study were the presence of at least one typical pleural-based/subpleural hypoechoic lesion with or without pleural effusion (Figs. 3 and 4); these nodules are typically hypovascular on color Doppler imaging (Fig. 5). However, the presence of nonspecific subpleural lesions less than 5 mm in size and isolated free pleural effusion or the presence of normal sonographic findings should make the diagnosis of PE unlikely.
a–c CT pulmonary angiography shown in axial sections in the mediastinal window demonstrating a, b a saddle-shaped embolus extending from the distal pulmonary artery bifurcation to the right and the left main pulmonary branches then into the upper and lower segmental and subsegmental divisions (red arrows). c A mild right pleural effusion and multiple pleural-based subsegmental atelectasis were detected. d, e Ultrasound images in B-mode for the same patient showing a right basal hypoechoic pleural-based lesion (yellow arrow) in d; coexistent right basal mild reactive simple pleural effusion was seen (open yellow arrow) in e
CT pulmonary angiography (a–c) and ultrasound (d) images. a An axial section in the mediastinal window showing a small filling defect seen within the middle lobe lateral segmental branch. b An axial section in the lung window showing subsequent distal pleural-based wedge-shaped opacity. c Coronal section in the mediastinal window showing a filling defect in the middle lobe lateral segmental branch (open red arrow), that is related to the middle lobe pleural-based opacity. d An ultrasound image in B-mode showing a right middle lung zone pleural-based wedge-shaped hypoechoic lesion (yellow arrow)
CT pulmonary angiography (a) and ultrasound (b, c) images. a An axial section in the mediastinal window showing right lateral basal segmental branch dilatation with a filling defect seen inside and associated with a distal infarction (red arrows). b A right lower lung polygonal pleural-based hypoechoic lesion (yellow arrow). c A color Doppler image showing no detectable internal vascularity (open yellow arrow)
A differential diagnosis of the above-described lesions may encompass some other diseases including pneumonia, pulmonary neoplasm, and atelectasis; however, some sonographic criteria may be suggested for the aim of differentiation between them in the light of the clinical examination, where the carcinoma may have a rather rounded appearance with speculated borders, possible central breaking down, and can show internal vascularity. In pneumonia, the hypoechoic lesions are typically polygonal, inhomogenous with serrated margins and characteristically show an air-bronchogram or fluid bronchogram with increased internal vascularity. Atelectatic lesions are considered as the real challenge or the great mimicker lesions as they may have variable shapes and echogenicity patterns, but they often show internal vascularity inside (vascular sign), a feature that may help in the differentiation; pulmonary embolism lesions may present a hypoechoic rather homogenous lesions that may occasionally have central echoic area but often lack the internal vascularity and the air-bronchograms are seldom seen [6, 7].
The studies done by Pfeil et al., Comert et al., Abootalebi et al., and Ghanem et al. had adopted the same diagnostic criteria [8,9,10,11].
The current study included 25 patients, 15 males (60%), and 10 females (40%); thus, we had the same gender distribution as the Comert et al. and Ghanem et al. studies [9, 11].
In the current study, TUS demonstrated 17 subpleural hypoechoic lesions suggestive of PE in 12 out of 16 patients finally diagnosed as PE by CTPA with a mean of 1.3 lesion per patient which is lower than that was detected in Ghanem et al. [11], and this could be attributed to the difference in the sample size between the two studies where the latter was larger.
In agreement with Comert et al. and Ghanem et al. studies, the detected lesions were located mainly in the right basal lung region [9, 11], and unfortunately, we did not have an explanation for this.
Regarding the size of the lesions, our study showed that the mean size of the lesions was 15.3 × 10.5 mm while, in Comert et al. study, it was 22.9 × 31.2 mm [9] and, in Abootalebi et al. study, it was 16.4 × 11.1 mm [10]; thus, there was no big difference in the mean size of the lesions in the three studies, and all of them had a mean size that was more than 10 mm.
As regards the shape of the lesions, we found that the majority of lesions were wedge-shaped. This finding was also depicted by Abootalebi et al. and Ghanem et al. studies [10, 11].
