Abstract
The majority of high-risk neuroblastomas can be divided into three distinct molecular subgroups defined by the presence of MYCN amplification, upstream TERT rearrangements or alternative lengthening of telomeres (ALT). The common defining feature of all three subgroups is altered telomere maintenance; MYCN amplification and upstream TERT rearrangements drive high levels of telomerase expression whereas ALT is a telomerase independent telomere maintenance mechanism. As all three telomere maintenance mechanisms are independently associated with poor outcomes, the development of strategies to selectively target either telomerase expressing or ALT cells holds great promise as a therapeutic approach that is applicable to the majority of children with aggressive disease.
Here we summarise the biology of telomere maintenance and the molecular drivers of aggressive neuroblastoma before describing the most promising therapeutic strategies to target both telomerase expressing and ALT cancers. For telomerase-expressing neuroblastoma the most promising targeted agent to date is 6-thio-2′-deoxyguanosine, however clinical development of this agent is required. In osteosarcoma cell lines with ALT, selective sensitivity to ATR inhibition has been reported. However, we present data showing that in fact ALT neuroblastoma cells are more resistant to the clinical ATR inhibitor AZD6738 compared to other neuroblastoma subtypes. More recently a number of additional candidate compounds have been shown to show selectivity for ALT cancers, such as Tetra-Pt (bpy), a compound targeting the telomeric G-quadruplex and pifithrin-α, a putative p53 inhibitor. Further pre-clinical evaluation of these compounds in neuroblastoma models is warranted.
In summary, telomere maintenance targeting strategies offer a significant opportunity to develop effective new therapies, applicable to a large proportion of children with high-risk neuroblastoma. In parallel to clinical development, more pre-clinical research specifically for neuroblastoma is urgently needed, if we are to improve survival for this common poor outcome tumour of childhood.
Similar content being viewed by others
Background
Neuroblastoma is a common childhood malignancy arising from the sympathetic nervous system. It most commonly arises from the adrenal gland and presents with an abdominal mass, but has a heterogeneous clinical phenotype. Children aged less than 18 months often present with widespread metastases to the skin, liver and bone marrow (stage MS disease) [1], however this can spontaneously resolve without treatment. Conversely, distant metastatic spread in children aged greater than 18 months (stage M disease) is associated with an aggressive clinical phenotype and poor survival [1, 2].
Children aged > 18 months with stage M disease and/or amplification of the MYCN oncogene are classified as having clinical high-risk disease. High-risk neuroblastoma remains a major therapeutic challenge with survival rates of < 50% despite intensification of therapy [2, 3]. However, until recently, in the absence of MYCN amplification, the molecular drivers of aggressive disease were unknown.
In 2015 it was reported that aggressive neuroblastoma can be divided into 3 almost mutually exclusive subgroups with either MYCN amplification, rearrangements upstream to the telomerase reverse transcriptase (TERT) gene or alternative lengthening of telomeres (ALT) [4, 5]. Each subgroup is associated with the activation of a telomere maintenance mechanism (TMM) and poor outcomes. Conversely, it is thought that the absence of a TMM is associated with spontaneous regression and excellent survival [6].
Here we summarise TMMs in cancer, specifically focusing on the molecular alterations driving telomere maintenance in neuroblastoma, before describing potential novel therapeutic strategies to directly target TMMs for children with neuroblastoma.
Biology of telomere maintenance
Telomeres are regions of repetitive nucleotide sequences (TTAGGG) located at the ends of chromosomes that protect chromosomes from DNA damage, unnecessary DNA repair and fusion with other chromosomes. In normal dividing cells, with each cell replication telomeres gradually shorten, until a critical level is reached, the Hayflick limit, after which cells undergo senescence [7]. This gradual shortening of telomeres associated with cellular aging is believed to be a protective mechanism against uncontrolled growth, preventing cancer development in humans and other mammals [8]. In keeping with this, the activation of a TMM to prevent the shortening of telomeres is necessary for the continued sustained proliferation of cancer cells and hence a hallmark of cancer [9].
Telomerase, a functional ribonucleoprotein enzyme complex, maintains telomere length by adding telomeric DNA repeats at the 3′ ends of linear chromosomes. It is a reverse transcriptase that consists of a catalytic protein subunit TERT, and an essential RNA component known as human telomerase RNA (hTR), encoded by hTERC. hTR acts as a template for the synthesis of telomere DNA and is involved in the catalysis, localisation, and assembly of the telomerase holoenzyme [10, 11]. Telomerase is widely expressed in human embryos between 16 and 20 weeks gestation, however by the early neonatal period telomerase activity can no longer be detected in most somatic tissues [12]. In contrast, the majority of cancers overexpress telomerase: in a systematic analysis of 31 tumour types, over-expression of TERT was identified in 73% of all cancers [13]. This was most commonly associated with genetic alterations in either the TERT gene/promoter or TERT promoter methylation.
ALT is defined as maintenance of telomeres in the absence of telomerase activity [14]. It can be detected in 10–15% cancers overall but is particularly prevalent in tumours of mesenchymal origin [14, 15]. There is a strong association between ALT and loss of function (LoF) genetic alterations in ATRX (Alpha Thalassemia mental Retardation-X linked) in multiple malignancies, including neuroblastoma [13, 16,17,18].
