Abstract
Background
Gut microbiota plays a pivotal role in the gut-brain axis and can influence neurodevelopment and mental health outcomes. This review summarizes the current evidence on the associations between gut microbiota alterations and various psychiatric illnesses.
Main body
The composition of the gut microbiome evolves from birth through old age, and disruptions during critical periods may increase disease risk. Factors like diet, medications, stress, and infections can disturb the gut microenvironment and lead to dysbiosis. Dysbiosis has been linked to conditions like depression, anxiety, autism, ADHD, and schizophrenia. Proposed mechanisms involve microbial regulation of neurotransmitters, inflammation, oxidative stress, blood-brain barrier permeability, and the immune system. Therapeutic strategies like probiotics, prebiotics, and faecal transplantation may modulate the gut-brain axis and microbial ecosystem. However, more research is needed to elucidate the causal microbiota-psychiatry relationship. Understanding gut-brain interactions may uncover new possibilities for preventing and managing psychiatric disorders.
Conclusion
A growing body of research points to a close relationship between gut microbiota and mental health. While the field is still emerging, dysbiosis of gut microbial ecosystem has been associated with various neuropsychiatric conditions. The underlying mechanisms likely involve the microbiota-gut-brain axis signalling pathways. Additional research with larger samples is required to establish causal links between specific microbial changes and psychiatric outcomes.
Similar content being viewed by others
Background
The gastrointestinal tract and brain are linked through a sophisticated, bidirectional communication network known as the gut-brain axis [1]. As a result of studies demonstrating revealing the substantial impact of gut microbiota on signalling connections between the gut and brain and its involvement in the gut-brain axis, the term was revised to the microbiota-gut-brain axis. This axis governs the functions of the central nervous system (CNS), gut, and immunity [2]. In healthy individuals, gut microbiotas establish stable host-bacterial mutualism. Any disruption to this mutualism would adversely affect the functioning of the brain, digestive system, and metabolism [2]. Bidirectional signalling between the gut microbiota and the CNS can affect the reaction to stress, feelings of pain, neurochemical amounts, and gut-brain axis disorders [1, 3]. The interaction between the gut microbiome and the nervous system involves metabolic processes such as tryptophan, serotonin, immunity, gut hormonal, and short-chain fatty acid metabolism (SCFAs) [4]. SCFAs play a crucial role in regulating the release of important neurotransmitters, including enteroendocrine serotonin (5-HT) and peptide YY (PYY), a neuropeptide critical to the gut-brain axis [5]. Research has demonstrated that the intestinal microbiota can influence the behaviour of germ-free (GF) animals and alter the physiology and biochemical properties of the nervous system [6].
The hypothalamic-pituitary-adrenal axis (HPA) is a pivotal component of the neuroendocrine system, regulating various physiological processes, including digestion, immunity, behaviour, and stress responsiveness. Abnormal development of the HPA is observed in GF rodents, leading to a modified stress response and decreased expression of brain-derived neurotrophic factor (BDNF) [7]. If GF mice are colonized with normal gastrointestinal microbiota from conventionally reared mice or the probiotic Bifidobacterium infantis, these abnormalities might be reversible [7]. These findings underscore the regulatory role of gut microbiota in HPA activity and emphasize its critical contribution to the development of the nervous system.
Gut microbiota and brain development
While the human body is nearly sterile at birth, the gut is quickly colonized by bacteria. This colonization process continues throughout childhood and adolescence. Consequently, establishing and progressing intestinal microbiota during infancy may shape an individual’s future physical and mental well-being. Brain development throughout childhood and adolescence is of equal importance, mirroring the progression and maturation of intestinal microbiota. Potential disruptions in the mutualism between the host and microbiota during these periods may have long-lasting health effects, increase the risk of neurodevelopmental disorders, and modify gut-brain axis pathways. Moreover, the fragility and immaturity of intestinal microbiota during these stages render individuals more susceptible to environmental influences, including antibiotics, stress, inadequate nutrition, infections, and more. This susceptibility leads to gut microbiota dysbiosis, which is detrimental to physical and mental health and ultimately contributes to brain disorders [8]. Despite the fact that gut microbiota is typically more established and stable in maturity, synaptic pruning and myelination still take place [9]. Changes in intestinal microbiota that occur during this period may therefore influence brain function and behaviour. It is critical to preserve a robust intestinal microbiota during all stages of development, maturation, and colonization to avert age-related brain diseases.
Even though the ageing process does not represent a pivotal phase in neurodevelopment, inflammation is a prevalent occurrence within the body [10]. It manifests as a progressive chronic proinflammatory response. The progressive alteration of gut microbiota composition [11] caused by this response degrades the stability and diversity of microbiota [12]. The gut microbiota composition in older adults is frequently influenced by factors such as the living environment, dietary patterns, and individual health status [12]. Furthermore, factors such as drug use, impaired immunity, malabsorption of nutrients, and deterioration of digestive motility have an effect on the composition of the gut microbiota [13].
Role of microbiota in brain function
Microbiota produce different neuroactive molecules or neurotransmitters that maintain the communication between gut and brain such as acetylcholine, GABA, and serotonin [14, 15]. Interestingly, 90% of serotonin required for mood, behaviour, sleep, and other CNS functions is produced in the gut [16]. Serotonin binding to 5-HT receptors on the microglia induces another mechanism for gut-induced modulation of neuroinflammation [17]. Similarly, tryptophan, which is a serotonin precursor, can influence microglia activity and the transcriptional programme of astrocytes [18].
In addition, bacterial fermentation of indigestible dietary fibres produces SCFAs such as butyrate, propionate, and acetate [19]. A small fraction of SCFAs reaches the systemic circulation and cross the blood-brain barrier (BBB) restoring its integrity [20]. Moreover, SCFAs can restore the normal maturation process of the microglia [21] and modulate neurotransmitters, like glutamate, glutamine, GABA, and neurotrophic factors [22]. Propionate and butyrate can influence the cell signalling system and regulate the expression levels of tryptophan 5-hydroxylase 1, involved in the synthesis of serotonin, and tyrosine hydroxylase, which is involved in the biosynthesis of dopamine, adrenaline, and noradrenaline [23].
Impact of the mode of delivery on microbiota composition
The “sterile womb dogma” held for an extended period that the human foetus remains sterile until delivery, and that microorganisms begin to colonize gastrointestinal tract after delivery. However, previous research reported that microbial colonization begins in utero [24,25,26]. Colonization by Staphylococcus epidermidis, Enterococcus faecium, and Escherichia coli might occur through translocation via the bloodstream and placenta from the mother’s gut [8]. Vaginal versus caesarean section (CS) delivery mode significantly impacts newborn GI tract colonization [27, 28]. Despite lack of medical indication, CS rates continue rising, exceeding 50% in some countries [29].
Vaginally delivered (VD) infants’ gut microbiota resembles the maternal vaginal microbiota, dominated by Lactobacillus. Meanwhile, CS leads to an imbalance and decreased diversity, lacking exposure to the vaginal microbiota of mother. Pathogens from the hospital dominate initial contact [30]. Infants delivered vaginally have higher Sneathia, Bacteroides, Corynebacterium, Staphylococcus, Clostridium difficile, lower Lactobacillus, Prevotella, and Bifidobacteria, compared to those born by CS [27]. Colonization of microbiota plays a vital role in infant metabolism, immunity, and brain development [31]. Maternal stress may also impact infant nervous system development by altering vaginal microbiota and subsequent intestinal colonization [32].
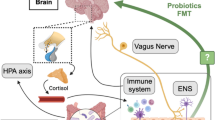
Factors affecting the changes of gut microbiota in psychiatric diseases (see Fig. 1)
Diet
Diet powerfully impacts the diversity and immunology of microbiota [33]. The Mediterranean diet with fish oil reduced symptoms of depression in one study [34]. However, another study found that vegetarian/vegan diets were associated with increased depression risk [35]. The high-fat, low-carbohydrate ketogenic diet improved cognition and memory in Alzheimer’s disease [36]. Despite the hypotheses that obesity is associated with increased Firmicutes/Bacteroidetes ratio, weight loss diets did not significantly alter this ratio [37]. However, the effect of diet-driven microbiota changes on the colon health and metabolism requires further studies [38].
Probiotics
Probiotics could be used in treatment of mental disorders which involve increased intestinal permeability like depression, anxiety, autism, and schizophrenia [39, 40]. Specific strains differentially impact the brain. A meta-analysis found that probiotics significantly alleviate symptoms of depression [41]. In healthy volunteers, Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 given for 30 days reduced Hospital Anxiety and Depression Scale scores versus placebo [42]. Also, other strains like Lactococcus lactis, B. longum, Lactobacillus bulgaricus, Bifidobacterium animalis, Streptococcus thermophilus, and L. helveticus decrease depression and stress [43]. Probiotics decreased inflammation and improved behavioural symptoms in patients with autism spectrum disorder (ASD) [44]. In schizophrenia, probiotics with vitamin D given for 12 weeks improved Positive and Negative Syndrome Scale (PANSS) scores, suggesting utility countering gastrointestinal inflammation [45]. Probiotics may also improve COVID-19-associated mood disturbances by restoring intestinal balance and preventing pathogen overgrowth [46, 47]. However, limitations exist, like avoiding probiotics in immunocompromised patients on corticosteroids [46].
Stress
The HPA axis dysregulation from early-life stressors increases risk for affective and anxiety disorders [48]. Corticotropin-releasing hormone (CRH) and arginine vasopressin (AVP) drive the HPA axis, influencing neurotransmission, sleep, mood, and feeding [49]. Communication between the nervous and endocrine systems regulates body functions [50]. Depressive disorders also show serotonin deficiency and HPA axis hyperactivity [51]. Stress reduces Lactobacillus and Bifidobacterium and increases Clostridium and Escherichia coli through catecholamine and glucocorticoid secretion [52].
Circadian system
The circadian system includes the suprachiasmatic nucleus central clock and peripheral clocks like the intestine. The gut microbiota shows diurnal fluctuations affected by shift work, light, sleep, diet, and stress [53]. Disrupting the circadian clock may contribute to psychiatric and metabolic disorders by altering gut microbiota signalling [54]. Bacterial clocks may also regulate human circadian rhythms and behaviour [55].
