Abstract
Background
Cardiovascular diseases are one of the prime causes of mortality globally. Therefore, concerted efforts are made to prevent or manage disruptions from normal functioning of the cardiovascular system. Disruption in lipid metabolism is a major contributor to cardiovascular dysfunction. This review examines how lecithin impacts lipid metabolism and cardiovascular health. It emphasizes lecithin's ability to reduce excess low-density lipoproteins (LDL) while specifically promoting the synthesis of high-density lipoprotein (HDL) particles, thus contributing to clearer understanding of its role in cardiovascular well-being. Emphasizing the importance of lecithin cholesterol acyltransferase (LCAT) in the reverse cholesterol transport (RCT) process, the article delves into its contribution in removing surplus cholesterol from cells. This review aims to clarify existing literature on lipid metabolism, providing insights for targeted strategies in the prevention and management of atherosclerotic cardiovascular disease (ASCVD). This review summarizes the potential of lecithin in cardiovascular health and the role of LCAT in cholesterol metabolism modulation, based on articles from 2000 to 2023 sourced from databases like MEDLINE, PubMed and the Scientific Electronic Library Online.
Main body
While studies suggest a positive correlation between increased LCAT activities, reduced LDL particle size and elevated serum levels of triglyceride-rich lipoprotein (TRL) markers in individuals at risk of ASCVD, the review acknowledges existing controversies. The precise nature of LCAT's potential adverse effects remains uncertain, with varying reports in the literature. Notably, gastrointestinal symptoms such as diarrhea and nausea have been sporadically documented.
Conclusions
The review calls for a comprehensive investigation into the complexities of LCAT's impact on cardiovascular health, recognizing the need for a nuanced understanding of its potential drawbacks. Despite indications of potential benefits, conflicting findings warrant further research to clarify LCAT's role in atherosclerosis.
Similar content being viewed by others
Background
Lecithin, a vital component derived from both plant and animal sources [1], is a natural amalgamation of diglycerides comprising palmitic, stearic, with oleic acids joined with the choline ester of phosphoric acid [2]. It is a compound found naturally in various tissues of animals and plants, including egg yolks, soybeans and peanuts. Simple oil from soybeans contains 2 to 3 percent lecithin, and significant quantities can also be found in whey and corn oil [3]. It is a complex mixture of phospholipids, triglycerides and glycolipids. Phospholipids are the primary component of lecithin and are responsible for its unique properties. Notably, it is ubiquitous on the outermost layer of plasma membranes [4, 5] and has been identified as a substantial phospholipid in amniotic fluid and lung surfactant [6, 7].
Lecithin offers potential health benefits, including cognitive enhancement through its choline content [8,9,10], liver protection from toxins [9, 11, 12] and potential cardiovascular improvements by lowering cholesterol levels [13,14,15,16]. It also has widespread applications in the food industry, where it acts as an emulsifier, stabilizer, wetting agent and antioxidant, commonly found in various products [3, 17]. Additionally, lecithin plays a significant role in the industrial sector; it is utilized in paints, cosmetics, pharmaceuticals and textiles for its properties as an emulsifier, wetting agent, dispersant and lubricant [18,19,20].
Cardiovascular diseases (CVD) encompass a variety of disarrangements acting on the blood vessels and the heart. Prominent types of CVD consist of atherosclerosis, coronary artery disease, arrhythmias and heart failure [21]. These conditions contribute to the global burden of CVD-related morbidity and mortality. CVD risk factors are multifaceted and encompass lifestyle elements such as unhealthy diet, uncontrolled intake of alcohol, stress, smoking and insufficient physical activity [22]. Genetic predisposition, age and pre-existing medical conditions like hypertension and diabetes also influence CVD susceptibility. Fact from WHO states that CVD is the dominant root of death globally with approximately 17.9 million mortalities adjudged to CVD in 2016 [23]. Understanding these diverse CVD types and their risk factors is important for growing worthwhile prevention and treatment strategies.
Atherosclerosis stands as one of the most life-threatening cardiovascular diseases, affecting individuals as early as 20 to 29 years old [24]. It is characterized by the accumulation of plaques in large and medium arteries, primarily composed of cholesterol, fibrin and calcium [25]. Key lipid-related cardiovascular threats include increased levels of low-density lipoprotein (LDL), elevated plasma triglycerides and reduced high-density lipoprotein (HDL) [26]. Remarkably, lecithin plays a pivotal role by diminishing excess LDL, the "bad cholesterol," and promoting the synthesis of HDL, the "beneficial cholesterol."
