Abstract
Background
Hepatic resection (HR) for hepatocellular carcinoma (HCC) is safe with good perioperative and long-term oncologic outcomes. There is a paucity of data with regards to intermediate-term outcomes (i.e., beyond 90-day and within 1-year mortality). This paper studies the risk factors for within 1-year mortality after elective HR with curative intent in patients with HCC.
Methods
An audit of patients who underwent curative HR for HCC from January 2007 to April 2016 was conducted. Univariate and multivariate analysis were sequentially performed on perioperative variables using Cox-regression analysis to identify factors predicting intermediate-term outcomes defined as within 1-year mortality. Kaplan–Meier survival curves and hazard ratios were obtained.
Results
Three hundred forty-eight patients underwent HR during the study period and 163 patients had curative hepatectomy for HCC. Fifteen patients (9.2%) died within 1-year after HR. Multivariate analysis identified Child-Pugh class B/C (HR 5.5, p = 0.035), multinodularity (HR 7.1, p = 0.001), macrovascular invasion (HR 4.2, p = 0.04) postoperative acute renal failure (HR 5.8, p = 0.049) and posthepatic liver failure (HR 9.6, p = 0.009) as significant predictors of 1-year mortality.
Conclusion
One-year mortality following HR for HCC remains high and can be predicted preoperatively by multinodularity, Child-Pugh class, and macrovascular invasion. Postoperative acute renal failure and liver failure are associated with 1-year mortality.
Similar content being viewed by others
Background
Hepatocellular carcinoma (HCC) is the fifth most prevalent cancer worldwide and the third most frequent cause of cancer-related death [1]. Locally, HCC is the third and fourth most frequent cause of cancer-related death among men and women, respectively [2]. Due to the shortage of donor livers for liver transplant (LT), hepatic resection (HR) is the first-line curative option and has low morbidity and mortality. However, recurrence rates remain high with an impact on delayed mortality [3, 4]. Tumor size and multinodularity are known predictors of early recurrence [3]. Many units, including ours, have witnessed that 90-day mortality is significantly higher than 30-day mortality [4], leading to underestimation of perioperative death when using the traditional 30-day mortality as a key performance indicator of HR outcomes. Short-term perioperative outcomes report events up to 90-days. Long-term oncologic outcomes universally report 1-year, 3-year, or 5-year disease-free survival (DFS) or overall survival (OS). Thus, there is a hiatus in surgical oncology for reporting intermediate-term outcomes after 90 days and before 12 months. In colon cancer patients, reports found underestimation of 1-year mortality and identified postoperative complications, emergency resection, and comorbidities as risk factors [5]. Age, future liver remnant (FLR), and hepatic insufficiency [6] are associated with 90-day mortality risk following HR. Beyond 90-day mortality in HR is poorly studied and reported. In addition to tumor biology, early recurrence or treatment-related complications impact beyond 90-day mortality outcomes. Identification of mortality predictors within the first year post-hepatectomy would aid in clinical decision-making, in particular when considering less invasive treatment options and alternative strategies to HR with lower morbidity risk, as well as in patient enrolment into clinical trials.
Radiofrequency ablation (RFA) with trans-arterial chemoembolization (TACE) has been studied and reported to have equivalent oncological outcomes compared with HR [7, 8]. A propensity score-matched analysis of 154 HCC patients with single 2–3-cm tumors by Lee et al. [9] reported no noticeable difference in local tumor progression, intrahepatic distant recurrence, disease-free survival, and overall survival when comparing HR with TACE–RFA combination therapy. A recent meta-analysis by Giu et al. [7] also concludes that TACE–RFA combination therapy is associated with lower morbidity compared with surgery with comparable oncologic outcomes. Hence, in addition to 90-day mortality risk predictors, knowledge of predictors within 1-year mortality is vital as this could impact treatment recommendation, patients’ quality of life, and patient choice. Our study aims to identify all factors predicting mortality within the first year for patients undergoing HR with curative intent for HCC.
Methods
This is a retrospective audit of patients operated for HR at a university-affiliated hospital from January 2007 to April 2016. Survival data of all the patients was obtained prospectively until February 2018. Patients included in the study were those who underwent curative open or laparoscopic surgery for HCC. Patients with cholangiocarcinoma and metastatic liver nodules were excluded. All the patients were discussed at the multidisciplinary tumor board meeting prior to surgery.