A number of different non-canonical homologous recombination (HR) based mechanisms have been proposed to play a role in ALT telomere maintenance [19,20,21,22]. Furthermore, a number of studies have focused on the underlying basis for the relationship between ATRX LoF and the development of the non-canonical HR mechanisms implicated in ALT (summarised in Fig. 1). Firstly an established role of ATRX is the maintenance of genomic stability via the deposition of H3.3 into telomeric regions [24, 25]. In the absence of ATRX, G4 quadruplex structures may occur in guanine rich regions of DNA such as telomeres, resulting in stalling of replication forks, which provides a substrate for HR [26, 27]. Secondly, in the absence of ATRX, the MRN (Mre11-RAD50-Nbs1) complex is redistributed to ALT associated PML body sites where it is thought to also facilitate HR mechanisms [26]. Finally, it has been shown that the long non-coding RNA TElomeric Repeat-containing RNA (TERRA) is functionally antagonistic with ATRX [28], and therefore in the absence of ATRX, TERRA can form DNA-RNA hybrids known as R loops, that promote homology directed repair of telomeres [29]. Further confirming the role of ATRX as an ALT repressor, ATRX knockdown has been shown to induce ALT activity in cells of mesenchymal origin [30]. However, ATRX depletion does not promote ALT activity in all cell types [31, 32] suggesting that ATRX LoF alone is not sufficient to induce ALT and that additional, as yet unidentified mechanisms are also needed. Reinforcing the notion that ALT arises as a result a combination of ATRX loss and other factors, it has recently been shown that during the immortalisation process, ATRX loss results in a progressive chromatin de-compaction and a gradual induction of telomere replication dysfunction which triggers an adaptive response eventually resulting in ALT activation [33]. Furthermore the authors show that the telomere dysfunction induced by ATRX loss cannot be overcome by endogenous telomerase activity.
Mechanisms underlying the relationship between ATRX loss of function and ALT. Diagram of (a) a normal and (b) an ALT telomere. In normal cells ATRX and H3.3 co-localise with telomeric DNA, within PML bodies [23]. Following ATRX LoF, MRN complexes co-localise with PML bodies and a failure of telomeric H3.3 deposition results in G-quadruplex formation, facilitating non-canonical homologous recombination mechansims. Additionally, in the absence of functional ATRX, TERRA binds to telomeric DNA, facilitaing the formation of DNA-RNA hybrids known as R-loops which also promote homologous recombination repair
Genetic alterations in the histone chaperone and ATRX binding partner DAXX (death domain associated protein) have also been shown to result in ALT due to a failure to localise ATRX to PML bodies [34]. In addition genetic alterations in tumour protein 53 (TP53) and retinoblastoma 1 (RB1) have also been associated with ALT [13] however the mechanistic basis for these associations is currently unknown.
Biomarkers of TMM
In large-scale studies telomerase expression data is often used as a surrogate biomarker for telomerase activity [13], however quantification of telomerase activity by standardised assays based on the telomere repeat amplification (TRAP) protocol is also feasible in clinical trial settings both as a predictive biomarker in tumour tissue and as pharmacodynamic biomarker in peripheral blood mononuclear cells [35].
The identification of robust biomarkers of ALT has proved more challenging. The gold standard assay for ALT is to confirm the maintenance of telomeres, in the absence of telomerase activity through successive population doublings. However, this is only practical in cell lines, non quantitative, and requires long-term cell culture [14]. Although telomere length and telomere heterogeneity are often used as biomarkers of ALT the lack of specificity of these assays for ALT is becoming increasingly apparent [14, 36, 37].
PML bodies are ubiquitous throughout the genome and responsible for diverse functions including DNA repair. In ALT cancer cells PML bodies specifically co-localise to telomeric DNA, and are thought to facilitate HR [14, 26]. ALT associated PML bodies are now a well-established biomarker of ALT activity and have the advantage that they can be visualised in formalin fixed paraffin embedded (FFPE) material [14, 38].
ALT cells are also characterised by the presence of c-circles: single stranded, telomeric circular DNA strands which are thought to provide a template for ALT telomere synthesis [14]. The c-circle assay is a rolling circle PCR amplification assay, based on the self-priming nature of c-circles. The c-circle assay requires fresh-frozen tissue but is advantageous as c-circles are quantifiable, specific for ALT and c-circle levels can be used to evaluate response to ALT targeted agents [39, 40].
Telomeres and telomere lengthening in neuroblastoma
Amplification of the MYCN oncogene is found in almost 40% of clinical high-risk neuroblastomas [4, 41], and is associated with up-regulation of TERT expression and telomere dysfunction [4, 42]. In an additional 23–31% of high-risk neuroblastomas, TERT is activated through chromosomal rearrangements involving 5p15.33, proximal to the TERT gene, which induces transcriptional up-regulation of TERT by juxtaposing the TERT coding sequence with strong enhancer elements [4, 5]. The third distinct sub-group, accounting for 24% of high-risk neuroblastoma cases are those with ALT [36]. Approximately half of ALT neuroblastomas are associated with somatic alterations in ATRX [4, 5, 36]. In neuroblastoma, genetic alterations in ATRX are associated with a distinct clinical phenotype including older age at diagnosis and a chronic progressive disease course [43].
Neuroblastoma harbouring a TMM is associated with a poor prognosis regardless of clinical stage [4, 5, 44,45,46]. More recently it has been shown that in the presence of a TMM a concurrent mutation in a TP53 or RAS pathway associated gene is associated with an even worse prognosis [6]. Conversely, neuroblastoma with TP53 or RAS pathway mutations (including canonical ALK mutations) in the absence of a concurrent TMM are not associated with worse survival and can spontaneously regress [6]. This molecular risk stratification of neuroblastoma, as described by Ackermann et al. [6] is summarised in Fig. 2.