Literature reveals lecithin cholesterol acyltransferase's (LCAT) pivotal role in anti-atherogenic reverse cholesterol transport (RCT) [27]. LCAT translates unbound cholesterol in emerging HDL molecules to cholesteryl esters, enhancing HDL's cholesterol transport capacity [28]. LCAT elevates HDL cholesterol and triglyceride-rich lipoproteins levels and reduces LDL particle size [29]. These conflicting roles call for further investigations into LCAT's influence on HDL metabolism, LDL size and their implications for optimizing cardiovascular health [28].
Despite the promising role of LCAT activity in decreasing LDL particle size, further investigations are warranted to clarify the precise mechanisms by which increased LCAT activity results in a decrease in LDL particle size. This will advance our understanding of the association between lecithin and atherosclerosis.
This review unravels the effects of lecithin in cardiovascular health and highlights the role of LCAT in modulating cholesterol metabolism. Given the persistent global threat posed by cardiovascular diseases, particularly atherosclerosis, comprehending the mechanisms connecting lecithin, LCAT, and cardiovascular health is crucial. This review provides insights for future research and clinical interventions aimed at mitigating the risk factors associated with atherosclerosis and related cardiovascular conditions.
Search strategy
The scheme of this narrative review is to recount the potential of lecithin in cardiovascular health and highlight the role of LCAT in modulating cholesterol metabolism. This review was executed by searching for articles in MEDLINE, PubMed and the Scientific Electronic Library Online databases using the Medical Subject terms “lecithin,” “lecithin” and “cardiovascular health,” “LCAT” and “modulation of cholesterol metabolism.” Articles published during the period 2000–2023 in English were put together.
For article selection, clear criteria were established to ensure that only relevant studies were included in the review. Articles were chosen if they directly addressed the relationship between lecithin and cardiovascular health or discussed LCAT's role in modulating cholesterol metabolism. The focus was on articles published between 2000 and 2023 to capture recent findings, and consideration was given exclusively to studies published in English due to language limitations. Both experimental studies and clinical trials were eligible for inclusion to provide a comprehensive overview of the topic. Articles that did not meet these criteria or were duplicates, conference abstracts, reviews without original data or focused on unrelated topics were excluded. Screening of articles was conducted based on titles, abstracts and full texts, when necessary, with eligibility determined by two independent reviewers. Any disagreements in article selection were resolved through discussion or consultation with a third reviewer to reach a consensus. This systematic approach ensured that only relevant articles were included, enhancing the transparency and reproducibility of the review.
Lecithin
Lecithin, identified as egg yolk lecithin, soybean lecithin, soybean phospholipid [2], lecithol, phosphatidylcholine [30], choline phosphoglyceride [31] and phospholutein [32, 33], has a phosphatide or phospholipid component which is found in naturally occurring substances from both plant and animal sources [1]. It occurs naturally as a blend of diglycerides composed of stearic, oleic and palmitic acids, associated with the choline ester of phosphoric acid [2]. In nature, lecithin can have phosphoric acid linked to glycerol in either the alpha or beta position [34].
As a phosphatide present in virtually all living organisms, lecithin constitutes a substantial component of both brain and nervous tissue. It accounts for more than 50 percent of the phospholipids in most cell membranes of mammals [35]. Lecithin is also found on the surface of plasma membranes, forming the outermost layer [36]. The chemical structure of lecithin is displayed in Fig. 1.
Reports have indicated that lecithin is a significant phospholipid in amniotic fluid and lung surfactant [37,38,39,40,41,42,43,44]. Lecithin constitutes about 2–3% of the total weight of crude soybean oil and has also been found in substantial amounts in corn and wheat oils. Notably, lecithin makes up about 10% of the components of egg yolk [44,45,46].
Lecithin's major property of regulating cholesterol levels is a pivotal aspect of its role in promoting cardiovascular health [47,48,49]. It achieves this regulation through its capacity to reduce excess low-density lipoprotein (LDL), often dubbed as "bad cholesterol." High levels of LDL are associated with an increased risk of atherosclerosis and heart disease [50, 51].
Simultaneously, lecithin facilitates the synthesis of high-density lipoprotein (HDL), recognized as the "beneficial cholesterol" [52]. HDL contributes substantially to the removal of excess cholesterol from the blood circulation, transporting it to the liver for excretion, thus contributing to a healthier cardiovascular profile [53]. An increased presence of HDL is linked to a reduced risk of cardiovascular diseases [53].
Studies, such as the one conducted by Brunet and associates in 2003, have shown that diets rich in lecithin stimulate the secretion of bile acids by enhancing the formation of mixed micelles, which facilitate the solubilization and excretion of cholesterol. This mechanism involves elevated levels of phospholipids and cholesterol compared to diets lacking lecithin [47]. This, in turn, underscores lecithin's significance in maintaining a balanced cholesterol profile and supporting heart health.