An anesthetist assessed every patient preoperatively at the pre-admissions counseling and evaluation clinic. Nonsurgical treatment modalities were offered for patients deemed unfit by the operating surgeon or those with a high cardiovascular risk as adjudged by an anaerobic threshold of < 11 ml/min/kg. An ICG retention value of > 15% at 15 min precludes major HR. Our institute offers liver-directed chemo- and radio-embolization as well as local thermal ablative therapies for suitable patients. The HPB consensus guidelines were used to recognize those with satisfactory FLR [10]. The Brisbane 2000 classification [11] was used to define the types of resection. Only minor HRs were offered to Child-Pugh class B/C patients. The choice of technique (i.e., open versus laparoscopic or anatomical versus nonanatomical resection) was left to the individual surgeon.
Intraoperative findings and postoperative complications were recorded. R1 resection indicates a histologically positive margin with tumor cells within 1 mm from the margin. The presence of any combined HCC and cholangiocarcinoma cases (n = 2) were excluded. Post-hepatectomy liver failure (PHLF) 1 (50–50 criteria), PHLF2 (peak serum bilirubin criteria), and PHLF3 (ISGLS criteria) were utilized as per their original definitions [12,13,14]. Length of stay was calculated from the date of surgery to discharge date, both dates inclusive. Following discharge, patients were followed up with a physical examination, serum liver function tests, alpha-fetoprotein (AFP), and computed tomography (CT) scan at regular intervals of 3 to 4 months for the first year and 4 to 6 months thereafter. Within 1-year mortality is defined as all-cause mortality within 12 months of the date of HR.
Descriptive statistics for quantitative data were expressed as median and range, while the qualitative data was expressed as n (%). For nominal data, chi-square or Fisher’s exact test was performed. Paired metric data were analyzed using the Wilcoxon signed-rank test. Variables with p values < 0.1 in univariate analysis were entered for multivariate analysis with the Cox proportional hazard model. Statistical significance was defined as p < 0.05. Kaplan–Meier survival curves were generated for the pre-, intra-, and postoperative independent risk factors from multivariate analysis. All analyses were done using IBM SPSS Statistical Analysis Version 20.0.
Results
Between January 2007 and April 2016, 400 patients underwent HR at our institution. Thirty patients (7.5%) were excluded due to missing data for perioperative variables, and twenty-two patients (5.5%) were excluded for missing long-term oncologic outcome data. Out of 348 patients, 163 patients (46.8%) with a median age of 66 years (37–87 years) underwent HR with curative intent, and the diagnosis of HCC was confirmed histologically. The baseline characteristics of the patients are summarized in Table 1. The median tumor diameter was 40 mm (1–200 mm), with 68 patients (41.7%) having tumors ≥ 50 mm. Seventeen (10.4%) were of Child-Pugh class B/C, and over half (51.5%) had Hepatitis B. Seventy-four patients (45.4%) underwent major HR, and 101 (62.0%) had laparoscopic hepatectomy (Table 1). Thirty-day mortality was 3.7% (n = 6), and 90-day mortality was 4.9% (n = 8). Fifteen patients (9.2%) died within the first year post-hepatectomy (Table 2). The median survival of patients with 1-year mortality was 9 months (range 0–11 months).
Table 3 summarizes the univariate and multivariate analysis of preoperative variables. The following factors were associated with 1-year mortality: albumin < 35 g/dL (p = 0.064), prognostic nutritional index (PNI) < 45 (p = 0.021), platelet–lymphocyte ratio (PLR) ≥ 150 (p = 0.089), neutrophil–lymphocyte ratio (NLR) ≥ 5 (p = 0.038) and Child-Pugh B/C (p = 0.004). Multivariate analysis identified Child-Pugh B or C (HR 5.5, p = 0.035, 95% confidence interval (CI) 1.130–26.551), and the presence of two or more tumors (HR 7.1, p = 0.001, 95% CI 2.305–21.899) as preoperative factors that were independently associated with mortality.
Table 4 summarizes the univariate and multivariate analysis of intraoperative variables. The following factors were associated with 1-year mortality: open HR (p = 0.060), major HR (p = 0.068), macrovascular invasion (MAVI) (p = 0.001), microvascular invasion (p = 0.035), and blood loss of more than 1000 ml (p = 0.053). Multivariate analysis identified macrovascular invasion (HR 4.2, p = 0.043, 95% CI 1.046–17.191) as the only intraoperative factor that was independently associated with 1-year mortality. Eighteen patients (11.0%) were reported to have MAVI intraoperatively. Six patients with MAVI died within 1 year.