Occupational and environmental factors
Workplace biological (animal contact), chemical (metalworking fluids, pesticides), and physical (pressure, travel) exposures alter the microbiome [56]. After 30 days at sea, sailors showed increased Streptococcus gordonii and Klebsiella pneumoniae [57]. Night shift workers had increased Firmicutes, Actinobacteria, Dorea, and Faecali bacterium versus day workers [54]. These microbiome changes could serve as occupational health biomarkers [53]. Environmental pollutants like heavy metals, pesticides, polycyclic aromatic hydrocarbons (PAH), and polychlorinated biphenyls (PCB) also modify the microbiota [58]. Early chlorpyrifos exposure caused chronic microglial dysregulation, increasing Alzheimer’s disease risk [59]. However, microbes can detoxify xenobiotics, sometimes generating more toxic byproducts [60].
The coronavirus-19 (COVID-19)
Depression in COVID-19-infected patients could be due to social factors such as the social quarantine or pathological factors such as changes in the HPA axis, CNS proinflammatory cytokines, microglial production of inflammatory cytokines, or injury to the hippocampus [61].
Recently, post-acute COVID-19 syndrome (PACS) or long COVID-19 was a recent term used to describe a syndrome which is characterized by the persistence of clinical manifestations that persist 4 weeks after the onset of acute symptoms of COVID-19. Psychological issues that could persist after COVID-19 include anxiety, depression, insomnia, cognitive impairment, and posttraumatic stress disorder (PTSD) [62,63,64,65,66,67,68].
Gut microbiota diversity and beneficial bacteria predominance affect COVID-19 infection. After clearance of COVID-19, the gut microbiome remains dysbiotic, with fewer beneficial bacteria. Patients with PACS had higher levels of Ruminococcus gnavus and Bacteroides vulgatus and lower levels of Faecalibacterium prausnitzii. Moreover, patients with PACS who suffer neuropsychiatric symptoms had higher level of Clostridium innocuum, and Actinomyces naeslundii [69].
Antibiotics, antivirals, antifungals, and steroids, as well as diabetes, hypertension, and old age, worsen this dysbiosis [70]. Therefore, microbiota modification using probiotic could play a potential beneficial role as an adjunctive therapy in COVID-19 infection and is still an ongoing research process [71].
Role of gut microbiota in psychiatric illnesses
Attention-deficit hyperactivity disorder (ADHD)
ADHD is a common neurodevelopmental disorder affecting millions of children [72]. Genes for dopamine receptors and transmitters are the main etiological factors [73]. Growing evidence indicates a gut-brain connection [73]. ADHD patients showed increased Actinobacteria (e.g. Bifidobacterium) and reduced Firmicutes versus controls, with enhanced dopamine precursor synthesis capacity [74]. However, a meta-analysis found no significant microbiome differences beyond increased Blautia in ADHD, which regulates the metabolism and inflammation [75]. More research is needed in demographically diverse cohorts [76] and in assessing ADHD with other comorbidity [77,78,79]. Early probiotic administration like Lactobacillus rhamnosus GG may reduce ADHD risk by modulating emotional behaviour and GABA receptor expression [80]. Dietary improvements like reducing food additives and increasing omega-3 polyunsaturated fatty acids (PUFAs) can also minimize ADHD hyperactivity [81].
Autism spectrum disorder (ASD)
ASD is a neurodevelopmental disorder that is hazardous and is characterized by gastrointestinal symptoms [82]. While genetic factors, gastrointestinal abnormalities, inflammation, and environmental exposures are plausible contributors, no single factor can account for ASD [83] Patients diagnosed with ASD exhibited dysbiosis characterized by a phylum number of Fusobacteria, Verrucomicrobia, Firmicutes, and Bacteroides, as well as a ratio of Firmicutes to Bacteroides. The authors also reported that the alterations impact the concentrations of volatile organic compounds (VOC) and short-chain fatty acids (SCFAs) in individuals diagnosed with ASD. These volatile organic compounds include indole, a precursor to serotonin and melatonin and a metabolite of tryptophan [83]. Nevertheless, these findings must be interpreted with caution due to the potential impact of antibiotic therapy or individualized dietary regimens on individuals diagnosed with ASD [1].
Bipolar disorder (BD)
BD is a persistent mood disorder characterized by alternating manic and depressive episodes [84]. It was clinically observed that longer untreated illness in bipolar I disorder lead to more symptoms severity [85]. On the other hand, TRYCATs are neuroregulatory tryptophan catabolites, oxidative and nitrosative stress, and immune-inflammatory indicators found in patients [86]. Dysbiosis of the gut microbiota could be linked to the development of BD [87]. Increased Coriobacteriaceae associates with higher cholesterol, while more Lactobacilli associates with obesity in BD [88, 89]. Reduced Faecalibacterium, an anti-inflammatory commensal, also correlates with BD [90]. Clostridiaceae, which produce mood-regulating SCFAs, were four times lower in BD [91]. Previous research observed Toxoplasma gondii among patients with schizophrenia and bipolar disorder [92] that might affect gut microbiota in these patients and trigger conditions.
Neurocognitive disorders
Cognitive function preclinical studies, such as GF/GI infection models, antibiotic treatment, dietary manipulation, and probiotic treatment, have shown that gut microbiota composition affects cognitive function [93,94,95,96]. Prebiotics can improve emotional attention performance in healthy individuals [97], and probiotics can modify brain activity during a comparable test [98]. Furthermore, previous research found a significant relationship between Alzheimer disease and gut microbiota dysbiosis. A higher abundance of Prevotella species and lactic acid bacteria was correlated with cognition [99]. Targeting gut microbiota for cognitive benefits may be effective at age extremes, when brain function is vulnerable and in flux, with rapid development in infancy and gradual decline in function with a steady decline in specific cognitive abilities in old age [100]. A small randomized controlled trial revealed that microbiota-targeted therapies may benefit age-related cognitive impairment [101]. Also, previous research found cognitive impairment in schizophrenia and bipolar [102] that might elicit indirect relation of gut microbiota in cognitive impairment. No research has been conducted on the effectiveness of microbiota supplementation in boosting cognitive development in infants. However, preclinical research indicates that gut microbiota significantly impacts neurodevelopment throughout important postnatal periods [7, 103, 104] that might relate to hormonal disturbance. Similarly, previous research found prevalence of psychosis and depression in postnatal period was increased and mostly related to hormonal impairment especially postpartum [105, 106].
Major depressive disorder (MDD)
Many of the factors described in the data which established a connection between this mental disorder and intestinal microbiota components were validated by Naseribafrouei et al. [107].
There was a notable increase in the prevalence of MDD among patients [106, 108], who were correlated with elevated levels of the valeric acid-containing bacterium Oscillibacter and the inflammation-associated genus Alistipes. In a mouse model, Zhang et al. [98] established a correlation between dysbiosis of the microbiota and systemic inflammation as well as raise intestinal permeability [109]. The alteration of microbiota composition of mice caused by endogenous melatonin reduction (EMR) included an increase in the relative abundance of Lactobacillus, a decrease in the abundance of Bacteroidetes, and a modification in the ratio of Firmicutes/Bacteroidetes. Furthermore, EMR rodents exhibited increased systemic inflammation and enhanced gut permeability, which was manifested as a leaky gut. Utilizing SCFA quantification to analyse the microbiota composition of individuals diagnosed with MDD may prove effective. In a study on the SCFAs profile involving 116 women, it was found that 40.52% of the participants reported experiencing depression [110]. The results of the study indicated that the proportion of propionic acid was reduced among the participants, while isocaproic acid was higher, in comparison to the composition of healthy subjects. However, the inability to definitively assert that SCFAs contribute to the depressive phenotype was attributable to the small sample size. Studies on animal models have established a correlation between the composition of intestinal microbiota and personality traits and behaviour, including anxiety and depression. After transplanting the gut microbiota of confident Mongolian gerbils (Meriones unguiculatus), Gan et al. [111] observed alterations in the behaviour of timid individuals. After “bold faecal microbiota” transplantation, timid gerbils frequently displayed courageous behaviour, suggesting a correlation between the gut microbiota and the disposition of the host [112].
Schizophrenia (SCZ)
Schizophrenia is a complex condition affecting emotional, vocational, and cognitive abilities [113]. Viruses, cardiovascular, and metabolic diseases increase the risk of premature mortality among SCZ patients [114]. Owen et al. identified three distinct dimensions in SCZ: negative symptoms, positive symptoms, and cognitive impairment [115]. Moreover, schizophrenia could be induced by other medical disease [116] or drugs [117, 118].
The objective of neuroimaging and biochemical research is to elucidate the pathogenesis of SCZ. So far, neurotransmitter dependencies have been found, which may explain SCZ clinical manifestations. In this pathophysiology, dopamine appears to be the most important neurotransmitter [119]; however, other studies suggest that dopamine had an indirect function and identified other neurotransmitter linkages [120, 121]. Kozłowska et al. linked the etiology of SCZ to immune/inflammatory processes, where host alarmins activate signalling pathways, causing several infection-induced or sterile inflammatory disorders. A rising body of research highlights the importance of the glutamatergic system [122]. Specifically, the neuregulin 1 gene on 8p12 and the G72 and G30 genes on 13q33, which activate DAOA, are of concern [123]. These genes confirm the neurodevelopmental idea of SCZ and the glutamatergic system’s participation [124]. Increasing mesolimbic dopaminergic transmission and inhibiting glutamatergic transmission are known to contribute to favourable SCZ symptoms. The growing number of premises suggests that kynurenic acid (KYNA) may modulate both pathways [125]. KYNA) functions as an α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptor and an antagonist of the NMDA receptor complex’s strychnine-independent glycine site. SCZ patients have higher CSF KYNA levels, according to research. KYNA study revealed its potential function in CNS physiology and disease. KYNA influences CNS function and illness symptoms, although the mechanism is unknown. However, significant KYNA concentration disparities between sick and healthy individuals suggest its role in the development of neurological and mental diseases [126]. KYNA is difficult to detect in the blood due to its low penetration of the blood-brain barrier. Therefore, scientists focus on other metabolites in the kynurenine pathway. Previous study found a predicted concentration of 3-hydroxykynureine for reducing psychopathological symptoms in the first episode of SCZ [127]. The discovery of biological variables that predict antipsychotic medication success is promising. Despite thorough investigation, there is still no known cure for psychotic disorders, including SCZ, and the etiopathogenesis of this baffling and incurable disease remains unknown [112].