Lecithin, a complex mixture of phospholipids, is subject to various chemical reactions, and its breakdown through hydrolysis is a fundamental one [54]. When subjected to complete hydrolysis, lecithin molecules disintegrate into their basic building blocks. This process yields two fatty acid molecules, often including palmitic, oleic and stearic acids [54], a molecule of phosphoric acid, glycerol, and a basic nitrogenous compound, choline [53]. These constituents are vital in understanding the composition and functional properties of lecithin.
Another intriguing chemical behavior of lecithin is its propensity to spontaneously bind with oxygen when exposed to atmospheric air [55]. This phenomenon is attributed to the double bonds present in the unsaturated fatty acids which are found within the triglyceride and phosphoglyceride components of lecithin. These double bonds are vulnerable to oxidation, which can lead to the formation of oxidative products such as epoxides and hydroperoxides [55]. This oxidative reactivity of lecithin has implications for its use in various applications, especially in the food industry and pharmaceuticals, where maintaining product stability and quality is of paramount importance [55]. Lecithin's oxidative reactivity finds various applications in the food sector, functioning as an emulsifier in baked goods and confectionery, stabilizing salad dressings and sauces, and aiding in the mixing of fats and oils in margarine. In therapeutics, it enhances drug solubility, bioavailability and stability in lipid-based drug delivery systems and supplements, while also regulating the release of medications in sustained-release formulations [55]
Global incidence of cardiovascular disease
Cardiovascular diseases (CVDs) present a formidable global health challenge, annually claiming millions of lives across the globe [56]. This overarching term comprises of variety of conditions, such as coronary heart disease, stroke and peripheral artery disease, where the intricate interplay of cholesterol and its counterparts often stands at the heart of the matter [57].
Cholesterol, indispensable for cell membranes and various biological functions [58], becomes problematic when low-density lipoprotein (LDL) cholesterol rises to excessive levels, significantly contributing to the development of cardiovascular diseases (CVDs) [59]. Its vital role in cellular structure ensures membrane integrity and fluidity, facilitating essential cell functions and communication. Moreover, as a precursor for steroid hormones, bile acids and vitamin D synthesis, cholesterol plays a crucial role in hormonal regulation, digestion and overall metabolic processes [58. However, dysregulated LDL cholesterol levels can lead to the accumulation of plaque in arteries, contributing to the pathogenesis of CVDs [59]. The accumulation of LDL cholesterol in arteries results in the formation of plaques, which narrow the lumen of blood vessels and impede the flow of blood, thereby setting the stage for heart attacks and strokes [57, 60].
While LDL cholesterol takes the center stage in the narrative of CVD development, its detrimental impact is often heightened by accomplices such as high blood pressure, diabetes and smoking [57]. Furthermore, elevated levels of triglycerides, another type of blood fat, can compound the risk of CVDs, particularly when coupled with high LDL cholesterol [51]. This intricate web of interconnected factors underscores the multifaceted nature of CVD development, emphasizing the critical need for comprehensive approaches in addressing risk factors for effective prevention and management.
This global health challenge extends its reach to Africa, where CVDs contribute significantly to the continent's high mortality rate. Sub-Saharan Africa is home to 25 million people living with CVDs, and this prevalence is anticipated to rise by 25% by 2030 [21]. CVDs, claiming 13% of all deaths, stand as the leading cause of mortality in Africa, with a staggering 7.4 million people succumbing to premature deaths each year before the age of 70 [61].
Several factors contribute to this disproportionate burden in Africa. Inadequate access to healthcare services, poor preventative measures and late diagnosis and treatment options significantly contribute to the high mortality rate from CVDs [62,63,64]. Socioeconomic disparities, including poverty, unemployment and restricted access to good food and physical activity, contribute to the escalating prevalence of CVD risk factors in Africa [65,66,67]. Furthermore, the lack of public awareness about CVDs and their risk factors hampers early diagnosis and intervention, leading to complications and increased mortality [68, 69].
Cardiovascular diseases (CVDs) have risen as a pressing global health issue, and their impact on public health is profound. In 2008, CVDs accounted for nearly half of all reported deaths worldwide, highlighting their significant burden [64]. It is worth noting that this burden is particularly concentrated in low-to-middle-income countries [70], where healthcare resources and access to preventive measures are often limited.