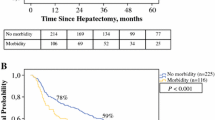
Table 5 summarizes the univariate and multivariate analysis of postoperative variables. Multiple factors were associated with 1-year mortality: intensive care unit admission (p < 0.001), pneumonia (p < 0. 001), acute renal failure (ARF) (p < 0. 001), intra-abdominal abscess (p = 0.006), PHLF1 (p < 0.001), PHLF2 (p < 0.001), and PHLF3 (p < 0.001). Multivariate analysis identified ARF (HR 5.8, p = 0.049, 95% CI 1.009–33.294) and PHLF3 (HR 9.6, p = 0.009, 95% CI 1.778–51.320) to be independently associated with 1-year mortality. Twenty-eight patients (17.1%) experienced PHLF3, out of which almost 40% died within 1 year. There was a significantly higher incidence of PHLF3 in patients experiencing one-year mortality (73.3% vs. 9.5%). Similarly, a total of seven patients experienced ARF, and six died within 1 year.
Child-Pugh B or C, multinodularity, MAVI, ARF, and PHLF3 were independent risk factors for 1-year mortality with a decrease in the 1-year survival rate from 93.2 to 70.6% (p = 0.001) (shown in Fig. 1), 95.8 to 76.7% (p < 0.001) (shown in Fig. 2), 93.8 to 66.7% (p < 0.001) (shown in Fig. 3), 94.2 to 14.3% (p < 0.001) (shown in Fig. 4), and 97.1 to 56.0% (p < 0.001) (shown in Fig. 5), respectively.
Discussion
Five independent factors were associated with mortality within 1 year in HCC patients undergoing HR with curative intent: Child-Pugh B/C, multinodularity, MAVI, ARF, and ISGLS PHLF criteria.
Cirrhotic patients have increased perioperative general risk for both elective and emergency surgical procedures [15, 16]. Our study showed that Child-Pugh stage B/C patients have a five-fold risk of 1-year mortality. Due to high perioperative risk, patients with Child-Pugh class C are not candidates for HR, and we included one patient with Child-Pugh class C. Cirrhosis is also associated with poor oncologic outcomes. A study by Kojiro Taura et al. [17] reported a 5-year survival rate of 81%, 54%, and 28% in non-cirrhotic, Child-Pugh class A, and Child-Pugh class B following HR, respectively. This is attributed to either a recurrence or development of metasynchronous HCC in cirrhotic liver. Few studies have evaluated the short-term prognosis of cirrhosis on post-hepatectomy patients. Chopinet et al. [18] reported acceptable short-term mortality and morbidity in cirrhotic patients (up to Child-Pugh class B, 7 points) and suggested that HR can be considered if patients have normal indocyanine green test and no portal hypertension. TACE, while conventionally considered a palliative procedure, has shown to improve survival outcomes and improve quality of life in patients with unresectable HCC [7]. Saviano et al. [8] reported no statistical difference in a 3-year overall survival in cirrhotic patients who underwent TACE–RFA combination versus HR in the presence of large solitary tumors. In a recent meta-analysis comparing TACE–RFA combination versus surgery, Gui et al. have concluded that TACE–RFA combination has similar long-term oncologic outcomes with the benefit of lower morbidity compared with surgery [7]. Child-Pugh class B/C patients should be carefully evaluated and preoperatively optimized to reduce the risk of PHLF and mortality, ensuring that HR remains safe [18], while considering TACE–RFA combination as an alternative therapy. TACE should be re-classified as a ‘curative adjunct’ rather than palliative in its intent.
