Abstract
Background
Pulmonary embolism (PE) is a common and usually fatal condition that is commonly misdiagnosed and consequently ignored. Lung ultrasonography is quickly becoming a valuable tool in the ER and ICU for assisting in emergency decisions.
Methods
One hundred fourteen patients with moderate/high probability of PE in the Critical Care Department meeting the inclusion/exclusion criteria were enrolled in the study. A detailed medical history and a full physical examination involving vital signs, transthoracic ultrasound, CT pulmonary angiography (CTPA), and echocardiography were done for the eligible participants. The goal of this study was to evaluate the diagnostic role of transthoracic ultrasound (TUS) in PE and to compare its specificity and sensitivity with CT pulmonary angiography. Multivariate logistic regression analysis was performed.
Results
Within the 75 patients with confirmed PE based on CTPA, 30 had LUS findings of confirmed PE (40%), and 45 had LUS findings not consistent with confirmed PE (60%). Within the 39 patients with no PE based on CTPA, all patients had LUS findings of non-confirmed PE (100%), and no patients had LUS findings of confirmed PE (0%). The diagnosis of pulmonary embolism based on confirmed LUS signs had sensitivity and specificity of 40% and 100% respectively with 100% positive predictive value and 46.4% negative predictive value.
Conclusion
Positive TUS findings with moderate/high suspicion for PE may prove a valuable tool in the diagnosis of PE at the bedside, especially in the emergency setting, but a negative TUS study cannot rule out PE with certainty.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pulmonary embolism (PE) is a serious medical condition characterized by obstructing one or more of the pulmonary arteries. It happens when a blood clot, or foreign material that plugs, known medically as deep vein thrombosis, or DVT gets stuck in the pulmonary arteries after entering the bloodstream from the legs [20]. Impaired oxygen exchange results from blood flow to the lungs being interrupted by pulmonary artery obstruction. Numerous symptoms may result, such as abrupt dyspnea, chest pain that may get worse with breathing deeply, fast breathing, an increased heart rate, bloody coughing, dizziness, or fainting. Pulmonary embolism can cause shock or even death in extreme situations [18].
Because the typical clinical appearance of shortness of breath and chest pain cannot be conclusively distinguished from other causes, the diagnosis of PE is based mostly on validated clinical criteria in conjunction with selected testing. Medical imaging decisions are made using clinical reasoning, which considers the patient’s symptoms, history, and physical examination results before evaluating the clinical likelihood of the results [22].
Lung ultrasound (LUS) is considered the fastest, non-invasive, simple diagnostic approach in the intensive care unit (ICU) and other inpatient settings. It is associated with minimal complications, a radiation-free technique, and relatively low cost [28]. It is regarded as a useful tool for diagnosing heart, lung, and pleura diseases, particularly in emergency care medicine. Also, it enables the quantitative evaluation of diaphragmatic function and mobility. Various sonographic approaches for assessing diaphragmatic dysfunction have been validated [5]. This study was conducted to evaluate the diagnostic role of transthoracic ultrasound in the diagnosis of pulmonary embolism as an effective prompt diagnostic tool in the ICU and ER.
Patients and methods
One hundred fourteen individuals who met the inclusion and exclusion criteria and exhibited a moderate to high clinical suspicion of a pulmonary embolism at the Critical Care Department were enrolled in the research between January 2021 and June 2022. The pulmonary embolism clinical suspicion was according to the Revised Geneva score for the evaluation of patient pulmonary embolism [12, 27].
Inclusion/exclusion criteria
The inclusion criteria involved patients with moderate/high clinical pulmonary embolism and patients with high-risk factors like venous thromboembolism, lower extremity fracture, malignancy, obesity, congestive heart failure, postpartum period, operation, and PE. Exclusion criteria were patients with contraindications to CT pulmonary angiography (CTPA), patients having experienced adverse reactions after the administration of contrast media, renal complications, and pregnant women. The eligible patients were subjected to a detailed medical history and a full physical examination involving vital signs, transthoracic ultrasound, CTPA, and echocardiography. The transthoracic ultrasound was performed by the linear probe of (7.5–12 MHz frequency), while CTPA was done using a 16-section (Siemens) CT scanner. As for the echocardiography, Toshiba instruments, Japan (Nemio SSA-550A), with a 2.5-MHz transducer and harmonic imaging were employed.