A striking characteristic of CVDs is their association with an aging population, with more than 50 percent of CVD-related deaths occurring in individuals over the age of 70. This demographic shift places additional strain on healthcare systems, as older individuals often require specialized medical care and interventions. The African continent, with its vast and diverse population, is significantly impacted by the CVD epidemic. Sub-Saharan Africa alone reported approximately one million CVD-related deaths in 2013, comprising a substantial portion of global CVD fatalities and a noteworthy fraction of all deaths on the continent [71]. CVDs have become a major contributor to non-communicable disease-related mortality in Africa, reflecting a significant change in the region's disease landscape over the past few decades.
Moreover, there exists a notable disparity in CVD mortality between genders, with more than a 10 percent difference in death rates between females and males [71]. This variation underscores the need for gender-specific health strategies and interventions to address the unique risk factors and healthcare needs of women and men.
The evolving landscape of CVDs in Africa is shaped by epidemiological transitions and population dynamics, particularly in low-resource communities. Sub-Saharan Africa hosts a substantial proportion of the world's impoverished population, making it essential to develop strategies that are tailored to the region's specific socioeconomic and healthcare challenges [72]. Addressing the CVD burden in Africa necessitates a comprehensive and multi-faceted approach that takes into consideration the complex nature of factors contributing to this public health challenge.
Lipid-related cardiovascular risk factors
In recent years, heightened attention has been directed towards cardiovascular risk factors linked to lipids, particularly low-density lipoprotein (LDL) and high-density lipoprotein (HDL) [26]. These factors, including elevated plasma triglycerides, have been extensively researched for prevention and treatment strategies.
LDL, a central player in cardiovascular health, undergoes a complex process resulting in the formation of small, dense LDL particles. This intricate process involves the exchange of lipids between triglyceride-rich lipoproteins and LDL, a phenomenon influenced by genetic traits. Aged small, dense LDL particles, with diminished protection against free radical attack, linger longer in the bloodstream. Their increased susceptibility to oxidative modification contributes to the formation of atherosclerotic plaques, as evidenced by studies like the Quebec Heart Study, highlighting a robust connection between small and dense LDL cholesterol concentration and the risk of coronary heart disease [73, 74].
Conversely, high-density lipoprotein (HDL) plays a protective role in cardiovascular health. Reduced levels of HDL are related to an elevated risk of cardiovascular diseases. HDL's atheroprotective function is achieved through the pathway of reverse cholesterol transport (RCT), wherein cholesterol is moved from peripheral tissues to the liver for elimination. This process involves crucial components such as lecithin-cholesteryl ester acyl-transferase (LCAT) and apolipoprotein Apo A1 [26, 75].
Beyond RCT, HDL exhibits various atheroprotective properties, including preventing the development of reactive oxygen species, inhibiting LDL oxidation, protecting endothelial cells from apoptosis and participating in inflammatory and apoptotic processes [76,77,78,79,80,81].
Maintaining healthy lipid levels is crucial for cardiovascular well-being. Factors such as estrogen, reduced body fat, moderate alcohol intake, strenuous exercise and certain medications like niacin and fibrates have been identified to positively impact HDL cholesterol levels and overall cardiovascular health [82, 83].
In unraveling the complexities of lipid dynamics, valuable insights emerge for developing comprehensive strategies aimed at addressing cardiovascular health and managing the associated risk factors linked to cardiovascular diseases.
Atherosclerosis as a cardiovascular disease
Atherosclerosis is one of the most life-threatening cardiovascular diseases, which can affect individuals as early as their twenties. It contributes to an estimated 15.2 million deaths annually worldwide, making it a leading cause of mortality, as reported by the World Health Organization [24]. The initial stage of atherosclerosis is characterized by the accumulation of plaque in the arteries, particularly in large- and medium-sized vessels. This plaque primarily consists of cholesterol derived from low-density lipoproteins (LDLs), fibrin and calcium. The development of plaque can lead to ischemia due to the obstruction of blood flow and may result in the formation of thrombi when the plaque ruptures, leading to the blockage of blood vessels [25].
Atherosclerosis, or the formation of plaque, primarily occurs in the endothelium of arterial walls [26]. Under normal circumstances, the endothelium contributes to blood vessel dilation, reduces the growth of smooth muscle cells and prevents inflammatory responses [84]. However, in the context of atherosclerosis, dysfunction in the endothelium leads to reduced production of nitric oxide, a major vasodilator, resulting in increased vasoconstriction, heightened permeability and the uptake of LDL cholesterol by macrophages. This process leads to the formation of early lesions known as fatty streaks, the first visible sign of atherosclerosis [26].