Molecular risk classification of neuroblastoma [6]. The presence of a telomere maintenance mechansim is defined as either MYCN amplification, a TERT rearrangement, telomerase upregulation or ALT. In neuroblastoma with a TMM the presence of a concurrent mutation in 1 of 17 defined RAS or TP53 pathway genes defines a group of patients with particularly poor outcomes. The most common RAS/TP53 pathway alterations found in neuroblastoma are activating ALK mutations
Although the somatic alterations driving TMM’s in the majority of neuroblastoma are well defined, high telomerase expression can occur in the absence of either a TERT translocation or MYCN amplification [6]. Also in the absence of an ATRX alteration the underlying drivers of ALT neuroblastoma are currently unknown, and somatic alterations in other genes, known to be associated with ALT in other malignancies are rarely found in neuroblastoma [47]. Numerous additional mechansims for telomere maintenance in neuroblastoma have been suggested. A study has identified interstitial telomeric sequences at sites of unbalanced translocations in neuroblastoma cell lines and postulated that these may contribute to a defective telomere maintenance pathway [48]. Another possibility is that genetic predisposition may contribute to the development of TMMs. In keeping with this, six common single nucleotide polymorphisms known to be associated with telomere lengthening have been found to be associated with an increased risk of neuroblastoma [49]. In keeping with the hypothesis that additional underlying predisposing factors may contribute to TMMs, a substantial increase in telomeric DNA damage and active telomere trimming has been described consistently across all high-risk neuroblastomas, regardless of telomerase or ALT status [50].
In summary, data is accumulating to support the hypothesis that TMMs are the common defining molecular feature of aggressive disease in the majority of children with clinical high-risk neuroblastoma. Therefore, both telomerase and ALT represent attractive targets for the development of novel therapeutic strategies with the potential to benefit a significant proportion of high-risk neuroblastoma patients. Here, we review the current pre-clinical and clinical research focused on targeting telomere maintenance with a specific focus on the potential relevance to patients with neuroblastoma.
Drugs targeting telomerase activity
Imetelstat (GRN163L) is a competitive telomerase inhibitor with a complementary structure to the template region of the RNA component of telomerase that binds to and blocks the active site of the enzyme [51]. Confirmation of target inhibition and pre-clinical efficacy of imetelstat has been demonstrated in multiple cancer subtypes [52,53,54].
A phase I trial of imetelstat in 20 children with refractory or recurrent solid tumours demonstrated telomerase inhibition, which was sustained through to day 8 of cycle 1 of therapy [55]. Two of 16 patients had a partial response, however no responses were seen in any of the 6 patients with neuroblastoma enrolled on the trial. The main dose limiting toxicities were neutropenia, thrombocytopenia, and lymphopenia. A Phase II study on imetelstat in children with recurrent or refractory central nervous system malignancies was also associated with significant haematological toxicity and was discontinued after two children died of intra-tumoral haemorrhage [35], thus paediatric development for this compound has subsequently been discontinued.
BIBR1532 is a potent synthetic, non-nucleoside telomerase inhibitor [56]. However like imetelstat there are significant concerns regarding toxicity [57], and this agent has not yet been evaluated in clinical trials.
Sodium metaarsenite (KML001) binds to telomeric sequences, displacing hTERT from the nucleus into the cytoplasm [58] and has been shown to be cytotoxic in neuroblastoma cells in vitro [59]. In a phase I trial in adults with advanced solid tumours, objective responses to KML001 and cisplatin were seen in four out of 18 patients, however this trial was also discontinued due to toxicity [60].
Telomestatin stabilises G-quadruplexes, which in turn, inhibits telomerase activity [61]. This compound has been shown to induce apoptosis in-vitro in telomerase expressing neuroblastoma cell lines [62] but is not yet in clinical development.
6-thio-2′-deoxyguanosine (6-thio-dG) is a nucleoside analogue, which in telomerase active cells is recognised by telomerase and incorporated into telomeres resulting in telomere dysfunction [63]. Pre-clinical efficacy has been demonstrated in melanoma, non-small cell lung cancer and paediatric brain tumour models [64,65,66]. Although 6-thio-dG has not yet been evaluated in clinical trials, due to the novel mechanism of action of the compound it is thought to be less toxic than traditional telomerase inhibitors [67]. 6-thio-dG has been shown to be effective in-vivo in neuroblastoma models with TERT activation. However, the response in MYCN amplified xenografts was mixed, likely reflective of the additional oncogenic pathways activated by MYCN [47]. This is a priority compound for further development for neuroblastoma (Table 1).
XAV939 is an inhibitor of tankyrase, a positive regulator or telomerase. It has been shown to induce apoptosis in the telomerase expressing neuroblastoma cell line SH-SY5Y [70].
Drugs targeting ALT
Ataxia telangiectasia mutated (ATM) inhibitor combination therapy
It has been reported by Koneru et al. that ALT neuroblastoma cell lines are more resistant to topoisomerase inhibitors and that activation of ATM at ALT telomeres is associated with chemo-resistance. The ATM inhibitor AZD0156 was found to be synergistic with temozolomide and irinotecan therapy in both in vitro and in-vivo models of ALT neuroblastoma [71]. AZD0156 is currently in phase I trials in adults [72].
Ataxia telangiectasia and Rad3 related (ATR) inhibitor
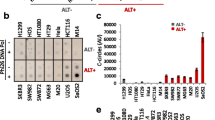
In 2015 Flynn et al. reported that the presence of ALT renders cells hypersensitive to ATR inhibition in osteosarcoma models [73]. We have evaluated the clinical ATR inhibitor AZD6738 [74], in a panel of neuroblastoma cell lines and found that in contrast, ALT cell lines are relatively resistant to ATR inhibition in comparison to other neuroblastoma cell lines (Fig. 3a-b). This is in keeping with a subsequent publication which directly refuted the findings of Flynn et al. and concluded that differences in ATR inhibitor sensitivity were not related to ALT [77]. Taken together this data does not currently support a role of ATR inhibitors as an ALT specific therapy.