Per the sonographic assessment, patients were divided into four groups for PE diagnosis: (1) two or more characteristic wedge-shaped, triangular, or rounded pleura-based hypoechoic lesions with or without pleural effusion as confirmed PE group; (2) one characteristic pleura-based hypoechoic lesions with pleural effusion as possible PE group; (3) one characteristic pleura-based hypoechoic lesions without pleural effusion as probable PE group; and (4) free pleural effusion alone or normal sonographic group. PE was suspected if at least one or more typical pleural-based/subpleural hypoechoic lesions with or without pleural effusion were reported. Regarding CTPA, PE was affirmed by the presence of intraluminal filling defects that show a sharp interface with intravenous contrast material, complete arterial occlusion with failure to opacify the entire lumen, and central arterial filling defect within a distended artery surrounded by I.V. contrast material. The local ethics committee approved the study, and all patients meeting the inclusion criteria gave written informed consent.
Statistical analysis
The data was coded and entered using the Statistical Program for the Social Sciences (SPSS) version 25 (IBM Corp., Armonk, NY, USA). The following metrics were employed to analyze quantitative data: mean, standard deviation, median, minimum, and maximum; frequency (count) and relative frequency (%) were used to analyze categorical data. To compare quantitative variables, non-parametric Kruskal–Wallis and Mann–Whitney tests were employed. To compare serial measurements within each patient, non-parametric Friedman and Wilcoxon signed-rank tests were used [8]. The chi-square test was used to compare categorical data. Quantitative variable correlations were found using the Spearman correlation coefficient [6].
Results
Following the revised Geneva score, 125 patients who had a moderate or high clinical suspicion of pulmonary embolism were recruited. After enrollment, 11 patients were excluded: 4 declined to sign the CTPA consent form, and 7 had high serum creatinine levels that are contraindicative of CTPA. The study sample represented the remaining 114 participants. The studied group’s age varied from 19 to 87 years old, with an average age of 60.6 ± 12.93 years. There were 63 men (55.3%) and 51 women (44.7%) in the study group. The two most prevalent comorbidities were diabetes mellitus (27.2%) and hypertension (30.7%) as shown in Fig. 1.
According to transthoracic ultrasonography data, 55.3% of patients had pleural effusion, 67.5% had < 3 B-lines, 46.5% had just one subpleural consolidation, and 13.2% had two subpleural consolidations, while 16% have a possible diagnosis of PE, and 24.6% have a probable diagnosis. Thirty-five percent of cases had no serious pulmonary embolism symptoms by LUS (Table 1).
The revised Geneva score ranged from 3 to 18 with mean ± SD 7.36 ± 0.35. The lower limb duplex showed DVT in 63 patients (55.3%) of the studied population. CTPA was the gold standard for the diagnosis of pulmonary embolism. It was positive for pulmonary embolism in 75 patients (57.9%). There was no pulmonary embolism in 39 patients (34.2%). The diagnostic accuracy of LUS and echocardiography were evaluated against the gold standard of CTPA. In 30 of the 75 patients with CTPA positive findings (40%), some signs were considered as confirmed PE by LUS. In another 14 and 15 patients (18.7% and 20%), the LUS findings were consistent with possible and probable PE respectively. Of the 39 patients with CTPA negative findings, no patients had LUS findings of confirmed PE, 2 patients (5.1%) had findings of possible PE, 13 patients (33.3%) had findings of probable PE, and 24 patients (61.5%) had LUS findings that exclude PE. Table 2 presents the LUS diagnostic categories in comparison to CTPA.