Inadequate intake of antioxidants such as vitamin E, selenium and a diet low in fiber and unsaturated fats is dietary risk factor for atherosclerosis and dyslipidemias [85,86,87,88,89].
Available remedies for the management of cardiovascular diseases
While statins continue to be the primary treatment for hypercholesterolemia, recent research has explored alternative remedies with promising results. These alternatives aim to address not only cholesterol levels but also potential side effects associated with statin therapy.
Dietary modifications, such as a lecithin-enriched diet, have shown promise in positively impacting lipoprotein metabolism and cholesterol homeostasis, potentially reducing overall cholesterol levels [17, 90]. Plant sterols and stanols, soluble fiber from sources like psyllium and oats, and adherence to the Mediterranean diet are additional dietary approaches with proven efficacy in lowering cholesterol and reducing cardiovascular risk [91,92,93].
Nutraceuticals and supplements, including red yeast rice, berberine, omega-3 fatty acids and Coenzyme Q10, offer additional options for managing hypercholesterolemia [94,95,96,97]. Lifestyle modifications, such as regular exercise, weight management and stress reduction techniques, also play a crucial role in lowering cholesterol levels and improving overall cardiovascular health [98,99,100].
While statins remain a valuable tool in managing hypercholesterolemia, alternative remedies offer promising options for individuals seeking additional support or experiencing side effects. A combination of dietary modifications, nutraceuticals, lifestyle changes and appropriate medical supervision can effectively manage cholesterol levels and reduce cardiovascular risk.
Atherogenic lipoproteins
Several factors influence the atherogenic properties of cholesterol-containing lipoproteins in plasma. One critical factor is particle size, with smaller particles accumulating more rapidly in artery walls than larger particles. The size of the particle also determines its affinity for binding to the subendothelial matrix, with smaller particles binding more readily to proteoglycans [101]. Apolipoprotein B (Apo B), a protein found in lipoproteins, plays a significant role in this context, possessing multiple binding sites for proteoglycans and promoting the retention of lipoprotein particles within the subendothelial matrix, rendering lipoproteins containing Apo B atherogenic in nature [102].
Beyond particle size, additional factors contribute to the atherogenicity of cholesterol-containing lipoproteins. Remnant lipoproteins, resulting from the loss of their triglyceride cargo, exhibit prolonged circulation time and delayed clearance, increasing their interaction with the arterial wall and enhancing their atherogenic potential [103, 104].
Oxidative modification is another crucial aspect, with lipoproteins, particularly LDL, susceptible to modification by free radicals, leading to the formation of oxidized LDL (oxLDL). OxLDL is highly atherogenic, readily taken up by macrophages in the artery wall, triggering an inflammatory response and contributing to plaque formation [60, 105].
The composition and apoprotein profile further influence atherogenic potential. Lipoproteins enriched in triglycerides, such as VLDL remnants and chylomicron remnants, have higher atherogenic potential, while specific apolipoproteins associated with a lipoprotein particle, such as ApoC-III and ApoA-I, play distinct roles in influencing atherogenicity [106, 107].
Understanding these factors beyond particle size provides a comprehensive perspective on the complex interplay between lipoproteins and atherosclerosis. Targeting these factors through lifestyle modifications, medications and potentially novel therapeutic approaches can contribute to more effective strategies for preventing and managing cardiovascular diseases.
Lecithin cholesterol acyltransferase (LCAT) activity in atherosclerosis and cardiovascular disease: insights from recent studies
For over 50 years, lecithin acyltransferase (LCAT) has been recognized as an enzyme capable of esterifying cholesterol in plasma. It plays a crucial role in HDL maturation and reverse cholesterol transport. Despite the enzyme's apparent benefits for cholesterol metabolism, its role in atherosclerosis pathogenesis remains debated [108].
Conflicting results arise from human studies on high-risk cardiovascular patients and the general population. Some studies suggest that LCAT activity could promote atherogenesis [109,110,111,112]. Conversely, other research indicates that LCAT deficiency and reduced HDL-C levels reduce the risk of atherosclerosis, coronary artery disease or ischemic heart disease [113,114,115,116,117,118]. Additionally, gender differences in LCAT activity have been reported, with women exhibiting higher LCAT activity having an increased risk of CAD compared to men [119, 120]. Table 1 shows a summary of studies showing the correlation between LCAT and atherosclerosis.
