Abstract
Background
Neuroblastoma, one of the most prevalent childhood cancers, is often treated with surgery, radiation, and chemotherapy. However, prognosis and survival are still dismal for children with neuroblastoma at high risk. Consequently, it is vital to identify new and effective treatment targets. As a component of the meiotic cohesion complex, REC8 is involved in a wide range of malignancies. The current work assessed the impact of REC8 knockdown on SH-SY5Y and SK-N-AS neuroblastoma cells and delved into the molecular mechanism behind this effect.
Methods
Knockdown of REC8 using the small interfering (si) RNA technology, and the results were verified by quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) and western blot. The Cell Counting Kit-8 (CCK-8) was used to examine cell proliferation, while flow cytometry was used to examine cell cycle progression and apoptosis. Analyses of angiogenesis included tube formation experiments. Transwell tests were used to examine cell migration and invasion.
Results
The data showed that downregulation of the REC8 led to a substantial decrease in cell proliferation by stopping the cell cycle in the G1 phase. REC8 knockdown significantly reduced neuroblastoma cell proliferation, migration, invasion, angiogenesis, induced cell cycle arrest, and enhanced apoptosis. We also discovered that repressing REC8 expression in neuroblastoma cell lines SH-SY5Y and SK-N-AS reduced their ability to activate the STAT3/VEGF signaling pathway.
Conclusions
Neuroblastoma therapy may benefit from targeting REC8 and its downstream targets.
Similar content being viewed by others
Introduction
Neuroblastoma (NB), an embryonic malignancy derived from the neural crest, is a viral disease threatening children’s health, causing nearly 15% of deaths of children among pediatric malignancies [1, 2]. Despite aggressive multimodal therapy, NB is characterized by heterogeneous biological behaviors, including spontaneous regression or aggressive progression [3, 4]. No cure or therapy exists now that would provide patients with a favorable prognosis regardless of the stage of the disease [5]. To lessen the harmful side effects and decrease the dosage necessary for medication treatment, researchers should identify the abnormal signaling pathways in neuroblastoma and screen for potential inhibitors to substitute for traditional chemotherapy [6, 7]. Understanding the fundamental biological underpinnings of neuroblastoma is critical for developing novel approaches to improve prognostic stratification and patient outcomes.
REC8 is cohesin which helps keep chromosomes in good shape by performing structural maintenance chores [8]. REC8 is usually suppressed in mitotic proliferation, whose role in cancer has recently been subject to controversy [9,10,11]. Recent research has demonstrated that REC8 enhances stemness and promotes metastasis of colorectal cancer through BTK/Akt/β-catenin signaling pathway [12]. At the same time, Liu et al. found the expression of this gene to suppress tumor angiogenesis and progression in gastric cancer cells [13]. Unfortunately, neither the expression nor the function of REC8 in NB has been confirmed. Therefore, it was decided to study the effects of REC8 on the human neuroblastoma cell lines, specifically looking at how these processes relate to NB cell motility, invasion, and angiogenesis.
Methods and materials
Patients and tissues
In our study, 8 patients with primary neuroblastomas with a pathological diagnosis were selected from The Sixth Affiliated Hospital of Harbin Medical University. The fresh tissue samples were immediately snap-frozen in liquid nitrogen and stored at – 80 °C for western blotting experiments.
Cell culture and transfection
Human NBL cell lines (BE (2)-C, IMR-32, SK-N-SH, and SH-SY5Y) and normal dorsal root ganglia (DG) cells were gained from the Chinese Academy of Sciences (Shanghai, China), which were cultured in an F12 medium (Gibco) and Eagle’s minimal essential medium (Gibco) supplemented with 10% heat-inactivated fetal bovine serum (Gemini). All cell lines were incubated at 37 °C in a humid incubator with air containing 5% CO2. The cells were transfected with siRNA (siREC8 and negative control siNC) using Lipofectamine™ RNAimax (Thermo Fisher) according to the manufacturer's instructions. Riobio Biotechnology synthesized siRNAs.
Cell proliferation assays
Cells were seeded at 1 × 104 cells/well in 96-well plates, and three multiple wells were set in each group. After 24 h of incubation, the old medium was discarded, and then the different treatments were added separately. After continued incubation for 24 h, 10 μL CCK⁃8 (Solarbio, China) was added, and the OD value at 450 nm was detected using a microplate reader after incubation for 4 h at 37 °C.