Our study, consistent with a study by Moriguchi et al. [19], showed that multifocal HCC is a risk factor associated with 1-year mortality. The European Association for the Study of the Liver (EASL) recommended LT instead of HR as a first-line treatment for multinodular tumors [20]. They showed that the 5-year survival of LT in early HCC was comparable to those without malignancy (70%), and recurrence rates were less than 25%. However, due to the scarcity of donor organs, HR is still the first-line surgical management in patients with HCC. Based on our results, surgery may not be an ideal option for patients with Child-Pugh class B/C and multinodular HCC. Recently, some studies have shown good surgical outcomes in select patients with multinodular tumors and well-preserved liver function. Cheung et al. [21] compared HR with combined resection and ablation in multinodular HCC. They showed that the combined therapy had longer median survival (53 vs. 44.5 months). Knowing the number of tumors would allow the surgeon to discuss and offer treatment that includes a combination of ablation, chemoembolization, and surgery that is individualized to each patient. Patients with multinodular HCC should be carefully monitored for recurrence and offered enrolment into clinical trials. We did not study the pattern of 1-year mortality and hence unable to comment if this was associated with early HCC recurrence.
Our study showed that MAVI was associated with a five-fold risk for 1-year mortality and this is consistent with other reports [22]. MAVI of the hepatic or portal vein is part of the natural progression of HCC and is associated with a high recurrence rate even when treated with LT. Previously, portal vein thrombosis was considered a contraindication for LT, but techniques like thromboendovenectomy have emerged and redefined the role of LT and HR in patients with portal vein thrombosis [23]. Both portal and hepatic vein thrombosis are known to induce hepatic hypertrophy, which has the benefit in increasing the FLR, and hence MAVI is not an absolute contraindication of HR. In patients with MAVI, the median survival is less than 1-year, and BCLC recommends palliative Sorafenib treatment [24]. Further stratification based on MAVI location has shown that tumor with hepatic vein or vena cava invasion had a meager median survival of less than 5 months, with postoperative mortality of 28% [25]. This led to Mount Sinai Medical Centre no longer offering HR in patients with MAVI, as this result is worse than medical treatment or no therapy at all. Based on our experience and available literature, we would caution against HR in patients with MAVI and multifocal HCC as 1-year mortality risk is high, and the patient may enjoy survival advantage from TACE–RFA combination therapy [7]. Hepatic venous invasion and portal venous invasion may have a different biologic basis and need to be considered separately. A recent study by Levi Sandri et al. [26] achieved a recurrence-free survival of 39.1 months in four BCLC stage C patients with portal vein thrombosis by doing yttrium-90 radio-embolization before LT. Our study did not compare the outcome between location-stratified or perioperatively and intraoperatively known MAVI. Nevertheless, if MAVI is known on preoperative imaging, the patient should be counseled so that they can make informed decisions.
PHLF occurs in 4–19% of patients undergoing HR and is a severe complication that impacts mortality [27]. The variation in incidence arises due to the lack of a standardized definition. As part of postoperative factors, our study looked at three well-established definitions of PHLF. This variability of the definition is demonstrated in our study, where PHLF incidence was 3.7%, 4.3%, and 15.3% for PHLF1, 2, and 3, respectively. Only PHLF3 was statistically significant in the multivariate analysis of postoperative factors. In a study reporting 807 patients, Rahbari et al. [14] reported ISGLS criteria predicted mortality (HR 13.80; 95% CI 4.27–44.61, p < 0.01). Validation of PHLF3 as a postoperative 1-year mortality predictor is essential. PHLF is best prevented by optimization of preoperative factors [27] and incorporating strategies like portal vein embolization, two-staged hepatectomy, and associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) [28] to ensure satisfactory FLR. Integrating active monitoring of INR and bilirubin on postoperative day five can allow for early recognition of PHLF3, stratification of severity, and initiation of supportive organ therapy, and improving survival risk.
Postoperative ARF is a risk factor associated with 1-year mortality, which is consistent with a study by Saner et al. [29], that reported 73% of patients with post-hepatectomy ARF requiring dialysis died. In our study, 85% of ARF patients died, and seven patients (4.3%) experienced ARF. Lim et al. [30] suggested adequate volume expansion, use of diuretics or vasoactive drugs, and earlier postoperative renal replacement therapy for higher-risk patients.
Limitations of our study are retrospective, single-center study over a decade, and small sample size. Due to small sample and low event rate for mortality, the multivariate analysis showed wide confidence intervals. This impacts the precision of our study results. As there was only one patient with Child-Pugh class C, we have combined the data of Child-Pugh classes B and C. We did not categorize complications according to Clavien-Dindo classification and have reported each complication separately. We did not collect data related to the cause of mortality and if this was related or associated with the recurrence of HCC. We caution against generalizing the results as clinical profile, surgical technique, and technology and perioperative management would differ.