Within the 75 patients with confirmed PE based on CTPA, 19 had echocardiographic positive findings (25.3%), and 56 had echocardiographic negative findings (74.7%). Among the 39 patients with no PE based on CTPA, 34 had echocardiographic negative findings (87.2%), and 5 had echocardiographic positive findings (12.8%). The echocardiography had a sensitivity of only 25.3% and specificity of 87.2% with positive predictive value (PPV) and negative predictive value (NPV) of 79.2% and 37.8% respectively.
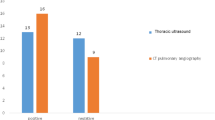
The diagnostic accuracy of LUS using confirmed findings of PE versus other LUS diagnostic categories (excluded, probable, and possible) against CTPA findings were compared. This was followed by an evaluation of the accuracy of the LUS to exclude PE based on LUS exclusion criteria versus other LUS categories (confirmed, possible, and probable) against CTPA findings. Within the 75 patients with confirmed PE based on CTPA, 30 had LUS findings of confirmed PE (40%), and 45 had LUS findings not consistent with confirmed PE (60%). Within the 39 patients with no PE based on CTPA, all patients had LUS findings of non-confirmed PE (100%), and no patients had LUS findings of confirmed PE (0%). The diagnosis of pulmonary embolism based on confirmed LUS signs had sensitivity and specificity of 40% and 100% respectively with 100% PPV and 46.4% NPV (Fig. 2).
Within CTPA-negative patients, LUS excluded PE in 24 (61.5%) and could not exclude PE in 15 patients (38.5%), while in CTPA-positive patients, LUS excluded PE in 16 patients (21.3%) and was not excluded in 59 patients (78.7%). The negative LUS signs for pulmonary embolism can exclude PE with 78.7% sensitivity, 61.5% specificity, 79.8% PPV, and 60% NPV (Fig. 2). Table 3 shows the comparison results between echocardiographic, LUS diagnosis of PE, and CTPA.
Out of 24 patients with sure echocardiographic findings of PE, 10 had LUS consistent with confirmed PE (41.7%), 4 (16.7%) had LUS findings of possible PE, and 5 (20.8%) had probable and excluded PE. Out of 90 patients with sure echocardiographic findings of PE (41.7%), 10 had LUS consistent with confirmed PE, 4 (16.7%) had LUS findings of possible PE, and 5 (20.8%) had probable and excluded PE. On the other hand, 35 out of 90 patients (38.9%) with echocardiography negative findings had PE excluded by LUS compared to 23 (25.6%), 12 (13.3%), and 20 (22.2%) for probable, possible, and confirmed LUS PE diagnosis.
Patients with CTPA positive for PE had significantly higher revised Geneva scores. It was 8.7 ± 3.3 in CTPA-positive patients compared to 4.7 ± 1 in CTPA-negative patients (P < 0.001). The revised Geneva score was also significantly higher with the increased probability of PE by LUS. It was 6.5 ± 3.1 in excluded PE compared to 6.0 ± 2.3, 7.9 ± 2.8, and 9.5 ± 3.7 for probable, possible, and confirmed PE, respectively (P < 0.001) (Fig. 3).
The diagnostic accuracy of the revised Geneva score for PE based on the gold standard of CTPA was evaluated using the ROC analysis. The AUC for the detection of CTPA patients was 0.902 (Fig. 4). A cut-off of the score of 5.25 was found to have a sensitivity of 82.7% and specificity of 84.6% with PPV and NPV of 91.2% and 71.7% respectively.
Discussion
A considerable proportion of fatal PEs are undetected until after autopsy, despite advances in diagnostics and more knowledge of the condition. There is a wide range in short-term mortality of PE, from 2.5% to as high as 33% [7, 13]. The internationally recognized gold standard for diagnosing patients with suspected PE is computed CTPA. Most hospitals have access to CTPA, which has been demonstrated to be more sensitive and specific than standard invasive pulmonary angiography (PA) in the diagnosis of PE [29]. On the other hand, several concerns about reducing the barrier and boosting CTPA usage have been brought up. Based on previous research, the overuse of CTPA as the only diagnostic test for patients with suspected PE may lead to allergic reactions to iodine contrast agents, contrast-induced nephropathy, and long-term radiation effects [16].