(a) Representative dose response curve for AZD6738 in a panel of neuroblastoma cell lines (b) Results of 3 independent SF50 experiments in the panel of neuroblastoma cell lines. Cell lines are grouped and colour coded according to MYCN and ALT [36, 75] status. For SF50experiments, cells were seeded into 96-well plates and the following day compound was added to wells in triplicate, across a concentration gradient including DMSO-only controls. After 5 days cell viability was assessed by Cell Titer Glo®assay. The SF50was calculated as the drug concentration that inhibits viability/cell growth by 50% compared with controls, according to non-linear regression analysis, using Graphpad Prism. Statistical comparison of results is by unpaired t-test (c) MYCN expression by western blot in the panel of cell lines (N-MyC antibody: santa cruz sc-53,993, GAPDH antibody: cell signaling #2118) (d) Representative images of a Th-MYCN GEMM tumour prior to, and after 7 days treatment with 75 mg/kg AZD6738. (e) Waterfall plot documenting the relative changes in tumour volume following 7-day treatment with AZD6738 at three different dose levels. Preliminary studies of AZD6738 were performed in the Th-MYCN model of MYCN amplified neuroblastoma [76]. AZD6738 was supplied by AstraZeneca under a Material Transfer Agreement. It was diluted in 10% DMSO, 40% propylene glycol and 50% water as per manufacturers instructions. In a dose finding study three different doses (25 mg/kg, 50 mg/kg and 75 mg/kg) were trialled in 2 mice each for 7 days by oral gavage. Comparison of tumour response was made between animals receiving vehicle only and AZD6738. For response assessment, magnetic resonance imaging (MRI) of tumours was performed at day zero and after 7 days of administration of the compound
Conversely, we found that MYCN amplified neuroblastoma cell lines show a significantly greater in vitro sensitivity to single agent AZD6738 than non-MYCN amplified neuroblastoma cell lines (Fig. 3a-c), and a preliminary dose finding study demonstrated sensitivity to single agent AZD6738 in the Th-MYCN transgenic mouse model of MYCN driven neuroblastoma [76] (Fig. 3d-e). This is in keeping with other cMYC driven tumours where oncogene driven replicative stress results in a reliance on ATR signalling [78, 79]. Also in keeping with this, the SKNAS neuroblastoma cell line is relatively sensitive to AZD6738 (Fig. 3a) and known to over-express cMYC [80]. In MYCN amplified neuroblastoma models, ATR has also been proposed to play a role in resolution of transcription/replication conflicts [81].
Pre-clinical research in other ALT cancers
Recent pre-clinical data focused on the therapeutic targeting of ALT in other cancer subtypes may give important insight into potential therapeutic vulnerabilities that can be exploited for ALT neuroblastoma (Table 1): Tetra-Pt (bpy) is a cisplatin derivative that inhibits telomeric homologous recombination by targeting the telomeric G-quadruplex and has been shown to inhibit growth of ALT-cell xenograft tumours in mice [82]. Thus far this agent has not entered clinical trials nor has been evaluated in neuroblastoma models. CX5461 is an RNA polymerase I inhibitor which has been shown to selectively kill ATRX mutant cells due to its effects on ribosomal RNA transcription. In-vitro sensitivity in ALT cancer cell lines has been demonstrated [83]. It has also recently been reported that some ALT cancer cells rely on p53 and AKT activity to suppress apoptosis. Furthermore the authors go on to show that the p53 inhibitor pifithrin-α suppresses tumour growth in an ALT xenograft model [84] although others have shown that pifithrin-α is not a specific inhibitor of p53 [85], calling into question the underlying mechanism of the demonstrated pifithrin-α response. This strategy may however represent a relevant novel therapeutic opportunity for ALT neuroblastoma, particularly in view of the fact that the majority of neuroblastoma is TP53 wild type [86]. Finally, preliminary data has shown that ALT cancer cell lines are in general more sensitive to trabectedin, although the mechanism for this is not clear [87].
Tumour heterogeneity, evolution and TMM targeted therapeutics
In some other paediatric malignancies, ALT (identified by the presence of ALT associated PML bodies) and telomerase activation have been shown to co-exist in the same tumour [88, 89]. In neuroblastoma, intra-tumoural diversity of telomere length in individual tumours has been identified using quantitative telomere fluorescence in-situ hybridisation (FISH) [90], however this is not a sufficiently sensitive or specific biomarker of ALT activity [14, 36]. In fact, one study has identified a subset of neuroblastomas with extremely long telomeres in the absence of either telomerase activity or ALT (detected by c-circle assay and ALT associated PML bodies) [36]. Further studies have shown that although TERT alterations and MYCN amplification do co-exist in a small proportion of cases, there is no overlap between the telomerase expressing and ALT positive neuroblastoma [47]. This is in keeping with a recent publication also showing that ATRX mutations and MYCN amplification are synthetically lethal in neuroblastoma [91]. Taken together, the evidence in neuroblastoma to date is that the presence of either ALT or telomerase activation is associated with differing distinct genetic drivers and occurs in mutually exclusive nature [4, 5].
Although data thus far indicates that subgroups of neuroblastoma are driven by either telomerase or ALT activation, it is highly likely that a selective pressure targeting one TMM will support the emergence of an alternative mechanism. In multiple other cancer subtypes it has been shown that long-term telomerase inhibition results in the emergence of features consistent with ALT [92,93,94]. Conversely, with the development of ALT targeted therapeutics it is probable that an up-regulation of telomerase will be seen. However, encouragingly, following ATRX LoF, it appears that ALT is a necessary adaption for cancer cell survival, and that reactivation of telomerase activity cannot overcome endogenous telomere dysfunction [33].
Finally although it has been shown that activation of a TMM is the key determinant of poor outcome in neuroblastoma, the key drivers of TMM’s in neuroblastoma; MYCN amplification and genetic alterations in ATRX are also associated with distinct patterns of wider transcriptional activation which drive malignant transformation [91, 95, 96]. Furthermore it is known that the co-occurrence of RAS/TP53 pathway alterations with a TMM is associated with a particularly dismal outcome [6] and accordingly, alterations in the RAS and TP53 pathways are enriched at the time of neuroblastoma relapse [97, 98]. Therefore, TMM targeting strategies will only be beneficial when given in combination with agents targeting these key pathways and the evaluation of combination therapies is urgently needed.