The central hyperechogenicity inside the lesions was found in 2 lesions (15%) in two different patients. The central hyperechoic structure indicates the presence of an air inside the patent bronchiole in the affected segment and was considered as a sign of segmental involvement. Our result was higher than the Ghanem et al. study which reported central hyperechogenicity in only 4.5% of their patients [11].
The sensitivity, specificity, PPV, NPV, and accuracy of TUS for the diagnosis of PE in comparison with MDCT, the gold standard, in our study were 75%, 89%, 92%, 67%, and 80%, respectively (Figs. 1 and 2).
Pfeil et al. in Germany, for the first time, had compared the results of TUS with MDCT in the detection of PE among 33 patients who had symptoms of suspected PE. The design of our study was similar to theirs. They reported 70% sensitivity and 69.6% specificity, 84.25% NPV, and 50% PPV for TUS in this regard [8].
Comert et al. included 50 patients in their study and had found that the sensitivity, specificity, PPV, NPV, and the accuracy of TUS were 90%, 60%, 77.1%, 80%, and 78%, respectively [9].
Another study done by Abootalebi et al. included 77 patients and documented that the sensitivity, specificity, NPV, PPV, and the accuracy of TUS were 84%, 94.2%, 87.5%, 92.5%, and 91%, respectively [10].
A more recent study by Ghanem et al. included 60 patients and reported that the sensitivity, specificity, NPV, PPV, and the accuracy of TUS were 82%, 90%, 72%, 94%, and 85%, respectively [11].
Comparing our results with the forementioned studies done by Pfeil et al., Comert et al., Abootalebi et al., and Ghanem et al. [8,9,10,11], rather similar statistical parameters were met apart from the low sensitivity and NPV in our study which could be explained by the small sample size.
In our study, four cases were considered false negative, where they had a negative TUS scan while their CTPA was positive for PE (Fig. 6). This could be explained in that two of them had isolated central PE not reaching the periphery of the lung and the PE associated lesions can be detected by TUS only when they extend to the lung periphery, while the other two patients were bedridden and their general condition hindered proper patient positioning and exposure for the classic examination.
CT pulmonary angiography (a, b) and ultrasound (c) images. a An axial section in the mediastinal window showing a hypodense filling defect seen involving the distal most part of the right main pulmonary artery extending into its descending branch (red arrow). b An axial section in the mediastinal window showing multiple hypodense filling defects that are seen involving the pulmonary arteries’ descending branches (red arrows). c An ultrasound image in B-mode showing no evidence of pleural-based lesions
Beyond the scope of the current study, some studies had investigated the use of the trans-thoracic echocardiography (TTE) for the detection of acute pulmonary thrombo-embolism and described some echocardiographic findings including right ventricular reduced size and dysfunction and dilated pulmonary arteries, and sometimes, the thrombus may be visualized; moreover, the interventricular septum changes (like flattening and paradoxical movement) as well as inferior vena cava dilatation had been described; however, they reported some limitations regarding the acoustic window for depiction of such findings in addition to the low sensitivity for these findings [12, 13].
Although our study had reported encouraging results regarding the diagnosis of PE-related lesions by TUS, we do not believe that TUS can be considered as an efficient alternative to CTPA. As a considerable proportion (16%) of patients who were considered false negative by TUS and then PE could have been missed without CTPA, another pitfall is the false-positive results, where the TUS findings (subpleural hypoechoic nodules) were depicted in the absence of any emboli detected by CTPA (Fig. 7).
CT pulmonary angiography (a–c) and ultrasound (d) images. a, b Axial and coronal sections in the mediastinal window showing normal appearance of the main, segmental, and the sub-segmental arteries with no evidence of any filling defects. c An axial section showing a left basal pleural-based subsegmental atelectasis (red arrow). d An ultrasound image in B-mode showing a left lower lung zone well-defined pleural-based rounded hypoechoic lesion (between the calibers)
Some limitations were met for the use of TUS in the diagnosis of PE in our study including the small sample size, the central location of the thrombus as the lesions can be detected by TUS only when they extend peripherally to the lung surface, and the fact that ultrasonography is an operator-dependent technique.