Sleep disorders
Gut microbiota and sleep interact bidirectionally. Microbial colonization in early development coincides with critical cognitive phases [128]. Sleep loss alters microbiota diversity and composition in adulthood [129, 130]; also, short sleep fragmentation impacts gut bacteria and metabolism over time [130].
In obstructive sleep apnea, microbiota dysbiosis relates to inflammation in children [131]. Foecal transplants from sleep apnea mice increase sleep in naive mice, suggesting that the microbiota mediates sleep-wake changes [132]. Patients with acute and chronic insomnia show gut dysbiosis [133]. Therefore, microbes that produce sleep neurochemicals like GABA, serotonin, and histamine may hold therapeutic potential [134].
As mentioned early, the circadian disruptions in humans and mice alter microbiota diversity [54]. Light exposure, melatonin, and microbiota-targeted treatments may readjust circadian rhythms [135, 136]. Microbiota also relates to sleep disorders in psychiatric conditions [137, 138]. Overall, the microbiota-gut-brain axis bidirectionally regulates sleep physiology through various pathways [139].
Addiction disorders
Metabolites of gut microbiota have an essential role in the substance abuse disorders including SCFAs and bile acids. Vagal nerve stimulation, brain-derived neurotropic factor, and gut epithelial barrier dysfunction with bacterial translocation represent other factors that link microbiota with addiction [140].
Alcohol
Initial gut microbiome studies in substance abuse focused heavily on alcohol use. Chronic alcoholics exhibit reduced gut microbiome diversity and alter composition compared to healthy controls, with fewer beneficial Firmicutes (Lactobacilli, Enterococci) and Actinobacteria (Bifidobacteria) [141]. Acute alcohol exposure may temporarily disrupt intestinal permeability, enabling bacterial products to enter circulation [142]. This leakage is linked to increased depression and cravings in the recovering alcoholics [143]. In a meta-analysis, dopamine, GABA, serotonin, and norepinephrine were found to be the main neurotransmitters upregulated in the presence of alcohol. Alcohol-induced alteration of microbiota can have adverse effect on the brain function and play a vital role in addiction and alcohol dependence [144].
CNS stimulants
Cocaine induces upregulation of the proinflammatory mediators in the GI tract along with a compromise of mucosal barrier integrity [145]. Cocaine use is independently associated with intestinal dysbiosis and increased Bacteroidetes [146] and alteration of Verrucomicrobia and Firmicutes [147]. Furthermore, patients with cocaine use disorder showed marked dysbiosis of both foecal and oral microbiota composition and function [148]. Opioid use correlates with altered gut microbiome composition in some studies [149,150,151] but not in others [152]. Confounding factors like polysubstance abuse require further research. However, opioids appear to change gut microbial communities and function in ways that may impact the drug effects [140].
Anxiety
No published research has examined the relationship between gastrointestinal microbiota and any specific anxiety disorder. Among the various anxiety disorders, obsessive-compulsive disorder has exhibited the strongest correlation with infection, particularly respiratory tract infection caused by group A beta-hemolytic streptococcus [153], while there is a lack of research examining the efficacy of probiotics in patients with obsessive-compulsive disorder. A study on rodents indicates that L. rhamnosus may have some potential impact [154]. Lyte et al. discovered that anxiety-like behaviour in rodents was induced by subclinical concentrations of the pathogen Campylobacter jejuni administered orally via gavage. These doses failed to elicit an overt immune response. Involvement of brainstem regions, including the nucleus tractus solitarius and lateral parabrachial nucleus, in the processing that generates autonomic, neuroendocrine, and behavioural responses was also observed [155]. Bruch examined the Medical Expenditure Panel Survey to determine prospectively whether intestinal infection is associated with the future onset of an anxiety disorder and compelling evidence supporting a correlation between intestinal infection and the subsequent onset of anxiety [156].
Role of microbiota as a treatment option for psychiatric illness
Diet
Food has a substantial impact on the gut microbiota, and studies have shown that making dietary modifications in a short period of time (24 h) can modify the structure of the intestinal microbiota system [157], which could be offered as an adjuvant antidepressant medication. It has been demonstrated that specific dietary components can maintain the gut microbiota’s structural homeostasis; this effect is believed to have clinical implications. Patients can alleviate the influence of minor symptoms by reducing psychological resistance to treatment through nutritional relief. Prebiotics are nondigestible fibres that promote the growth of beneficial gut microbes by being partially digested in the gastrointestinal tract [42].
Prebiotics
Prebiotics in diet, such as insulins, oligofructose, fructooligosaccharide (FOS), and galacto-oligosaccharide (GOS), could influence the intestinal microbiota ecosystem structure, which is drastically diminished in patients with depression [158]. Tarr’s experiment in 2015 proved that the oligosaccharides 3-sialyllactose (3SL) or 6-sialyllactose (6SL) in human breast milk inhibit the development of anxiety [159]. Healthy diet like the Mediterranean diet which includes high levels of plant compounds, vitamins, minerals, PUFAs, and dietary fibres is recommend for patients who suffer depression [34].
Probiotics and psychobiotic
People usually support probiotics’ antidepressant and antianxiety properties, but additional confirmation is still needed due to inaccuracy and a lack of clinical tables [43]. Schnorr and Bachner administered a combination of psychotherapy and dietary intervention to an apprehensive patient. Instead of hyperglycaemic diets, they incorporated meals that were abundant in probiotics. The results indicated that this therapeutic regimen decreased the prevalence of unfavourable microorganisms (e.g. Clostridium), increased the abundance of beneficial microorganisms (e.g. Lactobacillus), and improved anxiety and insomnia. Additionally, the drug regimen altered the composition and diversity of bacteria [160].
Variable probiotics possess potent stress-modulating and anxiolytic effects which make those probiotics to be promising living psychobiotics for alleviating psychological disorders. They act by maintaining the intestinal homeostasis, improving mucosal and systemic immunity, and regulating the metabolism of gut microbiota. The main microbial genera with psychobiotic characteristics are Lactobacillus, Lactococcus, and Bifidobacterium [161].
Aberrant intestinal microbiota diminishes the stability of the gastrointestinal barrier, allowing increased entry of lipopolysaccharides (LPS) into the body. This, in turn, triggers systemic inflammation and a stress response [162]. However, probiotic medication can effectively prevent damage to the intestinal barrier and enhance its function through various mechanisms, ultimately reducing the reactivity of the HPA axis to stress [163]. Probiotics such as Lactobacillus rhamnosus can regulate plasma corticosterone levels induced by excitation and alleviate depression by influencing hormones released by the vagus nerve and hippocampus, including BDNF and oxytocin [164]. Also, the vagal nerve regulates the activity of Bifidobacterium infantis, which is associated with hormones including acetylcholine and corticosterone [7]. It is important to note that different probiotic strains may affect individuals differently. For instance, a study revealed that the ingestion of Lactobacillus casei did not significantly improve health in all patients when they were in a healthy state [165]. Notably, the effect of probiotics proved in experiencing anxiety or depression [165]. Conversely, in another investigation, healthy subjects consuming a meal containing L. helveticus and B. longum for 30 days reported improved stress states and negative mood regulation Daily intake of L. casei strain Shirota has been shown to enhance gut microbiota composition and function, potentially reducing stress exposure symptoms in healthy participants [166].
In addressing treatment-resistant depression (TRD), Bamling et al. employed a unique combination of antidepressant treatment with probiotics, magnesium, and selective serotonin reuptake inhibitors (SSRIs), resulting in a significant improvement in depressive symptoms [167]. Additionally, Lactobacillus plantarum JYLP-326 was reported to alleviate anxiety, depression, and insomnia in college students experiencing test anxiety [168]. However, the efficacy of probiotics in treating gastrointestinal or behavioural symptoms in children with autism spectrum disorder (ASD) is limited [169].
Synbiotic
Synbiotics, a combination of prebiotics and probiotics, have been found to alleviate depression in patients undergoing haemodialysis. The supplementation of synbiotics was associated with increased serum levels of BDNF in a subgroup of patients with depression [170, 171].
Engineered bacteria
Engineered bacteria, such as L-4-chlorokynurenine (L-4-Cl-KYn) expressed by marine bacteria, have been utilized to treat depression. Combined with the strain’s native enzymes, it enhances the therapeutic effect [172]. Targeting peptide release in the intestines could be a promising method for integrating with future psychological discoveries, focusing on peptide-mediated immune responses, enhanced vagal signalling, or regulation of neuropeptide expression in specific brain regions. However, intestinal peptides face practical limitations, including a brief half-life and slow traversal of the blood-brain barrier [173]. Modified strains with targeted peptide chemistry modifications may enhance the efficacy of intestinal peptides, and ongoing efforts aim to improve both modified peptide chain sequences and strain selection [174].
Faecal microbiota transplantation (FMT)
FMT involves transferring foecal microorganisms from a donor to a recipient. While FMT has been effective in treating microbial structural abnormalities and depression, it can induce anxiety, depressed behaviours, and stress responses in healthy individuals.
Nevertheless, FMT has demonstrated positive outcomes in treating microbial structural abnormalities [175] and depression [176, 177] and reprogramming the host’s metabolism [178]. Previous study demonstrated that FMT could regulate serotonin levels, reduce gut epithelial validation response, and control inflammatory response. Additionally, it can affect the variety and ecosystem structure of colon microbiota [179]. FMT has also exhibited antidepressant effects on depression induced by chronic, unpredictable mild stress in rats, impacting various neurotransmitters, inflammatory factors, neurotrophic factors, and glucagon-like peptides [180]. In patients with major depression, oral frozen FMT capsules, used as an add-on therapy, significantly improved depressive symptoms after 4 weeks of treatment [181].
Irritable bowel syndrome (IBS), a gastrointestinal functional disorder, is a common consequence of depression [182]. FMT has been incorporated into typical treatment programmes for IBS, showing significant efficacy with remission rates of up to 89% in treated patients [183]. Although some negative consequences have been observed after FMT treatment due to alterations in the intestinal microbiota [184], most of these effects are modest [185] or reversible [184].