Conclusions
In conclusion, our study showed that Child-Pugh B or C, multinodularity, and macrovascular invasion were independent predictors of mortality within the first year post curative hepatectomy, and postoperative renal or liver failure was associated with 1-year mortality. Knowing these risk factors can aid in clinical decision-making and the design of clinical trials.
Availability of data and materials
The data that support the findings of this study are available from the National Healthcare Group but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of the National Healthcare Group.
Abbreviations
- HR:
-
Hepatic resection
- HCC:
-
Hepatocellular carcinoma
- LT:
-
Liver transplant
- ALPPS:
-
Associating liver partition and portal vein ligation for staged hepatectomy
- DFS:
-
Disease-free survival
- OS:
-
Overall survival
- FLR:
-
Future liver remnant
- RFA:
-
Radiofrequency ablation
- TACE:
-
Trans-arterial chemoembolization
- PHLF:
-
Post-hepatectomy liver failure
- AFP:
-
Alpha-fetoprotein
- CT:
-
Computed tomography
- NLR:
-
Neutrophil–lymphocyte ratio
- CI:
-
Confidence interval
- MAVI:
-
Macrovascular invasion
- ARF:
-
Acute renal failure
References
Bosch FX, Ribes J, Cléries R, Díaz M. Epidemiology of hepatocellular carcinoma. Clin Liver Dis. 2005;9(2):191–211.
LH P, CK Y, KT T, LE Y. W H. Singapore Cancer Registry Interim Annual Registry Report: trends in cancer incidence in Singapore 2009-2013. Singapore: National Registry of Diseases Office; 2013.
Kamiyama T, Nakanishi K, Yokoo H, Kamachi H, Tahara M, Kakisaka T, et al. Analysis of the risk factors for early death due to disease recurrence or progression within 1 year after hepatectomy in patients with hepatocellular carcinoma. World J Surg Oncol. 2012;10:107.
Egger ME, Ohlendorf JM, Scoggins CR, McMasters KM, Martin RCG 2nd. Assessment of the reporting of quality and outcome measures in hepatic resections: a call for 90-day reporting in all hepatectomy series. HPB (Oxford). 2015;17(9):839–45.
Dekker JWT, van den Broek CBM, Bastiaannet E, van de Geest LGM, Tollenaar RAEM, Liefers GJ. Importance of the first postoperative year in the prognosis of elderly colorectal cancer patients. Ann Surg Oncol. 2011;18(6):1533–9.
Mayo SC, Shore AD, Nathan H, Edil BH, Hirose K, Anders RA, et al. Refining the definition of perioperative mortality following hepatectomy using death within 90 days as the standard criterion. HPB (Oxford). 2011;13(7):473–82.
Gui CH, Baey S, D'Cruz RT, Shelat VG. Trans-arterial chemoembolization + radiofrequency ablation versus surgical resection in hepatocellular carcinoma - a meta-analysis. Eur J Surg Oncol. 2020;46(5):763–71.
Saviano A, Iezzi R, Giuliante F, Salvatore L, Mele C, Posa A, et al. Liver resection versus radiofrequency ablation plus transcatheter arterial chemoembolization in cirrhotic patients with solitary large hepatocellular carcinoma. J Vasc Intervent Radiol. 2017;28(11):1512–9.
Lee H-J, Kim JW, Hur YH, Shin SS, Heo SH, Cho SB, et al. Combined therapy of transcatheter arterial chemoembolization and radiofrequency ablation versus surgical resection for single 2-3 cm hepatocellular carcinoma: a propensity-score matching analysis. J Vasc Interv Radiol. 2017;28(9):1240–7.e3.
Vauthey JN, Dixon E, Abdalla EK, Helton WS, Pawlik TM, Taouli B, et al. Pretreatment assessment of hepatocellular carcinoma: expert consensus statement. HPB (Oxford). 2010;12(5):289–99.
Strasberg SM, Belghiti J, Clavien PA, Gadzijev E, Garden JO, Lau WY, et al. The Brisbane 2000 terminology of liver anatomy and resections. HPB. 2000;2(3):333–9.
Balzan S, Belghiti J, Farges O, Ogata S, Sauvanet A, Delefosse D, et al. The "50-50 criteria" on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg. 2005;242(6):824–9.