Detecting pleural effusions, guiding pulmonary thoracentesis, and diagnosing a range of mediastinal and pleural diseases have all benefited from the use of TUS as a diagnostic tool in recent years, supplementing standard radiography approaches [19]. In addition to being non-invasive, it is also quick, inexpensive, and radiation-free. Furthermore, it has been suggested as a substitute for CT in certain situations, such as when a patient is too unstable to be transferred to a CT room to track the development of acute respiratory distress syndrome [10]. TUS has emerged as an alternative method in many clinical settings, including intensive care units, due to its relative ease of use and the availability of affordable, portable, and user-friendly equipment, providing accurate information that is relevant for both therapy and diagnosis [14].
The goal of the current study was to assess, against the gold standard of CTPA, the use of lung and pleural US in the diagnosis of PE. In the analysis, 114 patients having a clinical suspicion of pulmonary embolism, either moderate or high, were included. The possibility of PE was calculated using the Geneva score, taking into account the clinical findings and risk variables of the patient [25]. Among the enrolled patients, the revised Geneva score varied from 3 to 18, with a mean ± SD of 7.36 to 3.35. Regarded as the gold standard for pulmonary embolism diagnosis, CTPA revealed positive results for PE in 75 patients or 65.8% of the total. We divided our patient population into four categories: probable PE [28 (24.6%), confirmed PE [30 (26.3%)], excluding PE [40 (35.1%), and possible PE [16 (14%)].
TSU results showed that 13.2% of patients had two subpleural consolidations, 67.5% had < three B-lines, 46.5% had only one subpleural consolidation, and 55.3% of patients had pleural effusion. For the sonographic diagnosis of PE, several criteria can be used. The two most typical signs of PE are pleural-based parenchymal changes and hypoechoic lesions.
In 12 out of 16 CTPA-positive patients, Baz et al. [3] showed that subpleural hypoechoic lesions by TUS were suggestive of PE. Most of the lesions had a wedge shape,however, occasionally, they were spherical or had polygonal configurations. According to some research, 20% of patients may have an air-filled bronchiole since they showed a single hyperechoic structure in the lesion’s center [17, 23, 24]. Also, pleural involvement in PE first resulted in local effusion close to the damaged lung region and may later progress to basal pleural effusion.
Based on the presence of two or more wedge pleural-based hypoechoic lesions, TUS identified verified instances of PE in our study with 40% sensitivity, 100% specificity, 100% PPV, and 46.4% NPV. Based on this result, the diagnosis of PE is confirmed even if the lack of sonographic evidence for PE does not rule out the condition. TUS showed 42% sensitivity and 98% specificity for detecting PE using the wedge sign, according to Acar et al. [2]. When the authors performed TUS, edema, alveolar hemorrhage, and tissue necrosis had not yet materialized because they had assessed the patients within a few hours after the onset of symptoms [2]. In a similar vein, TUS was claimed by Bekgoz et al. [4] to have 46% sensitivity and 100% specificity for PE diagnosis. Most of the previous trial results stated that the diagnosis of PE based on TUS findings has a high specificity ranging from 69.6 to 94.2% compared to lower sensitivity ranging from 70 to 84% [1, 3, 11, 21].
The data showing low sensitivity of LUS can be attributed to the inclusion of a small number of PE patients or the less advanced CT technique used compared to other studies that use advanced CT techniques. For instance, in a published meta-analysis, the authors suggested that a negative US test could not definitively rule out PE with an overall sensitivity of 82%, specificity of 89%, and AUC of 0.91 for TUS [7]. Ultrasound in this study revealed that confirmed diagnosis of PE by LUS has a significantly higher mean revised Geneva score (9.1 ± 5.89) followed by possible diagnosis (8.19 ± 3.54) than probable diagnosis (5.66 ± 2.13) (p-value < 0.0001) that indicated the ability of LUS to detect the severity of PE.