Conclusions
In conclusion, the gut microbiota plays a fundamental role in brain development, with its composition evolving from birth to ageing. Factors such as diet, stress, disrupted circadian rhythms, environmental and occupational factors, and even COVID-19 can influence the microbiota composition, leading to dysbiosis implicated in various diseases, including those affecting the central nervous system. Gut-brain axis and microbiota dysbiosis could have a major role in pathogenesis of various mental disorders. As a result, the use of psychobiotics and faecal microbiota transplantation has emerged as a potential significant aspect of managing psychiatric diseases. Further research is still needed to address the exact causal link between certain microbiotal changes and various psychiatric disorders with further implication on the management for these conditions.
Availability of data and materials
Not applicable
Abbreviations
- CNS:
-
The central nervous system
- PYY:
-
Peptide YY
- 5-HT:
-
Serotonin
- GF:
-
Germ-free
- HPA:
-
The hypothalamic-pituitary-adrenal axis
- BDNF:
-
Brain-derived neurotrophic factor
- CS:
-
Caesarean section
- VD:
-
Vaginally delivered
- ASD:
-
Autism spectrum disorder
- PACS:
-
Post-acute COVID-19 syndrome
- CRH:
-
Corticotropin-releasing hormone
- AVP:
-
Arginine vasopressin
- PUFAs:
-
Polyunsaturated fatty acids
- PTSD:
-
Posttraumatic stress disorder
- ADHD:
-
Attention-deficit hyperactivity disorder
- VOC:
-
Volatile organic compounds
- SCFAs:
-
Short-chain fatty acids
- BD:
-
Bipolar disorder
- MDD:
-
Major depressive disorder
- EMR:
-
Endogenous melatonin reduction
- TRD:
-
Treatment-resistant depression
- FMT:
-
Faecal microbiota transplantation
- IBS:
-
Irritable bowel syndrome
- L-4-Cl-Kyn:
-
L-4-chlorokynurenine
References
Cryan JF, Dinan TG (2012) Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 13(10):701–712. https://doi.org/10.1038/nrn3346
Cryan JF, O'Mahony SM (2011) The microbiome-gut-brain axis: from bowel to behavior. Neurogastroenterol Motil 23(3):187–192. https://doi.org/10.1111/j.1365-2982.2010.01664.x
Collins SM, Surette M, Bercik P (2012) The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol 10(11):735–742. https://doi.org/10.1038/nrmicro2876
Dash S, Clarke G, Berk M, Jacka FN (2015) The gut microbiome and diet in psychiatry: focus on depression. Curr Opin Psychiatry 28(1):1–6. https://doi.org/10.1097/YCO.0000000000000117
Holzer P, Reichmann F, Farzi A, Neuropeptide Y (2012) peptide YY and pancreatic polypeptide in the gut-brain axis. Neuropeptides 46(6):261–274. https://doi.org/10.1016/j.npep.2012.08.005
Diaz Heijtz R, Wang S, Anuar F, Qian Y, Björkholm B, Samuelsson A et al (2011) Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci U S A 108(7):3047–3052. https://doi.org/10.1073/pnas.1010529108
Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN et al (2004) Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol 558(Pt 1):263–275. https://doi.org/10.1113/jphysiol.2004.063388
Borre YE, O'Keeffe GW, Clarke G, Stanton C, Dinan TG, Cryan JF (2014) Microbiota and neurodevelopmental windows: implications for brain disorders. Trends Mol Med 20(9):509–518. https://doi.org/10.1016/j.molmed.2014.05.002
Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW (2003) Mapping cortical change across the human life span. Nat Neurosci 6(3):309–315. https://doi.org/10.1038/nn1008
Franceschi C (2007) Inflammaging as a major characteristic of old people: can it be prevented or cured? Nutr Rev 65(12 Pt 2):S173–S176. https://doi.org/10.1111/j.1753-4887.2007.tb00358.x
Guigoz Y, Doré J, Schiffrin EJ (2008) The inflammatory status of old age can be nurtured from the intestinal environment. Curr Opin Clin Nutr Metab Care 11(1):13–20. https://doi.org/10.1097/MCO.0b013e3282f2bfdf
Claesson MJ, Jeffery IB, Conde S, Power SE, O'Connor EM, Cusack S et al (2012) Gut microbiota composition correlates with diet and health in the elderly. Nature. 488(7410):178–184. https://doi.org/10.1038/nature11319
Biagi E, Candela M, Turroni S, Garagnani P, Franceschi C, Brigidi P (2013) Ageing and gut microbes: perspectives for health maintenance and longevity. Pharmacol Res 69(1):11–20. https://doi.org/10.1016/j.phrs.2012.10.005
Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L et al (2015) Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 161(2):264–276. https://doi.org/10.1016/j.cell.2015.02.047
Pokusaeva K, Johnson C, Luk B, Uribe G, Fu Y, Oezguen N et al (2017) GABA-producing Bifidobacterium dentium modulates visceral sensitivity in the intestine. Neurogastroenterol Motil 29(1):e12904. https://doi.org/10.1111/nmo.12904
Gershon MD (2013) 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract. Curr Opin Endocrinol Diabetes Obes 20(1):14–21. https://doi.org/10.1097/MED.0b013e32835bc703
Glebov K, Löchner M, Jabs R, Lau T, Merkel O, Schloss P et al (2015) Serotonin stimulates secretion of exosomes from microglia cells. Glia. 63(4):626–634. https://doi.org/10.1002/glia.22772
Rothhammer V, Borucki DM, Tjon EC, Takenaka MC, Chao CC, Ardura-Fabregat A et al (2018) Microglial control of astrocytes in response to microbial metabolites. Nature. 557(7707):724–728. https://doi.org/10.1038/s41586-018-0119-x
Cummings JH, Englyst HN (1987) Fermentation in the human large intestine and the available substrates. Am J Clin Nutr 45(5 Suppl):1243–1255. https://doi.org/10.1093/ajcn/45.5.1243
Silva YP, Bernardi A, Frozza RL (2020) The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front Endocrinol (Lausanne) 11:25. https://doi.org/10.3389/fendo.2020.00025
Erny D, Hrabě de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E et al (2015) Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci 18(7):965–977. https://doi.org/10.1038/nn.4030
Frost G, Sleeth ML, Sahuri-Arisoylu M, Lizarbe B, Cerdan S, Brody L et al (2014) The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat Commun 5:3611. https://doi.org/10.1038/ncomms4611
Nankova BB, Agarwal R, MacFabe DF, La Gamma EF (2014) Enteric bacterial metabolites propionic and butyric acid modulate gene expression, including CREB-dependent catecholaminergic neurotransmission, in PC12 cells--possible relevance to autism spectrum disorders. PLoS One 9(8):e103740. https://doi.org/10.1371/journal.pone.0103740
Stinson LF, Boyce MC, Payne MS, Keelan JA (2019) The not-so-sterile womb: evidence that the human fetus is exposed to bacteria prior to birth. Front Microbiol 10:1124. https://doi.org/10.3389/fmicb.2019.01124
Nagpal R, Mainali R, Ahmadi S, Wang S, Singh R, Kavanagh K et al (2018) Gut microbiome and aging: physiological and mechanistic insights. Nutr Health Aging 4:267–285. https://doi.org/10.3233/NHA-170030
Walker RW, Clemente JC, Peter I, Loos RJF (2017) The prenatal gut microbiome: are we colonized with bacteria in utero? Pediatr Obes 12(S1):3–17. https://doi.org/10.1111/ijpo.12217
Kevin L, Eugene MD, Ryan CA, Ross RP, Catherine S (2022) First encounters of the microbial kind: perinatal factors direct infant gut microbiome establishment. Microbiome Res Rep 1(2):10. https://doi.org/10.20517/mrr.2021.09
Yao Y, Cai X, Ye Y, Wang F, Chen F, Zheng C (2021) The role of microbiota in infant health: from early life to adulthood. Front Immunol 12:708472. https://doi.org/10.3389/fimmu.2021.708472
Shaterian N, Abdi F, Ghavidel N, Alidost F (2021) Role of cesarean section in the development of neonatal gut microbiota: a systematic review. Open Med 16(1):624–639. https://doi.org/10.1515/med-2021-0270
Shao Y, Forster SC, Tsaliki E, Vervier K, Strang A, Simpson N et al (2019) Stunted microbiota and opportunistic pathogen colonization in caesarean-section birth. Nature 574(7776):117–121. https://doi.org/10.1038/s41586-019-1560-1
Martín-Peláez S, Cano-Ibáñez N, Pinto-Gallardo M, Amezcua-Prieto C (2022) The impact of probiotics, prebiotics, and synbiotics during pregnancy or lactation on the intestinal microbiota of children born by cesarean section: a systematic review. Nutrients 14(2):341
Hechler C, Borewicz K, Beijers R, Saccenti E, Riksen-Walraven M, Smidt H et al (2019) Association between psychosocial stress and fecal microbiota in pregnant women. Sci Rep 9(1):4463. https://doi.org/10.1038/s41598-019-40434-8
Gubert C, Kong G, Renoir T, Hannan AJ (2020) Exercise, diet and stress as modulators of gut microbiota: implications for neurodegenerative diseases. Neurobiol Dis 134:104621. https://doi.org/10.1016/j.nbd.2019.104621
Parletta N, Zarnowiecki D, Cho J, Wilson A, Bogomolova S, Villani A et al (2019) A Mediterranean-style dietary intervention supplemented with fish oil improves diet quality and mental health in people with depression: a randomized controlled trial (HELFIMED). Nutr Neurosci 22(7):474–487. https://doi.org/10.1080/1028415X.2017.1411320
Jain R, Larsuphrom P, Degremont A, Latunde-Dada GO, Philippou E (2022) Association between vegetarian and vegan diets and depression: a systematic review. Nutr Bull 47(1):27–49. https://doi.org/10.1111/nbu.12540
Horn J, Mayer DE, Chen S, Mayer EA (2022) Role of diet and its effects on the gut microbiome in the pathophysiology of mental disorders. Transl Psychiatry 12(1):164. https://doi.org/10.1038/s41398-022-01922-0
Ley RE, Turnbaugh PJ, Klein S, Gordon JI (2006) Human gut microbes associated with obesity. Nature 444(7122):1022–1023. https://doi.org/10.1038/4441022a
Duncan SH, Lobley GE, Holtrop G, Ince J, Johnstone AM, Louis P et al (2008) Human colonic microbiota associated with diet, obesity and weight loss. Int J Obes 32(11):1720–1724. https://doi.org/10.1038/ijo.2008.155
Alli SR, Gorbovskaya I, Liu JCW, Kolla NJ, Brown L, Müller DJ (2022) The gut microbiome in depression and potential benefit of prebiotics, probiotics and synbiotics: a systematic review of clinical trials and observational studies. Int J Mol Sci 23(9):4494. https://doi.org/10.3390/ijms23094494
Sanders ME, Merenstein DJ, Reid G, Gibson GR, Rastall RA (2019) Probiotics and prebiotics in intestinal health and disease: from biology to the clinic. Nat Rev Gastroenterol Hepatol 16(10):605–616. https://doi.org/10.1038/s41575-019-0173-3
Huang R, Wang K, Hu J (2016) Effect of probiotics on depression: a systematic review and meta-analysis of randomized controlled trials. Nutrients 8(8):483. https://doi.org/10.3390/nu8080483
Messaoudi M, Violle N, Bisson J-F, Desor D, Javelot H, Rougeot C (2011) Beneficial psychological effects of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in healthy human volunteers. Gut Microbes 2(4):256–261. https://doi.org/10.4161/gmic.2.4.16108
Liu RT, Walsh RFL, Sheehan AE (2019) Prebiotics and probiotics for depression and anxiety: a systematic review and meta-analysis of controlled clinical trials. Neurosci Biobehav Rev 102:13–23. https://doi.org/10.1016/j.neubiorev.2019.03.023
Critchfield JW, van Hemert S, Ash M, Mulder L, Ashwood P (2011) The potential role of probiotics in the management of childhood autism spectrum disorders. Gastroenterol Res Pract 2011:161358. https://doi.org/10.1155/2011/161358
Ghaderi A, Banafshe HR, Mirhosseini N, Moradi M, Karimi M-A, Mehrzad F et al (2019) Clinical and metabolic response to vitamin D plus probiotic in schizophrenia patients. BMC Psychiatry 19(1):77. https://doi.org/10.1186/s12888-019-2059-x
Łoniewski I, Skonieczna-Żydecka K, Sołek-Pastuszka J, Marlicz W (2022) Probiotics in the management of mental and gastrointestinal post-COVID symptomes. J Clin Med 11(17):5155
Ghannoum MA, Ford M, Bonomo RA, Gamal A, McCormick TS (2021) A microbiome-driven approach to combating depression during the COVID-19 pandemic. Front Nutr 8:576. https://doi.org/10.3389/fnut.2021.672390
Smith KE, Pollak SD (2020) Early life stress and development: potential mechanisms for adverse outcomes. J Neurodev Disord 12(1):34. https://doi.org/10.1186/s11689-020-09337-y
Laryea G, Arnett MG, Muglia LJ (2012) Behavioral studies and genetic alterations in corticotropin-releasing hormone (CRH) neurocircuitry: insights into human psychiatric disorders. Behav Sci 2(2):135–171
Tsigos CK, I Kyrou, E Kassi, GP Chrousos. Stress: endocrine physiology and pathophysiology. https://www.ncbi.nlm.nih.gov/books/NBK278995/ (2020). Accessed.