Conclusion
Despite evidence that telomere maintenance is a key driver of aggressive biology in neuroblastoma, clinical translation of novel therapeutics specifically targeting telomere maintenance remains extremely challenging. The only compound to make it into paediatric clinical trials so far, imetelstat is excessively toxic and pre-clinical data on other compounds targeting telomerase activity is extremely limited. The most promising telomerase targeting candidate to date is 6-thio-dG, however this agent is yet to make it into clinical trials. It must also be noted that in an aggressive malignancy such as neuroblastoma, rapid development of resistance to single-agent targeting of telomerase activation is highly likely and that combination therapies will be needed to overcome this. Also, in the case of MYCN amplified tumours, transcriptional up-regulation of TERT is only one of many oncogenic programmes up-regulated in MYCN amplified neuroblastoma cells.
For ALT driven cancers, there is preliminary data supporting specific roles of a handful of targeted therapeutic approaches but a dearth of robust evidence of pre-clinical efficacy specifically for neuroblastoma. The combination of ATM inhibition with chemotherapy is currently the most promising option for ALT neuroblastoma, although regimens combining cytotoxic chemotherapy agents and inhibition of master upstream regulators of DNA damage repair such as ATM are likely to be prone to significant toxicities.
Despite these challenges, the development of effective new strategies for neuroblastoma by either selective targeting of telomerase or ALT offers great potential to treat the underlying drivers of aggressive disease biology, and is applicable to the greater proportion of neuroblastoma patients. Furthermore, the hypothesis that TMM targeting strategies may be particularly effective in neuroblastoma is supported by the fact that significantly fewer mutations are found in neuroblastoma in comparison to adult malignancies, which often arise due to an accumulation of oncogenic mutations over time [99].
In addition, as recent data identifies an ‘ultra-high risk’ group of neuroblastoma patients with both telomere maintenance and mutations in RAS and TP53 pathway genes [6], rational combinations of telomere targeting agents with other targeted therapeutics must be sought. As pre-clinical data develops, rationally designed paediatric clinical trials will be required to personalise therapy to simultaneously target multiple drivers of aggressive biology in an individual patient.
As our understanding of the molecular drivers of fatal neuroblastoma has significantly expanded in recent years, pre-clinical research efforts must now focus on translating this knowledge into effective, less toxic new therapies for children with neuroblastoma.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Abbreviations
- TERT:
-
Telomerase reverse transcriptase
- ALT:
-
Alternative lengthening of telomeres
- TMM:
-
Telomere maintenance mechanism
- hTR:
-
Human telomerase RNA
- LoF:
-
Loss of function
- ATRX:
-
Alpha Thalassemia mental Retardation-X linked
- HR:
-
Homologous recombination
- MRN:
-
Mre11-RAD50-Nbs1
- PML:
-
Pro-myelocytic Leukaemia
- TERRA:
-
RNA TElomeric Repeat-containing RNA
- DAXX:
-
Death domain associated protein
- TP53:
-
Tumour protein 53
- RB1:
-
Retinoblastoma 1
- TRAP:
-
Telomere repeat amplification protocol
- FFPE:
-
Formalin fixed paraffin embedded
- ATM:
-
Ataxia telangiectasia mutated
- ATR:
-
Ataxia Telangiectasia and Rad3 related
- FISH:
-
Fluorescence in-situ hybridisation
References
Monclair T, et al. The International Neuroblastoma Risk Group (INRG) staging system: an INRG Task Force report. J Clin Oncol. 2009;27(2):298–303.
Ladenstein R, et al. Busulfan and melphalan versus carboplatin, etoposide, and melphalan as high-dose chemotherapy for high-risk neuroblastoma (HR-NBL1/SIOPEN): an international, randomised, multi-arm, open-label, phase 3 trial. Lancet Oncol. 2017;18(4):500–14.
Amoroso L, et al. Topotecan-Vincristine-Doxorubicin in Stage 4 High-Risk Neuroblastoma Patients Failing to Achieve a Complete Metastatic Response to Rapid COJEC: A SIOPEN Study. Cancer Res Treat. 2018;50(1):148–55.
Valentijn LJ, et al. TERT rearrangements are frequent in neuroblastoma and identify aggressive tumors. Nat Genet. 2015;47(12):1411–4.
Peifer M, et al. Telomerase activation by genomic rearrangements in high-risk neuroblastoma. Nature. 2015;526(7575):700–4.
Ackermann S, et al. A mechanistic classification of clinical phenotypes in neuroblastoma. Science. 2018;362(6419):1165–70.
Shay JW, Wright WE. Hayflick, his limit, and cellular ageing. Nat Rev Mol Cell Biol. 2000;1(1):72–6.
Shay JW, Wright WE. Telomeres and telomerase: three decades of progress. Nat Rev Genet. 2019;20(5):299–309.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74.
Shay JW, Bacchetti S. A survey of telomerase activity in human cancer. Eur J Cancer. 1997;33(5):787–91.
Kim NW, et al. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266(5193):2011–5.
Wright WE, et al. Telomerase activity in human germline and embryonic tissues and cells. Dev Genet. 1996;18(2):173–9.
Barthel FP, et al. Systematic analysis of telomere length and somatic alterations in 31 cancer types. Nat Genet. 2017;49(3):349–57.
Henson JD, Reddel RR. Assaying and investigating Alternative Lengthening of Telomeres activity in human cells and cancers. FEBS Lett. 2010;584(17):3800–11.
Amorim JP, et al. The Role of ATRX in the Alternative Lengthening of Telomeres (ALT) Phenotype. Genes (Basel). 2016;7(9):66.
Chen X, et al. Recurrent somatic structural variations contribute to tumorigenesis in pediatric osteosarcoma. Cell Rep. 2014;7(1):104–12.