In patients with acute PE, echocardiographic signals of right ventricular strain are frequently utilized as prognostic markers; nevertheless, the ability of echocardiography to identify PE is not well known [26]. It is thought to be relatively specific for PE in patients who do not have a history of chronic cardiorespiratory illness. The European Society of Cardiology recently issued guidelines that do not recommend echocardiography as part of the diagnostic workup in non-high-risk patients,however, signs of right ventricular overload in high-risk patients without another leading diagnosis justify emergent treatment if CTPA or another confirmatory test is not immediately available [15].
In the current study, echocardiography was done in 92.1% of cases and showed that 41.2% among patients have dilated right ventricle, 21.1% have sure signs of pulmonary embolism, and pulmonary artery systolic pressure ranged from 25 to 64 mmHg with mean ± SD 40.54 ± 12.37 mmHg with 25.3% sensitivity and 87.2% specificity for the diagnosis of PE with 79.2% positive predictive value and 37.8% negative predictive value.
Fields et al. identified 22 papers that explored test performance characteristics for echocardiography in suspected PE in a recent systematic review and meta-analysis. The frequency of acute PE was 40.8% in the whole population investigated. The authors suggested that echocardiography is insensitive to exclude PE because only an increased RV end-diastolic width of more than 80% is considered sensitive for acute PE. The meta-analysis’s findings deserve further consideration. Many echocardiographic findings are very specific in suspected acute PE: right heart thrombus, McConnell’s sign, paradoxical septal movement, and RV-free wall hypokinesis. Even in non-cardiologists’ point-of-care evaluations, these clinical indicators preserve good specificity [9].
Conclusion
To sum up, a positive TUS result with a moderate to high suspicion for PE may prove to be an effective, straightforward, non-invasive, and easily accessible tool for diagnosing pulmonary embolism at the patient’s bedside, particularly in an emergency involving critically ill and immobile patients. This will facilitate the decision to begin treatment immediately saving many lives and providing more quality-of-life features for patients experiencing PE.
Limitations
Our study was limited by the relatively low sample size, single-center study, and non-blinded. In a larger sample size, the TUS findings may be evaluated together with other clinical and imaging techniques in a multivariate regression analysis to perform a unified scoring system for the bedside emergency evaluation of patients with suspected PE.
Availability of data and materials
The data supporting the findings of the article are available from the corresponding author MS on reasonable request. The data are not publicly available due to restrictions of their containing information that could compromise the privacy of research participants.
References
Abootalebi A, Golshani K, Karami M, Masoumi B, Aliasgharlou M. Diagnostic validity of ultrasonography in evaluation of pulmonary thromboembolism. Adv Biomed Res. 2016;5:4. https://doi.org/10.4103/2277-9175.174975.
Acar H, Yılmaz S, Yaka E, Doğan NÖ, Özbek AE, Pekdemir M. Evaluation of the diagnostic role of bedside lung ultrasonography in patients with suspected pulmonary embolism in the emergency department. Balkan Med J. 2017;34(4):356–61. https://doi.org/10.4274/balkanmedj.2016.1181.
Baz AA, Hamdy IM, Mohammed AS, Assal HH. Diagnostic validity of thoracic ultrasound in the assessment of pulmonary embolism. Egypt J Radiol Nucl Med. 2019;50(1):5. https://doi.org/10.1186/s43055-019-0005-z.
Bekgoz B, Kilicaslan I, Bildik F, Keles A, Demircan A, Hakoglu O, Coskun G, Demir HA. BLUE protocol ultrasonography in Emergency Department patients presenting with acute dyspnea. Am J Emerg Med. 2019;37(11):2020–7. https://doi.org/10.1016/j.ajem.2019.02.028.