Cubała WJ, Landowski J (2006) Serotoninergic system and limbic-hypothalamic-pituitary-adrenal axis (LHPA axis) in depression. Psychiatr Pol 40(3):415–430
Zhou L, Kang L, Xiao X, Jia L, Zhang Q, Deng M (2019) “Gut microbiota-circadian clock axis” in deciphering the mechanism linking early-life nutritional environment and abnormal glucose metabolism. Int J Endocrinol 2019:5893028. https://doi.org/10.1155/2019/5893028
Mucci N, Tommasi E, Chiarelli A, Lulli LG, Traversini V, Galea RP et al (2022) WORKbiota: a systematic review about the effects of occupational exposure on microbiota and workers’ health. Int J Environ Res Public Health 19(3):1043
Mortaş H, Bilici S, Karakan T (2020) The circadian disruption of night work alters gut microbiota consistent with elevated risk for future metabolic and gastrointestinal pathology. Chronobiol Int 37(7):1067–1081. https://doi.org/10.1080/07420528.2020.1778717
Murakami M, Tognini P, Liu Y, Eckel-Mahan KL, Baldi P, Sassone-Corsi P (2016) Gut microbiota directs PPARγ-driven reprogramming of the liver circadian clock by nutritional challenge. EMBO Rep 17(9):1292–1303. https://doi.org/10.15252/embr.201642463
Fenga C (2022) Gut microbiota modulation: a tailored approach for the prevention of chronic diseases. Biomed Rep 16(4):23. https://doi.org/10.3892/br.2022.1506
Zhang J, Zhao J, Jin H, Lv R, Shi H, De G et al (2020) Probiotics maintain the intestinal microbiome homeostasis of the sailors during a long sea voyage. Gut Microbes 11(4):930–943. https://doi.org/10.1080/19490976.2020.1722054
Lu K, Mahbub R, Fox JG (2015) Xenobiotics: interaction with the intestinal microflora. ILAR J 56(2):218–227. https://doi.org/10.1093/ilar/ilv018
Voorhees JR, Remy MT, Erickson CM, Dutca LM, Brat DJ, Pieper AA (2019) Occupational-like organophosphate exposure disrupts microglia and accelerates deficits in a rat model of Alzheimer’s disease. npj Aging Mech Dis 5(1):3. https://doi.org/10.1038/s41514-018-0033-3
Gui X, Yang Z, Li MD (2021) Effect of cigarette smoke on gut microbiota: state of knowledge. Front Physiol 12:673341. https://doi.org/10.3389/fphys.2021.673341
Mohammadkhanizadeh A, Nikbakht F (2021) Investigating the potential mechanisms of depression induced-by COVID-19 infection in patients. J Clin Neurosci 91:283–287. https://doi.org/10.1016/j.jocn.2021.07.023
Shimohata T (2022) Neuro-COVID-19. Clin Exp Neuroimmunol 13(1):17–23. https://doi.org/10.1111/cen3.12676
Chippa V, Aleem A, Anjum F (2023) Post-Acute Coronavirus (COVID-19) Syndrome. StatPearls. Treasure Island (FL) ineligible companies. Disclosure: Abdul Aleem declares no relevant financial relationships with ineligible companies. In: Disclosure: Fatima Anjum declares no relevant financial relationships with ineligible companies.: StatPearls Publishing Copyright © 2023. StatPearls Publishing LLC
Ahmed GK, Khedr EM, Hamad DA, Meshref TS, Hashem MM, Aly MM (2021) Long term impact of Covid-19 infection on sleep and mental health: a cross-sectional study. Psychiatry Res 305:114243. https://doi.org/10.1016/j.psychres.2021.114243
Ahmed GK, Elbeh K, Gomaa HM, Soliman S (2021) Does COVID-19 infection have an impact on children’s psychological problems? Middle East Curr Psychiatry 28(1):77. https://doi.org/10.1186/s43045-021-00155-z
Ahmed GK, Mostafa S, Elbeh K, Gomaa HM, Soliman S (2022) Effect of COVID-19 infection on psychological aspects of pre-schooler children: a cross-sectional study. Middle East Curr Psychiatry 29(1):42. https://doi.org/10.1186/s43045-022-00207-y
Gk A, Salman SA, Elbeh K, Amer ZS, Abbas AM (2022) Correlation between psychiatric impact of COVID-19 during pregnancy and fetal outcomes in Egyptian women. Psychiatry Res 317:114920. https://doi.org/10.1016/j.psychres.2022.114920
Shehata RR, Ahmed GK, Hussien AARM, Mahmoud MA (2023) Does post-acute COVID-19 syndrome women’s sex problems link to psychiatry after 6 months? The Egyptian Journal of Neurology. Psychiatry Neurosurg 59(1):119. https://doi.org/10.1186/s41983-023-00722-7
Liu Q, Mak JWY, Su Q, Yeoh YK, Lui GC, Ng SSS et al (2022) Gut microbiota dynamics in a prospective cohort of patients with post-acute COVID-19 syndrome. Gut 71(3):544–552. https://doi.org/10.1136/gutjnl-2021-325989
Kim S, Rigatto K, Gazzana MB, Knorst MM, Richards EM, Pepine CJ et al (2020) Altered gut microbiome profile in patients with pulmonary arterial hypertension. Hypertension. 75(4):1063–1071. https://doi.org/10.1161/HYPERTENSIONAHA.119.14294
de Oliveira GLV, Oliveira CNS, Pinzan CF, de Salis LVV, Cardoso CRB (2021) Microbiota modulation of the gut-lung axis in COVID-19. Front Immunol 12:635471. https://doi.org/10.3389/fimmu.2021.635471
Kalenik A, Kardaś K, Rahnama A, Sirojć K, Wolańczyk T (2021) Gut microbiota and probiotic therapy in ADHD: a review of current knowledge. Prog Neuro-Psychopharmacol Biol Psychiatry 110:110277. https://doi.org/10.1016/j.pnpbp.2021.110277
Bonvicini C, Cortese S, Maj C, Baune BT, Faraone SV, Scassellati C (2020) DRD4 48 bp multiallelic variants as age-population-specific biomarkers in attention-deficit/hyperactivity disorder. Transl Psychiatry 10(1):70. https://doi.org/10.1038/s41398-020-0755-4
Aarts E, Ederveen THA, Naaijen J, Zwiers MP, Boekhorst J, Timmerman HM et al (2017) Gut microbiome in ADHD and its relation to neural reward anticipation. PLoS One 12(9):e0183509. https://doi.org/10.1371/journal.pone.0183509
Wang N, Gao X, Zhang Z, Yang L (2022) Composition of the gut microbiota in attention deficit hyperactivity disorder: a systematic review and meta-analysis. Front Endocrinol (Lausanne) 13:838941. https://doi.org/10.3389/fendo.2022.838941
Schleupner HVC, Carmichael MJ (2022) Attention-deficit/hyperactivity disorder and the gut microbiota–gut–brain axis: closing research gaps through female inclusion in study design. Women 2(3):231–253
Ahmed GK, Darwish AM, Khalifa H, Haridy NA (2022) Relationship between attention deficit hyperactivity disorder and epilepsy: a literature review. Egypt J Neurol Psychiatr Neurosurg 58(1):52. https://doi.org/10.1186/s41983-022-00482-w
Ahmed GK, Darwish AM, Khalifa H, Khashbah MA (2021) Evaluation of psychiatric comorbidity in attention-deficit hyperactivity disorder with epilepsy: a case-control study. Epilepsy Res 169:106505. https://doi.org/10.1016/j.eplepsyres.2020.106505
Ahmed GK, Darwish AM, Khalifa H, Khashbah MA (2020) Comparison of cognitive function, socioeconomic level, and the health-related quality of life between epileptic patients with attention deficit hyperactivity disorder and without. Middle East Curr Psychiatry 27(1):45. https://doi.org/10.1186/s43045-020-00054-9
Pärtty A, Kalliomäki M, Wacklin P, Salminen S, Isolauri E (2015) A possible link between early probiotic intervention and the risk of neuropsychiatric disorders later in childhood: a randomized trial. Pediatr Res 77(6):823–828. https://doi.org/10.1038/pr.2015.51
Checa-Ros A, Jeréz-Calero A, Molina-Carballo A, Campoy C, Muñoz-Hoyos A (2021) Current evidence on the role of the gut microbiome in ADHD pathophysiology and therapeutic implications. Nutrients. 13(1):249. https://doi.org/10.3390/nu13010249
Christensen DL, Braun KVN, Baio J, Bilder D, Charles J, Constantino JN et al (2018) Prevalence and characteristics of autism spectrum disorder among children aged 8 years - Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2012. MMWR Surveill Summ 65(13):1–23. https://doi.org/10.15585/mmwr.ss6513a1
De Angelis M, Francavilla R, Piccolo M, De Giacomo A, Gobbetti M (2015) Autism spectrum disorders and intestinal microbiota. Gut Microbes 6(3):207–213. https://doi.org/10.1080/19490976.2015.1035855
Dickerson F, Severance E, Yolken R (2017) The microbiome, immunity, and schizophrenia and bipolar disorder. Brain Behav Immun 62:46–52. https://doi.org/10.1016/j.bbi.2016.12.010
Ahmed GK, Elbeh K, Khalifa H, Samaan MR (2021) Impact of duration of untreated illness in bipolar I disorder (manic episodes) on clinical outcome, socioecnomic burden in Egyptian population. Psychiatry Res 296:113659. https://doi.org/10.1016/j.psychres.2020.113659
Hebbrecht K, Skorobogatov K, Giltay EJ, Coppens V, De Picker L, Morrens M (2021) Tryptophan catabolites in bipolar disorder: a meta-analysis. Front Immunol 12:667179. https://doi.org/10.3389/fimmu.2021.667179