Quantitative real-time PCR
TRIzol reagent (Invitrogen, USA) extracted total RNA from cells. Reverse transcription reactions were performed with a reverse transcription kit (Takara). SYBR Green Master Mix (Takara) was used for quantitative real-time PCR (qRT-PCR). The results were analyzed by the 2-ΔΔCt method.
Western blot
Protein extraction and western blot were performed as previously described [14]. Antibodies were as follows: REC8 (1:1,000; Abcam), VEGF (1:1,000; CST), STAT3 (Ser-473; 1:1,000; CST), p-STAT3 (1:1,000; CST), β-Actin (1:5,000; Abcam), and GAPDH (1:5,000; Abcam).
Colony formation assay
For 2 weeks, cells were grown after being seeded onto 6-well plates. After washing, fixing with 4% paraformaldehyde for 30 min, staining with 0.1% crystal violet for 1 min, and counting.
Flow cytometry
The cells in each group were collected and washed in PBS, and 100 μl cells were mixed with 5 μl Annexin V-FITC and 5 μl Propidium iodide (PI) (Vazyme), then incubated in the dark for 15 min, and then the cell apoptosis was determined by flow cytometry.
Transwell migration and invasion assay
Cell migration: Cells in each group were digested with trypsin, centrifuged, and resuspended with a serum-free medium at a density of 2.5 × 106/ml. 200 μL of suspension was added to the upper chamber of Transwell, and 600 μL of culture medium containing 10 g/dL fetal bovine serum was added to the lower chamber, and the cells were cultured for 24 h. After fixation with formaldehyde and crystal violet staining, the number of transmembrane cells was observed under an inverted microscope. Cell invasion: The matrigel was diluted with a culture medium, spread flat on the top of the chamber, and air-dried. The other experimental procedures were the same as the detection of cell migration.
Statistical analysis
Mean and standard deviation (SD) were used to illustrate the data. The two-sample Student’s t test was used to analyze the differences between the two groups. Statistical significance was assumed at the 0.05 level or below.
Results
REC8 expression was upregulated in NB cells
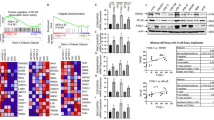
The expression level of REC8 was investigated using qRT-PCR and Western blot. As the results indicated, the level of REC8 was significantly reduced in NB tissues (Fig. 1A, B) and cells (Fig. 1C, D).
REC8 is upregulated in NB tissues and cells. A, B qRT-PCR and Western blot were used to detect the mRNA and protein level of REC8 in 8 cases of neuroblastoma. C, D qRT-PCR and Western blot were used to detect the mRNA and protein level of REC8 in neuroblastoma cell lines (BE (2)-C, IMR-32, SK-N-AS, and SH-SY5Y). T, tumor, N, normal
Downregulation of REC8 significantly inhibits cell proliferation and induces apoptosis in neuroblastoma in vitro
To explore the effect of REC8 on NB progression, we transiently transfected SH-SY5Y and SK-N-AS cells with the controls of siRNA and REC8 siRNA, respectively. As shown in Fig. 2A, protein levels of REC8 were significantly reduced in the si-REC8 group compared with the NC group. Next, we found that REC8 silencing could suppress NB cell viability (Fig. 2B), colony formation (Fig. 2C), and cell cycle G1/S phase transformation (Fig. 2D) and induce cell apoptosis (Fig. 2E).
Downregulation of REC8 significantly inhibits cell proliferation and induces apoptosis in neuroblastoma in vitro. A Western blotting detected the protein expression of REC8 in NB cells transfected with si-REC8 and negative control. B CCK8 was used to evaluate the cell viability of NB cells after REC8 knockdown transfection. C Colony formation of NB cells. D Flow cytometry was used to analyze the cell cycle of the SH-SY5Y and SK-N-AS cell lines. E Flow cytometry detected the cell apoptosis of NB cells
Effects of REC8 knockdown on NB cells migration and invasion
According to the wound healing assay and Transwell assay, the downregulation of REC8 reduced the migration and invasion of SH-SY5Y and SK-N-AS cells (Fig. 3A–C).
Silencing the REC8 gene by siRNA inhibits NB cells' angiogenesis
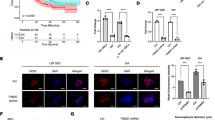
To explore the possible role of REC8 in tumor angiogenesis, we transiently transfected SH-SY5Y and SK-N-AS cells with the controls of siRNA and REC8 siRNA, respectively. As shown in Fig. 4, the tube-forming assay showed that the downregulation of REC8 inhibited the angiogenesis of NB cells.