Cammarota G, Sguazzotti I, Zanoni M, Messina A, Colombo D, Vignazia GL, Vetrugno L, Garofalo E, Bruni A, Navalesi P, Avanzi GC, Della Corte F, Volpicelli G, Vaschetto R. Diaphragmatic ultrasound assessment in subjects with acute hypercapnic respiratory failure admitted to the emergency department. Respir Care. 2019;64(12):1469–77. https://doi.org/10.4187/respcare.06803.
Chan YH. Biostatistics 104: correlational analysis. Singapore Med J. 2003;44(12):614–19.
Chen W, Xu K, Li Y, Hao M, Yang Y, Liu X, Huang X, Huang Y, & Ye Q. Clinical value of thoracic ultrasonography in the diagnosis of pulmonary embolism: a systematic review and meta-analysis. Med Ultrasonography. 2022;24(2):226–234. https://doi.org/10.11152/mu-3049.
Chen X. Non-parametric tests for three or more samples: The Friedman and Wilcoxon tests. J Exp Educ. 2003;62(1):75–86.
Fields JM, Davis J, Girson L, Au A, Potts J, Morgan CJ, Vetter I, Riesenberg LA. Transthoracic echocardiography for diagnosing pulmonary embolism: a systematic review and meta-analysis. J Am Soc Echocardiography. 2017;30(7):714-723.e4. https://doi.org/10.1016/j.echo.2017.03.004.
Gardelli G, Feletti F, Gamberini E, Bonarelli S, Nanni A, Mughetti M. Using sonography to assess lung recruitment in patients with acute respiratory distress syndrome. Emerg Radiol. 2009;16(3):219–21. https://doi.org/10.1007/s10140-008-0734-1.
Ghanem MK, Makhlouf HA, Hasan AA-A, Alkarn AA. Acute pulmonary thromboembolism in emergency room: Gray-scale versus color doppler ultrasound evaluation. Clin Respir J. 2018;12(2):474–82. https://doi.org/10.1111/crj.12547.
Gruettner J, Walter T, Lang S, Meyer M, Apfaltrer P, Henzler T, Viergutz T. Importance of Wells score and Geneva score for the evaluation of patients suspected of pulmonary embolism. In Vivo (Athens, Greece). 2015;29(2):269–72.
Hepburn-Brown M, Darvall J, Hammerschlag G. Acute pulmonary embolism: a concise review of diagnosis and management. Intern Med J. 2019;49(1):15–27. https://doi.org/10.1111/imj.14145.
Hu Q-J, Shen Y-C, Jia L-Q, Guo S-J, Long H-Y, Pang C-S, Yang T, Wen F-Q. Diagnostic performance of lung ultrasound in the diagnosis of pneumonia: a bivariate meta-analysis. Int J Clin Exp Med. 2014;7(1):115–21.
Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galiè N, Gibbs JSR, Huisman MV, Humbert M, Kucher N, Lang I, Lankeit M, Lekakis J, Maack C, Mayer E, Meneveau N, Perrier A, Pruszczyk P, Rasmussen LH, … Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35(43):3033–3069, 3069a–3069k. https://doi.org/10.1093/eurheartj/ehu283.
Kooiman J, Klok FA, Mos ICM, van der Molen A, de Roos A, Sijpkens YWJ, Huisman MV. Incidence and predictors of contrast-induced nephropathy following CT-angiography for clinically suspected acute pulmonary embolism. J Thrombosis Haemostasis. 2010;8(2):409–11. https://doi.org/10.1111/j.1538-7836.2009.03698.x.
Kroegel C, Reissig A. Principle mechanisms underlying venous thromboembolism: epidemiology, risk factors, pathophysiology and pathogenesis. Respiration. 2003;70(1):7–30. https://doi.org/10.1159/000068427.
Lubetsky A. Pulmonary embolism in cancer patients: a review. Israel Med Assoc J. 2022;24(3):179–82.
Mayo PH, Copetti R, Feller-Kopman D, Mathis G, Maury E, Mongodi S, Mojoli F, Volpicelli G, Zanobetti M. Thoracic ultrasonography: a narrative review. Intens Care Med. 2019;45(9):1200–11. https://doi.org/10.1007/s00134-019-05725-8.