Dai W, Liu J, Qiu Y, Teng Z, Li S, Yuan H et al (2022) Gut microbial dysbiosis and cognitive impairment in bipolar disorder: current evidence. Front Pharmacol 13:893567. https://doi.org/10.3389/fphar.2022.893567
Clavel T, Lepage P, Charrier C (2014) The Family Coriobacteriaceae. In: Rosenberg E, DeLong EF, Lory S, Stackebrandt E, Thompson F (eds) The prokaryotes: Actinobacteria. Springer Berlin Heidelberg, Berlin, Heidelberg, pp 201–238
Million M, Angelakis E, Maraninchi M, Henry M, Giorgi R, Valero R et al (2013) Correlation between body mass index and gut concentrations of Lactobacillus reuteri, Bifidobacterium animalis, Methanobrevibacter smithii and Escherichia coli. Int J Obes 37(11):1460–1466. https://doi.org/10.1038/ijo.2013.20
Painold A, Mörkl S, Kashofer K, Halwachs B, Dalkner N, Bengesser S et al (2019) A step ahead: exploring the gut microbiota in inpatients with bipolar disorder during a depressive episode. Bipolar Disord 21(1):40–49. https://doi.org/10.1111/bdi.12682
McIntyre RS, Subramaniapillai M, Shekotikhina M, Carmona NE, Lee Y, Mansur RB et al (2021) Characterizing the gut microbiota in adults with bipolar disorder: a pilot study. Nutr Neurosci 24(3):173–180. https://doi.org/10.1080/1028415X.2019.1612555
Hussein EAM, Khalifa H, Ramadan GK, Hassaan SH, Shaaban I, Farrag HMM (2020) Seroprevalence of Toxoplasma gondii among patients with schizophrenia and bipolar disorder in Upper Egypt: a comparative study with a control group. Ann Parasitol 66(2):183–192. https://doi.org/10.17420/ap6602.253
Ohland CL, Kish L, Bell H, Thiesen A, Hotte N, Pankiv E et al (2013) Effects of Lactobacillus helveticus on murine behavior are dependent on diet and genotype and correlate with alterations in the gut microbiome. Psychoneuroendocrinology. 38(9):1738–1747. https://doi.org/10.1016/j.psyneuen.2013.02.008
Davari S, Talaei SA, Alaei H, Salami M (2013) Probiotics treatment improves diabetes-induced impairment of synaptic activity and cognitive function: behavioral and electrophysiological proofs for microbiome–gut–brain axis. Neuroscience 240:287–296. https://doi.org/10.1016/j.neuroscience.2013.02.055
Li W, Dowd SE, Scurlock B, Acosta-Martinez V, Lyte M (2009) Memory and learning behavior in mice is temporally associated with diet-induced alterations in gut bacteria. Physiol Behav 96(4):557–567. https://doi.org/10.1016/j.physbeh.2008.12.004
Desbonnet L, Clarke G, Traplin A, O’Sullivan O, Crispie F, Moloney RD et al (2015) Gut microbiota depletion from early adolescence in mice: implications for brain and behaviour. Brain Behav Immun 48:165–173. https://doi.org/10.1016/j.bbi.2015.04.004
Schmidt K, Cowen PJ, Harmer CJ, Tzortzis G, Errington S, Burnet PWJ (2015) Prebiotic intake reduces the waking cortisol response and alters emotional bias in healthy volunteers. Psychopharmacology 232(10):1793–1801. https://doi.org/10.1007/s00213-014-3810-0
Tillisch K, Labus J, Kilpatrick L, Jiang Z, Stains J, Ebrat B et al (2013) Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology 144:1394–1401
Khedr EM, Omeran N, Karam-Allah Ramadan H, Ahmed GK, Abdelwarith AM (2022) Alteration of gut microbiota in Alzheimer’s disease and their relation to the cognitive impairment. J Alzheimers Dis 88:1103–1114. https://doi.org/10.3233/JAD-220176
Prenderville JA, Kennedy PJ, Dinan TG, Cryan JF (2015) Adding fuel to the fire: the impact of stress on the ageing brain. Trends Neurosci 38(1):13–25. https://doi.org/10.1016/j.tins.2014.11.001
Chung Y-C, Jin H-M, Cui Y, Kim DS, Jung JM, Park J-I et al (2014) Fermented milk of Lactobacillus helveticus IDCC3801 improves cognitive functioning during cognitive fatigue tests in healthy older adults. J Funct Foods 10:465–474. https://doi.org/10.1016/j.jff.2014.07.007
Khedr EM, Ghanima NE, Elbeh KA, Gk A, El-Fawal B (2023) Cognitive impairment among an Egyptian sample of patients with schizophrenia and bipolar disorders: a comparative study. Middle East Current. Psychiatry. 30(1):70. https://doi.org/10.1186/s43045-023-00344-y
Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F et al (2013) The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry 18(6):666–673. https://doi.org/10.1038/mp.2012.77
Desbonnet L, Clarke G, Shanahan F, Dinan TG, Cryan JF (2014) Microbiota is essential for social development in the mouse. Mol Psychiatry 19(2):146–148. https://doi.org/10.1038/mp.2013.65
Khedr EM, Ramadan ES, Osman MN, Ahmed GK (2023) Risk factors-related first episode postpartum psychosis among Egyptian women: the role of psychosocial and the biological factors. Egypt J Neurol Psychiatr Neurosurg 59(1):51. https://doi.org/10.1186/s41983-023-00653-3
Ahmed GK, Elbeh K, Shams RM, Malek MAA, Ibrahim AK (2021) Prevalence and predictors of postpartum depression in Upper Egypt: a multicenter primary health care study. J Affect Disord 290:211–218. https://doi.org/10.1016/j.jad.2021.04.046
Naseribafrouei A, Hestad K, Avershina E, Sekelja M, Linløkken A, Wilson R et al (2014) Correlation between the human fecal microbiota and depression. Neurogastroenterol Motil 26(8):1155–1162. https://doi.org/10.1111/nmo.12378
Osman DM, Ahmed GK, Farghal MM, Ibrahim AK (2022) Prevalence and predictors of depressive symptoms among married Egyptian women: a multicenter primary healthcare study. BMC Psychiatry 22(1):602. https://doi.org/10.1186/s12888-022-04239-w
Zhang B, Chen T, Cao M, Yuan C, Reiter RJ, Zhao Z et al (2022) Gut microbiota dysbiosis induced by decreasing endogenous melatonin mediates the pathogenesis of Alzheimer’s disease and obesity. Front Immunol 13:900132. https://doi.org/10.3389/fimmu.2022.900132
Skonieczna-Żydecka K, Grochans E, Maciejewska D, Szkup M, Schneider-Matyka D, Jurczak A et al (2018) Faecal short chain fatty acids profile is changed in polish depressive women. Nutrients. 10(12):1939
Gan L, Bo T, Liu W, Wang D (2022) The gut microbiota may affect personality in Mongolian gerbils. Microorganisms. 10(5):1054
Góralczyk-Bińkowska A, Szmajda-Krygier D, Kozłowska E (2022) The microbiota-gut-brain axis in psychiatric disorders. Int J Mol Sci 23(19):11245. https://doi.org/10.3390/ijms231911245
Szeligowski T, Yun AL, Lennox BR, Burnet PWJ (2020) The gut microbiome and schizophrenia: the current state of the field and clinical applications. Front Psychiatry 11:156. https://doi.org/10.3389/fpsyt.2020.00156
Olfson M, Gerhard T, Huang C, Crystal S, Stroup TS (2015) Premature mortality among adults with schizophrenia in the United States. JAMA Psychiatry 72(12):1172–1181. https://doi.org/10.1001/jamapsychiatry.2015.1737
Owen MJ, Sawa A, Mortensen PB (2016) Schizophrenia. Lancet. 388(10039):86–97. https://doi.org/10.1016/S0140-6736(15)01121-6
Hassaan SH, Darwish AM, Khalifa H, Ramadan HKA, Hassany SM, Ahmed GK et al (2019) Assessment of cognitive functions and psychiatric symptoms in hepatitis C patients receiving pegylated interferon alpha and ribavirin: a prospective cohort study. Int J Psychiatry Med 54(6):424–440. https://doi.org/10.1177/0091217419858277
Mahran ZG, Khalifa H, Makhlouf NA, Mostafa DK, Aboalam HS, Moustafa EF et al (2022) Effect of gender difference on psychiatric outcomes for hepatitis C virus patients receiving direct-acting antivirals in Egyptian population: a cohort study. Egypt J Neurol Psychiatr Neurosurg 58(1):155. https://doi.org/10.1186/s41983-022-00585-4
Shehata GA, Ahmed GK, Hassan EA, Rehim ASE-DA, Mahmoud SZ, Masoud NA et al (2022) Impact of direct-acting antivirals on neuropsychiatric and neurocognitive dysfunction in chronic hepatitis C patients. Egypt J Neurol Psychiatr Neurosurg 58(1):143. https://doi.org/10.1186/s41983-022-00568-5
Wysokiński A, Kozłowska E, Szczepocka E, Łucka A, Agier J, Brzezińska-Błaszczyk E et al (2021) Expression of dopamine D(1-4) and serotonin 5-HT(1A-3A) receptors in blood mononuclear cells in schizophrenia. Front Psychiatry 12:645081. https://doi.org/10.3389/fpsyt.2021.645081
Patrono E, Svoboda J, Stuchlík A (2021) Schizophrenia, the gut microbiota, and new opportunities from optogenetic manipulations of the gut-brain axis. Behav Brain Funct 17(1):7. https://doi.org/10.1186/s12993-021-00180-2
Stefansson H, Steinthorsdottir V, Thorgeirsson TE, Gulcher JR, Stefansson K (2004) Neuregulin 1 and schizophrenia. Ann Med 36(1):62–71. https://doi.org/10.1080/07853890310017585