Mafficini A, Scarpa A. Genomic landscape of pancreatic neuroendocrine tumours: the International Cancer Genome Consortium. J Endocrinol. 2018;236(3):R161–7.
Chudasama P, et al. Integrative genomic and transcriptomic analysis of leiomyosarcoma. Nat Commun. 2018;9(1):144.
O'Sullivan RJ, et al. Rapid induction of alternative lengthening of telomeres by depletion of the histone chaperone ASF1. Nat Struct Mol Biol. 2014;21(2):167–74.
Dilley RL, et al. Break-induced telomere synthesis underlies alternative telomere maintenance. Nature. 2016;539(7627):54–8.
Royle NJ, et al. The role of recombination in telomere length maintenance. Biochem Soc Trans. 2009;37(Pt 3):589–95.
Durant ST. Telomerase-independent paths to immortality in predictable cancer subtypes. J Cancer. 2012;3:67–82.
Chang FT, et al. PML bodies provide an important platform for the maintenance of telomeric chromatin integrity in embryonic stem cells. Nucleic Acids Res. 2013;41(8):4447–58.
Goldberg AD, et al. Distinct factors control histone variant H3.3 localization at specific genomic regions. Cell. 2010;140(5):678–91.
Lewis PW, et al. Daxx is an H3.3-specific histone chaperone and cooperates with ATRX in replication-independent chromatin assembly at telomeres. Proc Natl Acad Sci U S A. 2010;107(32):14075–80.
Clynes D, et al. Suppression of the alternative lengthening of telomere pathway by the chromatin remodelling factor ATRX. Nat Commun. 2015;6:7538.
Rhodes D, Lipps HJ. G-quadruplexes and their regulatory roles in biology. Nucleic Acids Res. 2015;43(18):8627–37.
Chu HP, et al. TERRA RNA Antagonizes ATRX and Protects Telomeres. Cell. 2017;170(1):86–101 e16.
Graf M, et al. Telomere Length Determines TERRA and R-Loop Regulation through the Cell Cycle. Cell. 2017;170(1):72–85 e14.
Napier CE, et al. ATRX represses alternative lengthening of telomeres. Oncotarget. 2015;6(18):16543–58.
Graham MK, et al. Functional Loss of ATRX and TERC Activates Alternative Lengthening of Telomeres (ALT) in LAPC4 Prostate Cancer Cells. Mol Cancer Res. 2019;17(12):2480–91.
Brosnan-Cashman JA, et al. ATRX loss induces multiple hallmarks of the alternative lengthening of telomeres (ALT) phenotype in human glioma cell lines in a cell line-specific manner. PLoS One. 2018;13(9):e0204159.
Li F, et al. ATRX loss induces telomere dysfunction and necessitates induction of alternative lengthening of telomeres during human cell immortalization. EMBO J. 2019;38(19):e96659.
Yost KE, et al. Rapid and reversible suppression of ALT by DAXX in osteosarcoma cells. Sci Rep. 2019;9(1):4544.
Salloum R, et al. A molecular biology and phase II study of imetelstat (GRN163L) in children with recurrent or refractory central nervous system malignancies: a pediatric brain tumor consortium study. J Neurooncol. 2016;129(3):443–51.
Dagg RA, et al. Extensive Proliferation of Human Cancer Cells with Ever-Shorter Telomeres. Cell Rep. 2017;19(12):2544–56.
Onitake Y, et al. Telomere biology in neuroblastoma: telomere binding proteins and alternative strengthening of telomeres. J Pediatr Surg. 2009;44(12):2258–66.
Yeager TR, et al. Telomerase-negative immortalized human cells contain a novel type of promyelocytic leukemia (PML) body. Cancer Res. 1999;59(17):4175–9.
Henson JD, et al. DNA C-circles are specific and quantifiable markers of alternative-lengthening-of-telomeres activity. Nat Biotechnol. 2009;27(12):1181–5.
Henson JD, et al. The C-Circle Assay for alternative-lengthening-of-telomeres activity. Methods. 2017;114:74–84.
Lee JW, et al. Clinical significance of MYCN amplification in patients with high-risk neuroblastoma. Pediatr Blood Cancer. 2018;65(10):e27257.
Kuzyk A, Gartner J, Mai S. Identification of Neuroblastoma Subgroups Based on Three-Dimensional Telomere Organization. Transl Oncol. 2016;9(4):348–56.
Cheung NK, et al. Association of age at diagnosis and genetic mutations in patients with neuroblastoma. JAMA. 2012;307(10):1062–71.
Kawashima M, et al. Telomere biology including TERT rearrangements in neuroblastoma: a useful indicator for surgical treatments. J Pediatr Surg. 2016;51(12):2080–5.
Hertwig F, Peifer M, Fischer M. Telomere maintenance is pivotal for high-risk neuroblastoma. Cell Cycle. 2016;15(3):311–2.
Ohali A, et al. Telomere length is a prognostic factor in neuroblastoma. Cancer. 2006;107(6):1391–9.
Roderwieser A, Sand F, Walter E, Fischer J, Getcht J, Batenhagen C, Ackermann S, Otte F, Welte A, Kahlert Y, Lieberz D, Hertwig F, Reinhardt C, Simon T, Peifer M, Ortmann M, Buttner R, Her B, O'Sullivan RJ, Berthold F, Fischer M. Telomerase is a prognostic marker of poor outcome and a therapeutic target in neuroblastoma. JCO Precision Oncol. 2019;3:1–20.
Schleiermacher G, et al. Stepwise occurrence of a complex unbalanced translocation in neuroblastoma leading to insertion of a telomere sequence and late chromosome 17q gain. Oncogene. 2005;24(20):3377–84.