In exploring the complex landscape of cholesterol metabolism, recent research has explored LCAT-independent pathways of cholesterol efflux, revealing potential mechanisms and clinical implications [108]. This adds a layer of complexity to our understanding of cholesterol homeostasis and suggests alternative routes for managing cholesterol levels beyond traditional pathways. LCAT deficiency has effects that go beyond the metabolism of HDL and can have wider clinical consequences due to its involvement in lipid metabolism. LCAT is essential for the esterification of cholesterol, which helps transport it in lipoproteins and contributes to the development of fully formed HDL particles. LCAT deficiency can cause a decrease in the ability to convert cholesterol into cholesterol esters, leading to the buildup of unbound cholesterol and triglycerides in lipoproteins that are circulating in the body. The disruption of lipid metabolism can make individuals more susceptible to certain cardiovascular problems, such as accelerated atherosclerosis, coronary artery disease and premature cardiovascular events. In addition, LCAT deficiency might present with symptoms such as corneal opacities, anemia and renal impairment, which demonstrate the widespread effects of poor lipid metabolism [121]. Hence, it is crucial to comprehend the wider ramifications of LCAT deficiency beyond HDL metabolism in order to effectively treat it clinically and devise specific therapeutic strategies.
LCAT deficiency has also garnered attention, extending beyond being perceived merely as a disorder of HDL metabolism. Contemporary investigations into LCAT deficiency have unveiled broader implications, shedding light on its intricate involvement in processes beyond HDL metabolism. This expanded perspective opens new avenues for exploring the role of LCAT and its deficiency in various aspects of lipid metabolism and related disorders.
One of such perspectives is the presence of lipoprotein X in LCAT deficiency conditions which might promote atherogenesis. Although its atherogenic potential is not fully confirmed, studies report extreme hypercholesterolemia mediated by lipoprotein X [122]. Also, some studies indicate that functional LCAT activity is not required for macrophage cholesterol efflux and RCT, as preβ-HDL acts as a cholesterol acceptor via ABCA1 or the SR-BI-dependent pathway [108, 109].
While there is growing interest in the role of LCAT and HDL in reducing atherosclerosis, leading to studies on various LCAT-related therapies, including recombinant LCAT and LCAT activation [123, 124], another perspective focuses on triglyceride-rich lipoproteins and low-density lipoprotein in atherosclerosis pathogenesis. Some studies suggest that increased LCAT activity may be linked to the formation of triglyceride-rich proteins and a concomitant reduction in LDL size [125, 126].
In a parallel line of inquiry, the emerging role of remnant lipoproteins in atherothrombosis has been a subject of interest [127]. The recognition of remnant lipoproteins as key players in this cardiovascular context adds depth to our understanding of atherothrombosis, potentially paving the way for targeted interventions that address these specific lipoprotein subtypes.
This evolving landscape of research highlights the dynamic nature of lipid-related studies, pushing the boundaries of our knowledge and encouraging a more comprehensive exploration of the intricate mechanisms that govern cholesterol metabolism and its implications for cardiovascular health.
LCAT activity and the RCT system
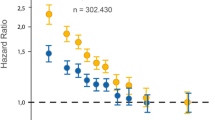
Studies suggest that elevated LCAT activity may contribute to increased triglyceride-rich lipoproteins (TRLs) and, consequently, decreased LDL particle size. This is likely due to the actions of hepatic lipase and cholesteryl ester transfer protein (CETP), as supported by findings from some observational studies [125, 126]. These investigations reveal a positive association between LCAT activity and markers of TRL metabolism, indirectly influencing LDL particle size. However, it is essential to acknowledge that the reverse cholesterol transport (RCT) system is a complex network governed by more than just LCAT. Recent research implicates the interplay of additional enzymes, such as phospholipid transfer protein (PLTP) and CETP in shaping HDL's atherosclerogenic potential [128, 129]. Therefore, a holistic understanding of these intricate interactions is crucial for deciphering the multifaceted roles of LCAT and the RCT system in cardiovascular health. Figure 2 shows the association of LCAT activity and an indicator of LDL-particle size (LDL-Rm value) with ASCVD development. Figure 2 is a representation of the association of LCAT activity as an indicator of LDL-particle size (LDL-Rm value) with ASCVD formation.
The association of LCAT activity as an indicator of LDL-particle size (LDL-Rm value) with ASCVD development [131]
RCT and atherosclerosis
The efficiency of the reverse cholesterol transport (RCT) system in combating atherosclerosis is tightly linked to the state of lipid metabolism. Dyslipidemias, a significant contributor to atherosclerotic cardiovascular disease (ASCVD), can paradoxically trigger LCAT activation. This increased activity accelerates free cholesterol esterification, potentially altering the direction of atherosclerotic plaque suppression [130, 131].
Interestingly, studies have identified a negative association between LCAT mass concentration and intravascular ultrasonography-assessed plaque volume in patients with coronary artery disease (CAD) [131]. This suggests that cautiously modulating LCAT activity may promote a more balanced RCT function, potentially leading to plaque regression in CAD patients [131].