Conclusion
TUS with its high specificity and diagnostic accuracy is a noninvasive, widely available, cost-effective method which can be rapidly performed. A negative TUS study cannot rule out PE with certainty, but positive TUS findings with moderate/high suspicion for PE may prove a valuable bedside tool in the diagnosis of PE, especially for critically ill and immobile patients, facilitating their immediate treatment.
Availability of data and materials
All data are available on a software system owned by each of the authors.
Abbreviations
- CTPA:
-
Computed tomography pulmonary angiography
- NPV:
-
Negative predictive value
- PE:
-
Pulmonary embolism
- PPV:
-
Positive predictive value
- TTE:
-
Trans-thoracic echocardiography
- TUS:
-
Thoracic ultrasound
References
Jaff MR, MS MM, Archer SL et al (2011) American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation, American Heart Association Council on Peripheral Vascular Disease, American Heart Association Council on Arteriosclerosis, Thrombosis, and Vascular Biology. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation 123(16):1788–1830
Stein PD, Sostman HD, Hull RD et al (2009) Diagnosis of pulmonary embolism in the coronary care unit. Am J Cardiol 103(6):881–886
Reissig A, Copetti R, Kroegel C (2011) Current role of emergency ultrasound of the chest. Crit Care Med. 39:839–845
Naidich DP, Srichai MB, Muller NL et al Computed tomography and magnetic resonance of the thorax 2007, 4th Edition. Lippincott Williams & Wilkins, pp 218–287
Srivali N, Ratanapo S, Cheungpasitporn W et al (2012) State of the art: practical approach for diagosis of pulmonary embolism. Am Med J. 3:141–146
Reissig A, Gramegna A, Aliberti S (2012) The role of lung ultrasound in the diagnosis and follow-up of community-acquired pneumonia. Eur J Intern Med 23:391–397
Görg C, Bert T, Kring R et al (2006) Transcutaneous contrast enhanced sonography of the chest for evaluation of pleural based pulmonary lesions: experience in 137 patients. Ultraschall Med. 27:437–444
Pfeil A, Reissig A, Heyne JP et al (2010) Transthoracic sonography in comparison to multislice computed tomography in detection of peripheral pulmonary embolism. Lung. 188:43–50
Comert SS, Caglayan B, Akturk U et al (2013) Thoracic Med. 8(2):99–104
Abootalebi A, Golshani K, Karami M et al (2016) Diagnostic validity of ultrasonography in evaluation of pulmonary thromboembolism. Adv Biomed Res 5:4 doi: 4763566
Ghanem MK, Makhlouf HA, Hasan AA et al (2018) Acute pulmonary thromboembolism in emergency room: gray-scale versus color Doppler ultrasound Evaluation. Clin Respir J 12:474–482
Wu J, Zhang J, Yang F, Li C, Ni M (2018) High-risk pulmonary embolism assessed by transthoracic echocardiography. Medicine 97(18):e0545
Lee J, Park J (2008) Role of echocardiography in patients with acute pulmonary thromboembolism. J Cardiovasc Ultrasound 16(1):9–16
Acknowledgements
Not applicable
Funding
All authors had no fund for this research.
Author information
Authors and Affiliations
Contributions
AA had performnce of the chest ultrasound for the patients in the study and shared in the sientific editing of the article. IM had read the CTA images and shared the reffernce collection and the sinentific editing. AS had supervised the CTA examinations as well as adequate history taking prior to examinations and the patient prepration for the examinations. HH had performaed the clinical assesment for the patients before and after the examinations and selection of the patients included in the study. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The protocol was reviewed and approved by the local ethics committee. All patients had given their written consents.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Baz, A.A., Hamdy, I.M., Mohammed, A.S. et al. Diagnostic validity of thoracic ultrasound in the assessment of pulmonary embolism. Egypt J Radiol Nucl Med 50, 5 (2019). https://doi.org/10.1186/s43055-019-0005-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-019-0005-z