Downregulation of REC8 significantly inhibits STAT3/VEGF Signaling in NB cells
Additionally, the potential molecular mechanisms underlying these activities of REC8 were also explored by determining the expression levels of key proteins in the STAT3/VEGF signaling pathway were evaluated. In the western blot and immunofluorescence assay of SH-SY5Y and SK-N-AS cells, VEGF and p-STAT3 expressions decreased when REC8 was inhibited (Fig. 5A, B), indicating that REC8 participated in the regulation of STAT3/VEGF signal pathway.
Discussion
Sister chromatid cohesion, homologous chromosome pairing, crossover recombination, and chromosome synapsis are mediated by REC8, a key component of the meiotic prophase chromosomal axis [8, 15]. Our results provide direct evidence that REC8 downregulation suppresses NB cell proliferation, motility, and angiogenesis, supporting our hypothesis that REC8 functions as a tumor-promoting gene. On the other hand, we discovered that REC8 mediated NB development through the STAT3/VEGF pathway.
Despite extensive research into neuroblastoma's oncogenesis, cell growth, proliferation, invasion, and migration [16, 17], the underlying molecular pathways leading to neuroblastoma pathogenesis, recurrence, and metastasis remain poorly known. Higher REC8 expression is inversely related to tumor initiation, progression, and metastasis [18, 19]. When we reduced REC8 expression, cell viability and proliferation were drastically reduced. We also discovered that a significant rise in the percentage of cells in the G0/G1 phase ensued when REC8 was silenced. We also demonstrated in vitro that REC8 sped up NB cell migration and invasion. Significant findings showed a favorable association between REC8 and cancer development, but there was also evidence revealing an adverse effect for REC8 in carcinogenesis. Recent evidence suggests that REC8 is epigenetically downregulated in gastric cancer [13]. The growth and colony formation of thyroid cancer cells is slowed by overexpression of REC8 in previous research [10]. Additionally, it has been demonstrated that REC8 inhibits gastric cancer cell angiogenesis by downregulating vascular endothelial growth factor expression [13]. Furthermore, it was shown that the PI3K pathway targets REC8 in thyroid cancer, and REC8 is a tumor-suppressing gene in this disease [10]. Discrepancies in the roles attributed to REC8 among cancer types may result from the fact that different forms of cancer have been studied in isolation.
The regulatory effects of REC8 on tumorigenesis and tumor progression were studied by examining STAT3/VEGF. Western blotting studies showed that REC8 can effectively down-regulate the expression of p-STAT3 and VEGF in SH-SY5Y and SK-N-AS cell lines. It has been reported that inhibition of the STAT3 pathway decreases VEGF expression and plays a significant role in NB [20] and in the genesis and progression of numerous other tumors [21, 22]. In addition, STAT3 was thought to be the primary transcription factor for the VEGF promoter [23]. STAT3 stimulated VEGF expression by binding to its promoter, and previous research indicated that CD24 regulated STAT3 activity and translocation in colorectal cancer [24].
Conclusion
In conclusion, our results demonstrated that blocking REC8 in neuroblastoma significantly reduced neuroblastoma cell proliferation, migration, invasion, angiogenesis, induced cell cycle arrest, and enhanced apoptosis through the STAT3/VEGF pathway. In this respect, REC8 is a potential target in the search for new therapeutics for NB.
Availability of data and materials
Data and materials supporting the results are available from the corresponding author on reasonable request.
Abbreviations
- NB:
-
Neuroblastoma
- QRT-PCR:
-
Quantitative real-time polymerase chain reaction
- REC8:
-
Meiotic recombination protein REC8 homolog
- CCK-8:
-
Cell Counting Kit-8
References
Swift CC, Eklund MJ, Kraveka JM, et al. Updates in diagnosis, management, and treatment of neuroblastoma. Radiographics. 2018;38:566–80.
Tsubota S, Kadomatsu K. Origin and initiation mechanisms of neuroblastoma. Cell Tissue Res. 2018;372:211–21.
Croteau N, Nuchtern J, LaQuaglia MP. Management of neuroblastoma in pediatric patients. Surg Oncol Clin N Am. 2021;30:291–304.
MacFarland S, Bagatell R. Advances in neuroblastoma therapy. Curr Opin Pediatr. 2019;31:14–20.
Zafar A, Wang W, Liu G, et al. Molecular targeting therapies for neuroblastoma: progress and challenges. Med Res Rev. 2021;41:961–1021.
Liang WH, Federico SM, London WB. Tailoring therapy for children with neuroblastoma on the basis of risk group classification: past, present, and future. JCO Clin Cancer Inform. 2020;4:895–905.