Walsh KM, et al. Common genetic variants associated with telomere length confer risk for neuroblastoma and other childhood cancers. Carcinogenesis. 2016;37(6):576–82.
Yu EY, et al. Telomere Trimming and DNA Damage as Signatures of High Risk Neuroblastoma. Neoplasia. 2019;21(7):689–701.
Roth A, Harley CB, Baerlocher GM. Imetelstat (GRN163L)--telomerase-based cancer therapy. Recent Results Cancer Res. 2010;184:221–34.
Dikmen ZG, et al. In vivo inhibition of lung cancer by GRN163L: a novel human telomerase inhibitor. Cancer Res. 2005;65(17):7866–73.
Gellert GC, et al. Effects of a novel telomerase inhibitor, GRN163L, in human breast cancer. Breast Cancer Res Treat. 2006;96(1):73–81.
Barszczyk M, et al. Telomerase inhibition abolishes the tumorigenicity of pediatric ependymoma tumor-initiating cells. Acta Neuropathol. 2014;128(6):863–77.
Thompson PA, et al. A phase I trial of imetelstat in children with refractory or recurrent solid tumors: a Children's Oncology Group Phase I Consortium Study (ADVL1112). Clin Cancer Res. 2013;19(23):6578–84.
Pascolo E, et al. Mechanism of human telomerase inhibition by BIBR1532, a synthetic, non-nucleosidic drug candidate. J Biol Chem. 2002;277(18):15566–72.
Hosoi T, et al. Inhibition of telomerase causes vulnerability to endoplasmic reticulum stress-induced neuronal cell death. Neurosci Lett. 2016;629:241–4.
Phatak P, et al. KML001 cytotoxic activity is associated with its binding to telomeric sequences and telomere erosion in prostate cancer cells. Clin Cancer Res. 2008;14(14):4593–602.
Repetto G, Sanz P, Repetto M. Comparative in vitro effects of sodium arsenite and sodium arsenate on neuroblastoma cells. Toxicology. 1994;92(1–3):143–53.
Edelman MJ, et al. Phase I and pharmacokinetic evaluation of the anti-telomerase agent KML-001 with cisplatin in advanced solid tumors. Cancer Chemother Pharmacol. 2016;78(5):959–67.
Kim MY, et al. Telomestatin, a potent telomerase inhibitor that interacts quite specifically with the human telomeric intramolecular g-quadruplex. J Am Chem Soc. 2002;124(10):2098–9.
Binz N, et al. Telomerase inhibition, telomere shortening, cell growth suppression and induction of apoptosis by telomestatin in childhood neuroblastoma cells. Eur J Cancer. 2005;41(18):2873–81.
Mender I, et al. Induction of telomere dysfunction mediated by the telomerase substrate precursor 6-thio-2′-deoxyguanosine. Cancer Discov. 2015;5(1):82–95.
Reyes-Uribe P, et al. Exploiting TERT dependency as a therapeutic strategy for NRAS-mutant melanoma. Oncogene. 2018;37(30):4058–72.
Mender I, et al. Telomerase-Mediated Strategy for Overcoming Non-Small Cell Lung Cancer Targeted Therapy and Chemotherapy Resistance. Neoplasia. 2018;20(8):826–37.
Sengupta S, et al. Induced Telomere Damage to Treat Telomerase Expressing Therapy-Resistant Pediatric Brain Tumors. Mol Cancer Ther. 2018;17(7):1504–14.
Sugarman ET, Zhang G, Shay JW. In perspective: An update on telomere targeting in cancer. Mol Carcinog. 2019;58(9):1581–8.
Barone A, et al. FDA Approval Summary: Trabectedin for Unresectable or Metastatic Liposarcoma or Leiomyosarcoma Following an Anthracycline-Containing Regimen. Clin Cancer Res. 2017;23(24):7448–53.
Baruchel S, et al. A phase 2 trial of trabectedin in children with recurrent rhabdomyosarcoma, Ewing sarcoma and non-rhabdomyosarcoma soft tissue sarcomas: a report from the Children’s Oncology Group. Eur J Cancer. 2012;48(4):579–85.
Tian X, et al. XAV939 promotes apoptosis in a neuroblastoma cell line via telomere shortening. Oncol Rep. 2014;32(5):1999–2006.
Koneru BLG, Nguyen L, Chen WH, Macha S, Farooqi A, Hindle A, Davidson H, Mccoy K, Yang S, Maris J, Reynolds P. Alternate Telomere Lengthening (ALT) neuroblastoma is a highly aggressive subgroup for which ATM kinase provides a novel therapeutic target. Advances in Neuroblastoma Conference, 2018. Abstract 272. Advances in Neuroblastoma Abstract Book. Adv Neuroblastoma Res. 2018. https://www.anrmeeting.org/dl/ANR2018/ANR_Abstract_Book_5-3-18.pdf.
Abida WBYJ, Azaro A, Krebs M, Im S, Chen T, Buil-Bruna N, Li Y, Eaton D, Stephens C, Ross G, Pass M, Rodon J, Dean E. Abstract A094: Phase 1 molecular study of AZD0156, a first-in-class oral selective inhibitor of ataxia telangiectasia mutated protein kinase (ATM), in combination with olaparib (AtoM Study, Module 1). Mol Cancer Ther. 2018;17(1):Abstract AO94.
Flynn RL, et al. Alternative lengthening of telomeres renders cancer cells hypersensitive to ATR inhibitors. Science. 2015;347(6219):273–7.
Foote KM, et al. Discovery and Characterization of AZD6738, a Potent Inhibitor of Ataxia Telangiectasia Mutated and Rad3 Related (ATR) Kinase with Application as an Anticancer Agent. J Med Chem. 2018;61(22):9889–907.
Farooqi AS, et al. Alternative lengthening of telomeres in neuroblastoma cell lines is associated with a lack of MYCN genomic amplification and with p53 pathway aberrations. J Neurooncol. 2014;119(1):17–26.