The atherogenic cascade
This intricate mechanism comes into play in an atherogenic state, where RCT system activation, alongside increased LCAT activity measured using the rate of esterification of serum cholesterol via endogenous substrates, leads to alterations in HDL metabolism. These changes result in elevated serum levels of TRL-related markers and a reduction in LDL particle size, ultimately contributing to the development of ASCVD. These findings provide valuable insights into the complex interplay of factors in atherosclerosis and cardiovascular health, offering potential targets for therapeutic interventions in the ongoing battle against cardiovascular diseases.
Conclusions
In the realm of cardiovascular health, lecithin emerges as a pivotal player in addressing the key risk factors—low-density lipoprotein (LDL), often regarded as the 'bad' cholesterol, and high-density lipoprotein (HDL), the 'good' cholesterol. Lecithin's role encompasses reducing LDL levels while simultaneously promoting the synthesis of HDL, thereby fortifying our defenses against cardiovascular ailments.
Notably, lecithin cholesterol acyltransferase (LCAT) takes the center stage in the intricate choreography of reverse cholesterol transport (RCT), an inherently anti-atherogenic process. At its core, RCT revolves around the removal of excess cholesterol by high-density lipoproteins (HDL) from cellular repositories.
Recommendations
Building upon the insights gleaned from this review, several recommendations can be made to further our understanding and enhance cardiovascular health.
Given the potential benefits of lecithin in modulating cholesterol levels, there is a need for rigorous research aimed at determining the ideal lecithin dosage for individuals. Tailored dosages can provide a more targeted approach to managing cardiovascular risk factors. Also, the intricate association between increased LCAT activity and a reduction in LDL particle size in the context of atherosclerosis warrants further investigation. In-depth research is required to unravel the precise mechanisms underlying this relationship. A clearer understanding of these processes can allow for more targeted with effective interventions strategies.
Incorporating these recommendations into future research and medical practice can contribute to advancing our knowledge of cardiovascular health and developing more tailored approaches for prevention and treatment, ultimately benefiting individuals at risk of cardiovascular diseases.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- LDL:
-
Low-density lipoprotein
- HDL:
-
High-density lipoprotein
- LCAT:
-
Cholesterol acyltransferase
- RCT:
-
Reverse cholesterol transport
- TRL:
-
Triglyceride-rich lipoprotein
- ASCVD:
-
Atherosclerotic cardiovascular disease
- CVD:
-
Cardiovascular diseases
- Apo B:
-
Apolipoprotein B
- Oxldl:
-
Oxidized LDL
- CETP:
-
Cholesteryl ester transfer protein
- PLTP:
-
Phospholipid transfer protein
References
Tanno H (2012) Lecithin. In: Ullmann's encyclopedia of industrial chemistry, vol 12 online version. Wiley‐VCH Verlag GmbH & Co. KGaA, Weinheim. https://doi.org/10.1002/14356007.a15_293
Wendel A (1995) Lecithin. In: Kirk‐Othmer encyclopedia of chemical technology, vol 15, 4th ed. Wiley‐Interscience, New York, pp 192–210
List GR (2015) Soybean lecithin: food, industrial uses, and other applications. Polar Lipids. https://doi.org/10.1016/b978-1-63067-044-3.50005-4
Nichols BJ, Lippincott-Schwartz J (2001) Endocytosis without clathrin coats. Trends Cell Biol 11(10):406–412. https://doi.org/10.1016/S0962-8924(01)02107-9
van Meer G, Voelker D, Feigenson G (2008) Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol 9:112–124. https://doi.org/10.1038/nrm2330
William Taeusch H, Lu KW, Goerke J, Clements JA (1999) Nonionic polymers reverse inactivation of surfactant by meconium and other substances. Am J Respir Crit Care Med 159(5 Pt 1):1391–1395. https://doi.org/10.1164/ajrccm.159.5.9808047
Basabe-Burgos O, Ahlström JZ, Mikolka P, Landreh M, Johansson J, Curstedt T, Rising A (2019) Efficient delipidation of a recombinant lung surfactant lipopeptide analogue by liquid-gel chromatography. PLoS ONE 14(12):e0226072. https://doi.org/10.1371/journal.pone.0226072