Meli M, Spicuzza L, Comella M, La Spina M, Trobia GL, Parisi GF, Di Cataldo A, Russo G. The role of ultrasound in the diagnosis of pulmonary infection caused by intracellular, fungal pathogens and mycobacteria: a systematic review. Diagnostics. 2023;13(9):1612. https://doi.org/10.3390/diagnostics13091612.
Pfeil A, Reissig A, Heyne J-P, Wolf G, Kaiser WA, Kroegel C, Hansch A. Transthoracic sonography in comparison to multislice computed tomography in detection of peripheral pulmonary embolism. Lung. 2010;188(1):43–50. https://doi.org/10.1007/s00408-009-9195-x.
Raja AS, Greenberg JO, Qaseem A, Denberg TD, Fitterman N, Schuur JD, & Clinical Guidelines Committee of the American College of Physicians. (2015). Evaluation of patients with suspected acute pulmonary embolism: best practice advice from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med. 2015;163(9):701–711. https://doi.org/10.7326/M14-1772.
Reissig A, Heyne JP, Kroegel C. Sonography of lung and pleura in pulmonary embolism: sonomorphologic characterization and comparison with spiral CT scanning. Chest. 2001;120(6):1977–83. https://doi.org/10.1378/chest.120.6.1977.
Reissig A, Kroegel C. Transthoracic ultrasound of lung and pleura in the diagnosis of pulmonary embolism: a novel non-invasive bedside approach. Respiration. 2003;70(5):441–52. https://doi.org/10.1159/000074195.
Rindi LV, Al Moghazi S, Donno DR, Cataldo MA, Petrosillo N. Predictive scores for the diagnosis of pulmonary embolism in COVID-19: a systematic review. Int J Infect Dis. 2022;115:93–100. https://doi.org/10.1016/j.ijid.2021.11.038.
Squizzato A, Galli L, Gerdes VEA. Point-of-care ultrasound in the diagnosis of pulmonary embolism. Crit Ultrasound J. 2015;7:7. https://doi.org/10.1186/s13089-015-0025-5.
Wong DD, Ramaseshan G, Mendelson RM. Comparison of the Wells and revised Geneva scores for the diagnosis of pulmonary embolism: an Australian experience. Intern Med J. 2011;41(3):258–63. https://doi.org/10.1111/j.1445-5994.2010.02204.x.
Xirouchaki, N., & Georgopoulos, D. (2007). The use of lung ultrasound: a brief review for critical care physicians and pneumonologists review. Pneumon, 20.
Zantonelli G, Cozzi D, Bindi A, Cavigli E, Moroni C, Luvarà S, Grazzini G, Danti G, Granata V, & Miele V. Acute pulmonary embolism: prognostic role of computed tomography pulmonary angiography (CTPA). Tomography (Ann Arbor, Mich.). 2022;8(1):529–539. https://doi.org/10.3390/tomography8010042.
Acknowledgements
Not applicable
Funding
The authors declare no funding.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by Mostafa Mohsen and Soliman Belal. The first draft of the manuscript was written by Mostafa Mohsen and Soliman Belal. Critical Review and Analysis were done by Khaled Taema and Amr El Hadidy. All authors read, reviewed, and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The local research ethics committee (REC) for experimental and clinical studies at the Critical Care Department of the Faculty of Medicine, Cairo University, reviewed and approved this research protocol (MD-271–2020). All patients were informed about the technique, and we obtained informed consent from all participants. Our research complied with the principles of the Declaration of Helsinki. Informed consent was obtained from all individual participants included in the study.
Consent for publication
Informed consent was obtained from all participants included in the study for the publication of the images in the intended figures.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mohsen, M., Hadidy, A.E., Taema, k. et al. Role of chest ultrasound in the diagnosis of pulmonary embolism: a cohort study. Egypt. J. Critical. Care. Med. 11, 1 (2024). https://doi.org/10.1007/s44349-024-00001-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44349-024-00001-1