Weiss WA, et al. Targeted expression of MYCN causes neuroblastoma in transgenic mice. EMBO J. 1997;16(11):2985–95.
Deeg KI, et al. Cancer Cells with Alternative Lengthening of Telomeres Do Not Display a General Hypersensitivity to ATR Inhibition. Front Oncol. 2016;6:186.
Murga M, et al. Exploiting oncogene-induced replicative stress for the selective killing of Myc-driven tumors. Nat Struct Mol Biol. 2011;18(12):1331–5.
Muralidharan SV, et al. BET bromodomain inhibitors synergize with ATR inhibitors to induce DNA damage, apoptosis, senescence-associated secretory pathway and ER stress in Myc-induced lymphoma cells. Oncogene. 2016;35(36):4689–97.
Zimmerman MW, et al. MYC Drives a Subset of High-Risk Pediatric Neuroblastomas and Is Activated through Mechanisms Including Enhancer Hijacking and Focal Enhancer Amplification. Cancer Discov. 2018;8(3):320–35.
Buchel G, et al. Association with Aurora-A Controls N-MYC-Dependent Promoter Escape and Pause Release of RNA Polymerase II during the Cell Cycle. Cell Rep. 2017;21(12):3483–97.
Zheng XH, et al. A Cisplatin Derivative Tetra-Pt (bpy) as an Oncotherapeutic Agent for Targeting ALT Cancer. J Natl Cancer Inst. 2017;109(10).
Udugama M, et al. Ribosomal DNA copy loss and repeat instability in ATRX-mutated cancers. Proc Natl Acad Sci U S A. 2018;115(18):4737–42.
Ge Y, et al. Inhibition of p53 and/or AKT as a new therapeutic approach specifically targeting ALT cancers. Protein Cell. 2019;10(11):808–24.
Walton MI, et al. An evaluation of the ability of pifithrin-alpha and -beta to inhibit p53 function in two wild-type p53 human tumor cell lines. Mol Cancer Ther. 2005;4(9):1369–77.
Pugh TJ, et al. The genetic landscape of high-risk neuroblastoma. Nat Genet. 2013;45(3):279–84.
Pompili L, et al. Diagnosis and treatment of ALT tumors: is Trabectedin a new therapeutic option? J Exp Clin Cancer Res. 2017;36(1):189.
M'Kacher R, et al. The Transition between Telomerase and ALT Mechanisms in Hodgkin Lymphoma and Its Predictive Value in Clinical Outcomes. Cancers (Basel). 2018;10(6):169.
Venturini L, et al. Telomere maintenance in Wilms tumors: first evidence for the presence of alternative lengthening of telomeres mechanism. Genes Chromosomes Cancer. 2011;50(10):823–9.
Pezzolo A, et al. Intratumoral diversity of telomere length in individual neuroblastoma tumors. Oncotarget. 2015;6(10):7493–503.
Zeineldin M, et al. MYCN amplification and ATRX mutations are incompatible in neuroblastoma. Nat Commun. 2020;11(1):913.
Chen W, et al. Telomerase inhibition alters telomere maintenance mechanisms in laryngeal squamous carcinoma cells. J Laryngol Otol. 2010;124(7):778–83.
Bechter OE, et al. Telomeric recombination in mismatch repair deficient human colon cancer cells after telomerase inhibition. Cancer Res. 2004;64(10):3444–51.
Hu Y, et al. Switch telomerase to ALT mechanism by inducing telomeric DNA damages and dysfunction of ATRX and DAXX. Sci Rep. 2016;6:32280.
Westermann F, et al. Distinct transcriptional MYCN/c-MYC activities are associated with spontaneous regression or malignant progression in neuroblastomas. Genome Biol. 2008;9(10):R150.
Qadeer ZA, et al. ATRX In-Frame Fusion Neuroblastoma Is Sensitive to EZH2 Inhibition via Modulation of Neuronal Gene Signatures. Cancer Cell. 2019;36(5):512–27 e9.
Eleveld TF, et al. Relapsed neuroblastomas show frequent RAS-MAPK pathway mutations. Nat Genet. 2015;47(8):864–71.
Carr-Wilkinson J, et al. High Frequency of p53/MDM2/p14ARF Pathway Abnormalities in Relapsed Neuroblastoma. Clin Cancer Res. 2010;16(4):1108–18.
Grobner SN, et al. The landscape of genomic alterations across childhood cancers. Nature. 2018;555(7696):321–7.
Acknowledgements
Not applicable.
Funding
SL George was supported by Christopher’s Smile and is currently supported by the Royal Marsden Cancer Charity. F Lorenzi is supported by Neuroblastoma UK. E Poon is supported by a Cancer Research UK Programme Grant (A18339). L Chesler is supported by the Higher Education Funding Council for England. Y. Jamin received a Children with Cancer UK Research Fellowship (2014/176). This work was supported in part by a Cancer Research UK Cancer Imaging Centre at ICR, in association with the MRC and Department of Health (England) (C1060/A16464).
Author information
Authors and Affiliations
Contributions
SLG and VP wrote the manuscript. SLG performed the in vitro experiments. In vivo experiments were performed by EP and YJ. LVM and PA critically appraised and edited the manuscript. LC supervised the study. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study did not involve human participants, data or tissue. For mouse experiments, protocols were approved and monitored by The Institute of Cancer Research Animal Welfare and Ethical Review Body (PPL 70/7945, later PPL P91E52C32), in compliance with the UK Home Office Animals (Scientific Procedures) Act 1986.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
George, S.L., Parmar, V., Lorenzi, F. et al. Novel therapeutic strategies targeting telomere maintenance mechanisms in high-risk neuroblastoma. J Exp Clin Cancer Res 39, 78 (2020). https://doi.org/10.1186/s13046-020-01582-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13046-020-01582-2