Mullen JT, Ribero D, Reddy SK, Donadon M, Zorzi D, Gautam S, et al. Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J Am Coll Surg. 2007;204(5):854–62 discussion 62-4.
Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery. 2011;149(5):713–24.
Madhavan S, Shelat VG, Soong SL, Woon WWL, Huey T, Chan YH, et al. Predicting morbidity of liver resection. Langenbecks Arch Surg. 2018;403(3):359–69.
Anbalakan K, Chua D, Pandya GJ, Shelat VG. Five year experience in management of perforated peptic ulcer and validation of common mortality risk prediction models – are existing models sufficient? A retrospective cohort study. International Journal of Surgery. 2015;14:38–44.
Taura K, Ikai I, Hatano E, Yasuchika K, Nakajima A, Tada M, et al. Influence of coexisting cirrhosis on outcomes after partial hepatic resection for hepatocellular carcinoma fulfilling the Milan criteria: an analysis of 293 patients. Surgery. 2007;142(5):685–94.
Chopinet S, Grégoire E, Bollon E, Hak J-F, Palen A, Vidal V, et al. Short-term outcomes after major hepatic resection in patients with cirrhosis: a 75-case unicentric western experience. HPB. 2019;21(3):352–60.
Moriguchi MTT, Higaki T, Kimura Y, Yamazaki S, Nakayama H, Ohkubo T, et al. Early cancer-related death after resection of hepatocellular carcinoma. Surgery. 2012;151(2):232–7.
Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35(3):421–30.
Cheung TT, Ng KK, Chok KS, Chan SC, Poon RT, Lo CM, et al. Combined resection and radiofrequency ablation for multifocal hepatocellular carcinoma: prognosis and outcomes. World J Gastroenterol. 2010;16(24):3056–62.
Shah SA, Tan JCC, McGilvray ID, Cattral MS, Levy GA, Greig PD, et al. Does microvascular invasion affect outcomes after liver transplantation for HCC? A histopathological analysis of 155 consecutive explants. J Gastrointest Surg. 2007;11(4):464–71.
Shelat VG, Diddapur RK. An early experience of liver transplantation in portal vein thrombosis. Singapore Med J. 2008;49(2):e37–41.
Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–90.
Roayaie S, Jibara G, Taouli B, Schwartz M. Resection of hepatocellular carcinoma with macroscopic vascular invasion. Ann Surg Oncol. 2013;20(12):3754–60.
Levi Sandri GB, Ettorre GM, Colasanti M, De Werra E, Mascianà G, Ferraro D, et al. Hepatocellular carcinoma with macrovascular invasion treated with yttrium-90 radioembolization prior to transplantation. Hepatobiliary Surg Nutr. 2017;6(1):44–8.
Fukushima K, Fukumoto T, Kuramitsu K, Kido M, Takebe A, Tanaka M, et al. Assessment of ISGLS definition of posthepatectomy liver failure and its effect on outcome in patients with hepatocellular carcinoma. J Gastrointest Surg. 2014;18(4):729–36.
Chan KS, Low JK, Shelat VG. Associated liver partition and portal vein ligation for staged hepatectomy: a review. Transl Gastroenterol Hepatol. 2020;5:37.
Saner F. Kidney failure following liver resection. Transplant Proc. 2008;40(4):1221–4.
Lim C, Audureau E, Salloum C, Levesque E, Lahat E, Merle JC, et al. Acute kidney injury following hepatectomy for hepatocellular carcinoma: incidence, risk factors and prognostic value. HPB (Oxford). 2016;18(6):540–8.
Acknowledgements
None
Funding
None
Author information
Authors and Affiliations
Contributions
SS, SM, and GL conducted data collection and interpretation and drafting of the manuscript. YHC was responsible for data analysis and interpretation and revision of the manuscript. SPJ, CWH, JKL, and VGS did conception of work, data interpretation, and critical revision of the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All patients undergoing liver resection provided written informed consent to participate in the liver standing order database approved by Institutional Review Board of National Healthcare Group with reference number - 2018-00048.
Consent for publication
Not applicable (no individual data included).
Competing interests
The authors declare that they have no competing interests
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sheriff, S., Madhavan, S., Lei, G.Y. et al. Predictors of mortality within the first year post-hepatectomy for hepatocellular carcinoma. J Egypt Natl Canc Inst 34, 14 (2022). https://doi.org/10.1186/s43046-022-00113-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43046-022-00113-8