Kozłowska E, Brzezińska-Błaszczyk E, Agier J, Wysokiński A, Żelechowska P (2021) Alarmins (IL-33, sST2, HMGB1, and S100B) as potential biomarkers for schizophrenia. J Psychiatr Res 138:380–387. https://doi.org/10.1016/j.jpsychires.2021.04.019
Priol AC, Denis L, Boulanger G, Thépaut M, Geoffray MM, Tordjman S (2021) Detection of morphological abnormalities in schizophrenia: an important step to identify associated genetic disorders or etiologic subtypes. Int J Mol Sci 22(17):9464. https://doi.org/10.3390/ijms22179464
Fatemi SH, Folsom TD (2009) The neurodevelopmental hypothesis of schizophrenia, revisited. Schizophr Bull 35(3):528–548. https://doi.org/10.1093/schbul/sbn187
Larsson MK, Schwieler L, Goiny M, Erhardt S, Engberg G (2015) Chronic antipsychotic treatment in the rat - effects on brain interleukin-8 and kynurenic acid. Int J Tryptophan Res 8:49–52. https://doi.org/10.4137/IJTR.S25915
Holtze M, Mickiené A, Atlas A, Lindquist L, Schwieler L (2012) Elevated cerebrospinal fluid kynurenic acid levels in patients with tick-borne encephalitis. J Intern Med 272(4):394–401. https://doi.org/10.1111/j.1365-2796.2012.02539.x
Condray R, Dougherty GG Jr, Keshavan MS, Reddy RD, Haas GL, Montrose DM et al (2011) 3-Hydroxykynurenine and clinical symptoms in first-episode neuroleptic-naive patients with schizophrenia. Int J Neuropsychopharmacol 14(6):756–767. https://doi.org/10.1017/S1461145710001689
Kelsey CM, Prescott S, McCulloch JA, Trinchieri G, Valladares TL, Dreisbach C et al (2021) Gut microbiota composition is associated with newborn functional brain connectivity and behavioral temperament. Brain Behav Immun 91:472–486. https://doi.org/10.1016/j.bbi.2020.11.003
Silva V, Palacios-Muñoz A, Okray Z, Adair KL, Waddell S, Douglas AE et al (2021) The impact of the gut microbiome on memory and sleep in Drosophila. J Exp Biol 224(Pt 3):jeb233619. https://doi.org/10.1242/jeb.233619
Maki KA, Burke LA, Calik MW, Watanabe-Chailland M, Sweeney D, Romick-Rosendale LE et al (2020) Sleep fragmentation increases blood pressure and is associated with alterations in the gut microbiome and fecal metabolome in rats. Physiol Genomics 52(7):280–292. https://doi.org/10.1152/physiolgenomics.00039.2020
Ko CY, Liu QQ, Su HZ, Zhang HP, Fan JM, Yang JH et al (2019) Gut microbiota in obstructive sleep apnea-hypopnea syndrome: disease-related dysbiosis and metabolic comorbidities. Clin Sci (Lond) 133(7):905–917. https://doi.org/10.1042/CS20180891
Badran M, Khalyfa A, Ericsson A, Gozal D (2020) Fecal microbiota transplantation from mice exposed to chronic intermittent hypoxia elicits sleep disturbances in naïve mice. Exp Neurol 334:113439. https://doi.org/10.1016/j.expneurol.2020.113439
Feng Y, Fu S, Li C, Ma X, Wu Y, Chen F et al (2021) Interaction of gut microbiota and brain function in patients with chronic insomnia: a regional homogeneity study. Front Neurosci 15:804843. https://doi.org/10.3389/fnins.2021.804843
Strandwitz P, Kim KH, Terekhova D, Liu JK, Sharma A, Levering J et al (2019) GABA-modulating bacteria of the human gut microbiota. Nat Microbiol 4(3):396–403. https://doi.org/10.1038/s41564-018-0307-3
Potter GD, Skene DJ, Arendt J, Cade JE, Grant PJ, Hardie LJ (2016) Circadian rhythm and sleep disruption: causes, metabolic consequences, and countermeasures. Endocr Rev 37(6):584–608. https://doi.org/10.1210/er.2016-1083
Pandi-Perumal SR, Trakht I, Spence DW, Srinivasan V, Dagan Y, Cardinali DP (2008) The roles of melatonin and light in the pathophysiology and treatment of circadian rhythm sleep disorders. Nat Clin Pract Neurol 4(8):436–447. https://doi.org/10.1038/ncpneuro0847
Hua X, Zhu J, Yang T, Guo M, Li Q, Chen J et al (2020) The gut microbiota and associated metabolites are altered in sleep disorder of children with autism spectrum disorders. Front Psychiatry 11:855. https://doi.org/10.3389/fpsyt.2020.00855
Heintz-Buschart A, Pandey U, Wicke T, Sixel-Döring F, Janzen A, Sittig-Wiegand E et al (2018) The nasal and gut microbiome in Parkinson’s disease and idiopathic rapid eye movement sleep behavior disorder. Mov Disord 33(1):88–98. https://doi.org/10.1002/mds.27105
Sugden D, Davidson K, Hough KA, Teh MT (2004) Melatonin, melatonin receptors and melanophores: a moving story. Pigment Cell Res 17(5):454–460. https://doi.org/10.1111/j.1600-0749.2004.00185.x
Meckel KR, Kiraly DD (2019) A potential role for the gut microbiome in substance use disorders. Psychopharmacology 236(5):1513–1530. https://doi.org/10.1007/s00213-019-05232-0
Kirpich IA, Solovieva NV, Leikhter SN, Shidakova NA, Lebedeva OV, Sidorov PI et al (2008) Probiotics restore bowel flora and improve liver enzymes in human alcohol-induced liver injury: a pilot study. Alcohol. 42(8):675–682. https://doi.org/10.1016/j.alcohol.2008.08.006
Bala S, Marcos M, Gattu A, Catalano D, Szabo G (2014) Acute binge drinking increases serum endotoxin and bacterial DNA levels in healthy individuals. PLoS One 9(5):e96864. https://doi.org/10.1371/journal.pone.0096864
Leclercq S, Matamoros S, Cani PD, Neyrinck AM, Jamar F, Stärkel P et al (2014) Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci U S A 111(42):E4485–E4493. https://doi.org/10.1073/pnas.1415174111
Qamar N, Castano D, Patt C, Chu T, Cottrell J, Chang SL (2019) Meta-analysis of alcohol induced gut dysbiosis and the resulting behavioral impact. Behav Brain Res 376:112196. https://doi.org/10.1016/j.bbr.2019.112196
Chivero ET, Ahmad R, Thangaraj A, Periyasamy P, Kumar B, Kroeger E et al (2019) Cocaine Induces inflammatory gut milieu by compromising the mucosal barrier integrity and altering the gut microbiota colonization. Sci Rep 9(1):12187. https://doi.org/10.1038/s41598-019-48428-2
Volpe GE, Ward H, Mwamburi M, Dinh D, Bhalchandra S, Wanke C et al (2014) Associations of cocaine use and HIV infection with the intestinal microbiota, microbial translocation, and inflammation. J Stud Alcohol Drugs 75(2):347–357. https://doi.org/10.15288/jsad.2014.75.347
Angoa-Pérez M, Zagorac B, Francescutti DM, Shaffer ZD, Theis KR, Kuhn DM (2023) Cocaine hydrochloride, cocaine methiodide and methylenedioxypyrovalerone (MDPV) cause distinct alterations in the structure and composition of the gut microbiota. Sci Rep 13(1):13754. https://doi.org/10.1038/s41598-023-40892-1
Gerace E, Baldi S, Salimova M, Di Gloria L, Curini L, Cimino V et al (2023) Oral and fecal microbiota perturbance in cocaine users: can rTMS-induced cocaine abstinence support eubiosis restoration? iScience. 26(5):106627. https://doi.org/10.1016/j.isci.2023.106627
Acharya C, Betrapally NS, Gillevet PM, Sterling RK, Akbarali H, White MB et al (2017) Chronic opioid use is associated with altered gut microbiota and predicts readmissions in patients with cirrhosis. Aliment Pharmacol Ther 45(2):319–331. https://doi.org/10.1111/apt.13858
Barengolts E, Green SJ, Eisenberg Y, Akbar A, Reddivari B, Layden BT et al (2018) Gut microbiota varies by opioid use, circulating leptin and oxytocin in African American men with diabetes and high burden of chronic disease. PLoS One 13(3):e0194171. https://doi.org/10.1371/journal.pone.0194171
Zhernakova A, Kurilshikov A, Bonder MJ, Tigchelaar EF, Schirmer M, Vatanen T et al (2016) Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science. 352(6285):565–569. https://doi.org/10.1126/science.aad3369
Xu Y, Xie Z, Wang H, Shen Z, Guo Y, Gao Y et al (2017) Bacterial diversity of intestinal microbiota in patients with substance use disorders revealed by 16S rRNA gene deep sequencing. Sci Rep 7(1):3628. https://doi.org/10.1038/s41598-017-03706-9
Lin H, Williams KA, Katsovich L, Findley DB, Grantz H, Lombroso PJ et al (2010) Streptococcal upper respiratory tract infections and psychosocial stress predict future tic and obsessive-compulsive symptom severity in children and adolescents with Tourette syndrome and obsessive-compulsive disorder. Biol Psychiatry 67(7):684–691. https://doi.org/10.1016/j.biopsych.2009.08.020
Kantak PA, Bobrow DN, Nyby JG (2014) Obsessive-compulsive-like behaviors in house mice are attenuated by a probiotic (Lactobacillus rhamnosus GG). Behav Pharmacol 25(1):71–79. https://doi.org/10.1097/FBP.0000000000000013
Lyte M, Varcoe JJ, Bailey MT (1998) Anxiogenic effect of subclinical bacterial infection in mice in the absence of overt immune activation. Physiol Behav 65(1):63–68. https://doi.org/10.1016/s0031-9384(98)00145-0