George SL, Parmar V, Lorenzi F, et al. Novel therapeutic strategies targeting telomere maintenance mechanisms in high-risk neuroblastoma. J Exp Clin Cancer Res. 2020;39:78.
Shahid S. The Rules of Attachment: REC8 Cohesin Connects Chromatin Architecture and Recombination Machinery in Meiosis. Plant Cell. 2020;32:808–9.
Yu J, To KF, Liang QY. Epstein-Barr virus-driven promoter hypermethylated genes in gastric cancer. Hong Kong Med J. 2017;23(Suppl 5):17–22.
Liu D, Shen X, Zhu G, et al. REC8 is a novel tumor suppressor gene epigenetically robustly targeted by the PI3K pathway in thyroid cancer. Oncotarget. 2015;6:39211–24.
Zhao J, Liang Q, Cheung KF, et al. Genome-wide identification of Epstein-Barr virus-driven promoter methylation profiles of human genes in gastric cancer cells. Cancer. 2013;119:304–12.
Zhou X, Xie X, Liu T, et al. REC8 enhances stemness and promotes metastasis of colorectal cancer through BTK/Akt/β-catenin signaling pathway. Transl Oncol. 2022;15:101305.
Liu M, Xu W, Su M, et al. REC8 suppresses tumor angiogenesis by inhibition of NF-κB-mediated vascular endothelial growth factor expression in gastric cancer cells. Biol Res. 2020;53:41.
Wang Q, Fan W, Liang B, et al. YY1 transcription factor induces proliferation and aerobic glycolysis of neuroblastoma cells via LDHA regulation. Exp Ther Med. 2023;25:37.
Sakuno T, Tashiro S, Tanizawa H, et al. Rec8 Cohesin-mediated Axis-loop chromatin architecture is required for meiotic recombination. Nucleic Acids Res. 2022;50:3799–816.
Po’uha ST, Le Grand M, Brandl MB, et al. Stathmin levels alter PTPN14 expression and impact neuroblastoma cell migration. Br J Cancer. 2020;122:434–44.
Wu DD, Gao YR, Li T, et al. PEST-containing nuclear protein mediates the proliferation, migration, and invasion of human neuroblastoma cells through MAPK and PI3K/AKT/mTOR signaling pathways. BMC Cancer. 2018;18:499.
Kalejs M, Ivanov A, Plakhins G, et al. Upregulation of meiosis-specific genes in lymphoma cell lines following genotoxic insult and induction of mitotic catastrophe. BMC Cancer. 2006;6:6.
Erenpreisa J, Cragg MS, Salmina K, et al. The role of meiotic cohesin REC8 in chromosome segregation in gamma irradiation-induced endopolyploid tumour cells. Exp Cell Res. 2009;315:2593–603.
Gorantla B, Bhoopathi P, Chetty C, et al. Notch signaling regulates tumor-induced angiogenesis in SPARC-overexpressed neuroblastoma. Angiogenesis. 2013;16:85–100.
Du YE, Tu G, Yang G, et al. MiR-205/YAP1 in Activated Fibroblasts of Breast Tumor Promotes VEGF-independent Angiogenesis through STAT3 Signaling. Theranostics. 2017;7:3972–88.
Lei Z, Duan H, Zhao T, et al. PARK2 inhibits osteosarcoma cell growth through the JAK2/STAT3/VEGF signaling pathway. Cell Death Dis. 2018;9:375.
Pagès G, Pouysségur J. Transcriptional regulation of the vascular endothelial growth factor gene--a concert of activating factors. Cardiovasc Res. 2005;65:564–73.
Wang X, Zhang Y, Zhao Y, et al. CD24 promoted cancer cell angiogenesis via Hsp90-mediated STAT3/VEGF signaling pathway in colorectal cancer. Oncotarget. 2016;7:55663–76.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Research idea and study design: QW and ZL; data acquisition: QW and WF; data analysis/interpretation: ZH and BL; statistical analysis: MF and ZZ. All authors have given approval for the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The ethics committee of our institution approved the study and the consent procedure of The Sixth Affiliated Hospital of Harbin Medical University. Consent to Participate: Informed written consent to participate in the study was provided by all participants (or their parents or legal guardian in the case of children under 16).
Consent for publication
Consent for publication was obtained from the participants.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, Q., Fan, W., Hao, Z. et al. REC8 regulates neuroblastoma cell proliferation, migration, invasion, and angiogenesis via STAT3/VEGF signaling. J Egypt Natl Canc Inst 35, 41 (2023). https://doi.org/10.1186/s43046-023-00197-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43046-023-00197-w