Bruch JD (2016) Intestinal infection associated with future onset of an anxiety disorder: results of a nationally representative study. Brain Behav Immun 57:222–226. https://doi.org/10.1016/j.bbi.2016.05.014
David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE et al (2014) Diet rapidly and reproducibly alters the human gut microbiome. Nature. 505(7484):559–563. https://doi.org/10.1038/nature12820
Akbari E, Asemi Z, Daneshvar Kakhaki R, Bahmani F, Kouchaki E, Tamtaji OR et al (2016) Effect of probiotic supplementation on cognitive function and metabolic status in Alzheimer’s disease: a randomized, double-blind and controlled trial. Front Aging Neurosci 8:256. https://doi.org/10.3389/fnagi.2016.00256
Tarr AJ, Galley JD, Fisher SE, Chichlowski M, Berg BM, Bailey MT (2015) The prebiotics 3'sialyllactose and 6'sialyllactose diminish stressor-induced anxiety-like behavior and colonic microbiota alterations: evidence for effects on the gut-brain axis. Brain Behav Immun 50:166–177. https://doi.org/10.1016/j.bbi.2015.06.025
Schnorr SL, Bachner HA (2016) Integrative therapies in anxiety treatment with special emphasis on the gut microbiome. Yale J Biol Med 89(3):397–422
Magalhães-Guedes KT (2022) Psychobiotic therapy: method to reinforce the immune system. Clin Psychopharmacol Neurosci 20(1):17–25. https://doi.org/10.9758/cpn.2022.20.1.17
Velloso LA, Folli F, Saad MJ (2015) TLR4 at the crossroads of nutrients, gut microbiota, and metabolic inflammation. Endocr Rev 36(3):245–271. https://doi.org/10.1210/er.2014-1100
Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T et al (2013) Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 155(7):1451–1463. https://doi.org/10.1016/j.cell.2013.11.024
Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG et al (2011) Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A 108(38):16050–16055. https://doi.org/10.1073/pnas.1102999108
Benton D, Williams C, Brown A (2007) Impact of consuming a milk drink containing a probiotic on mood and cognition. Eur J Clin Nutr 61(3):355–361. https://doi.org/10.1038/sj.ejcn.1602546
Kato-Kataoka A, Nishida K, Takada M, Kawai M, Kikuchi-Hayakawa H, Suda K et al (2016) Fermented milk containing Lactobacillus casei strain Shirota preserves the diversity of the gut microbiota and relieves abdominal dysfunction in healthy medical students exposed to academic stress. Appl Environ Microbiol 82(12):3649–3658. https://doi.org/10.1128/AEM.04134-15
Bambling M, Edwards SC, Hall S, Vitetta L (2017) A combination of probiotics and magnesium orotate attenuate depression in a small SSRI resistant cohort: an intestinal anti-inflammatory response is suggested. Inflammopharmacology. 25(2):271–274. https://doi.org/10.1007/s10787-017-0311-x
Zhu R, Fang Y, Li H, Liu Y, Wei J, Zhang S et al (2023) Psychobiotic Lactobacillus plantarum JYLP-326 relieves anxiety, depression, and insomnia symptoms in test anxious college via modulating the gut microbiota and its metabolism. Front Immunol 14:1158137. https://doi.org/10.3389/fimmu.2023.1158137
Ng QX, Loke W, Venkatanarayanan N, Lim DY, Soh AYS, Yeo WS (2019) A systematic review of the role of prebiotics and probiotics in autism spectrum disorders. Medicina (Kaunas) 55(5):129. https://doi.org/10.3390/medicina55050129
Haghighat N, Rajabi S, Mohammadshahi M (2021) Effect of synbiotic and probiotic supplementation on serum brain-derived neurotrophic factor level, depression and anxiety symptoms in hemodialysis patients: a randomized, double-blinded, clinical trial. Nutr Neurosci 24(6):490–499. https://doi.org/10.1080/1028415X.2019.1646975
Haghighat N, Mohammadshahi M, Shayanpour S, Haghighizadeh MH, Rahmdel S, Rajaei M (2021) The effect of synbiotic and probiotic supplementation on mental health parameters in patients undergoing hemodialysis: a double-blind, randomized, placebo-controlled trial. Indian J Nephrol 31(2):149–156. https://doi.org/10.4103/ijn.IJN_341_19
Luhavaya H, Sigrist R, Chekan JR, McKinnie SMK, Moore BS (2019) Biosynthesis of l-4-chlorokynurenine, an antidepressant prodrug and a non-proteinogenic amino acid found in lipopeptide antibiotics. Angew Chem Int Ed Eng 58(25):8394–8399. https://doi.org/10.1002/anie.201901571
Yin L, Yuvienco C, Montclare JK (2017) Protein based therapeutic delivery agents: contemporary developments and challenges. Biomaterials. 134:91–116. https://doi.org/10.1016/j.biomaterials.2017.04.036
Zhu F, Tu H, Chen T (2022) The microbiota-gut-brain axis in depression: the potential pathophysiological mechanisms and microbiota combined antidepression effect. Nutrients 14(10):2081. https://doi.org/10.3390/nu14102081
Bakken JS, Borody T, Brandt LJ, Brill JV, Demarco DC, Franzos MA et al (2011) Treating Clostridium difficile infection with fecal microbiota transplantation. Clin Gastroenterol Hepatol 9(12):1044–1049. https://doi.org/10.1016/j.cgh.2011.08.014
Fond GB, Lagier JC, Honore S, Lancon C, Korchia T, Sunhary De Verville PL et al (2020) Microbiota-orientated treatments for major depression and schizophrenia. Nutrients 12(4):1024. https://doi.org/10.3390/nu12041024
Rao J, Qiao Y, Xie R, Lin L, Jiang J, Wang C et al (2021) Fecal microbiota transplantation ameliorates stress-induced depression-like behaviors associated with the inhibition of glial and NLRP3 inflammasome in rat brain. J Psychiatr Res 137:147–157. https://doi.org/10.1016/j.jpsychires.2021.02.057
Yan ZX, Gao XJ, Li T, Wei B, Wang PP, Yang Y et al (2018) Fecal microbiota transplantation in experimental ulcerative colitis reveals associated gut microbial and host metabolic reprogramming. Appl Environ Microbiol 84(14):e00434. https://doi.org/10.1128/AEM.00434-18
Geng S, Cheng S, Li Y, Wen Z, Ma X, Jiang X et al (2018) Faecal microbiota transplantation reduces susceptibility to epithelial injury and modulates tryptophan metabolism of the microbial community in a piglet model. J Crohns Colitis 12(11):1359–1374. https://doi.org/10.1093/ecco-jcc/jjy103
Cai T, Zheng SP, Shi X, Yuan LZ, Hu H, Zhou B et al (2022) Therapeutic effect of fecal microbiota transplantation on chronic unpredictable mild stress-induced depression. Front Cell Infect Microbiol 12:900652. https://doi.org/10.3389/fcimb.2022.900652
Doll JPK, Vázquez-Castellanos JF, Schaub AC, Schweinfurth N, Kettelhack C, Schneider E et al (2022) Fecal microbiota transplantation (FMT) as an adjunctive therapy for depression-case report. Front Psychiatry 13:815422. https://doi.org/10.3389/fpsyt.2022.815422
Gracie DJ, Hamlin PJ, Ford AC (2019) The influence of the brain-gut axis in inflammatory bowel disease and possible implications for treatment. Lancet Gastroenterol Hepatol 4(8):632–642. https://doi.org/10.1016/S2468-1253(19)30089-5
Evrensel A, Ceylan ME (2016) Fecal microbiota transplantation and its usage in neuropsychiatric disorders. Clin Psychopharmacol Neurosci 14(3):231–237. https://doi.org/10.9758/cpn.2016.14.3.231
De Leon LM, Watson JB, Kelly CR (2013) Transient flare of ulcerative colitis after fecal microbiota transplantation for recurrent Clostridium difficile infection. Clin Gastroenterol Hepatol 11(8):1036–1038. https://doi.org/10.1016/j.cgh.2013.04.045
Fischer M, Kao D, Kelly C, Kuchipudi A, Jafri SM, Blumenkehl M et al (2016) Fecal microbiota transplantation is safe and efficacious for recurrent or refractory clostridium difficile infection in patients with inflammatory bowel disease. Inflamm Bowel Dis 22(10):2402–2409. https://doi.org/10.1097/MIB.0000000000000908
Acknowledgements
None
Funding
No sources of funding were required.
Author information
Authors and Affiliations
Contributions
GA conceived the original idea of this work and took the lead in writing the manuscript. HR and NE contributed equally in the literature review and writing of the manuscript. KE was responsible for revision. The final version of the manuscript was reviewed and approved by all co-authors.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ahmed, G.K., Ramadan, H.KA., Elbeh, K. et al. Bridging the gap: associations between gut microbiota and psychiatric disorders. Middle East Curr Psychiatry 31, 2 (2024). https://doi.org/10.1186/s43045-024-00395-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43045-024-00395-9