Canty D, Zeisel S (1994) Lecithin and choline in human health and disease. Nutr Rev 52:327–339. https://doi.org/10.1111/j.1753-4887.1994.tb01357.x
Gámiz F, Gallo M (2021) A systematic review of the dietary choline impact on cognition from a psychobiological approach: insights from animal studies. Nutrients 13(6):1966. https://doi.org/10.3390/nu13061966
Higgins JP, Flicker L (2003) Lecithin for dementia and cognitive impairment. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD001015
Lamireau T, Bouchard G, Yousef IM, Clouzeau-Girard H, Rosenbaum J, Desmoulière A, Tuchweber B (2007) Dietary lecithin protects against cholestatic liver disease in cholic acid-fed Abcb4-deficient mice. Pediatr Res 61(2):185–190. https://doi.org/10.1203/pdr.0b013e31802d7780
Kilchoer B, Vils A, Minder B, Muka T, Glisic M, Bally L (2020) Efficacy of dietary supplements to reduce liver fat. Nutrients 12(8):2302. https://doi.org/10.3390/nu12082302
Mourad AM, de Carvalho PE, Mazzola PG, Sabha M, Moriel P (2010) Influence of soy lecithin administration on hypercholesterolemia. Cholesterol 2010:824813. https://doi.org/10.1155/2010/824813
Knuiman J, Beynen A, Katan MB (1989) Lecithin intake and serum cholesterol. Am J Clin Nutr 49(2):266–268. https://doi.org/10.1093/ajcn/49.2.266
Nowacki D, Martynowicz H, Skoczyńska A et al (2017) Lecithin derived from ω-3 PUFA fortified eggs decreases blood pressure in spontaneously hypertensive rats. Sci Rep 7:12373. https://doi.org/10.1038/s41598-017-12019-w
Nowacki D, Martynowicz H, Wojakowska A, Turczyn B, Skoczyńska A, Trziszka T, Szuba A (2015) Effects of new generation egg-derived phospholipid fraction on blood pressure in hypertensive rats. https://doi.org/10.13140/RG.2.1.2095.5286
Bueschelberger H, Tirok S, Stoffels I, Schoeppe A (2014) Lecithins. In:Emulsifiers in food technology, pp 21–60. https://doi.org/10.1002/9781118921265.ch2
Joshi A, Paratkar SG, Thorat BN (2006) Modification of lecithin by physical, chemical and enzymatic methods. Eur J Lipid Sci Technol 108(4):363–373. https://doi.org/10.1002/ejlt.200600016
Szuhaj BF (1983) Lecithin production and utilization. J Am Oil Chem Soc 60(2Part1):306–309. https://doi.org/10.1007/bf02543508
Lehri D, Kumari N, Singh R, Sharma V (2019) Composition, production, physicochemical properties and applications of lecithin obtained from rice (Oryza sativa l.)—a review. Plant Sci Today 6(sp1):613–622. https://doi.org/10.14719/pst.2019.6.sp1.682
World Health Organization (2022) Cardiovascular diseases. https://www.who.int/health-topics/cardiovascular-diseases. Accessed 06 Nov 2023
American Heart Association (2023) Heart disease and stroke statistics—2023 update. https://doi.org/10.1161/CIR.0000000000001123
Centers for Disease Control and Prevention (2023) Cardiovascular diseases. https://www.cdc.gov/. Accessed 06 Nov 2023
Jebari-Benslaiman S, Galicia-García U, Larrea-Sebal A, Olaetxea JR, Alloza I, Vandenbroeck K, Benito-Vicente A, Martín C (2022) Pathophysiology of atherosclerosis. Int J Mol Sci 23(6):3346. https://doi.org/10.3390/ijms23063346
Li L, Liu S, Tan J, Wei L, Wu D, Gao S, Weng Y, Chen J (2022) Recent advance in treatment of atherosclerosis: key targets and plaque-positioned delivery strategies. J Tissue Eng 13:204173142210885. https://doi.org/10.1177/20417314221088509
Reza A, Nasrin S (2012) Cardiovascular disease risk factors. Ahvaz Jondishapour University of Medical Sciences, Iran, pp 279–310
Glomset JA (1968) The plasma lecithins:cholesterol acyltransferase reaction. J Lipid Res 9:155–167
Chung BH, Segrest JP, Franklin F (1998) In vitro production of beta-very low density lipoproteins and small, dense low density lipoproteins in mildly hypertriglyceridemic plasma: role of activities of lecithin:cholester acyltransferase, cholesterylester transfer proteins and lipoprotein lipase. Atherosclerosis 141:209–225
Welty FK (2013) How do elevated triglycerides and low hdl-cholesterol affect inflammation and atherothrombosis? Curr Cardiol Rep. https://doi.org/10.1007/s11886-013-0400-4
van der Veen JN, Kennelly JP, Wan S, Vance JE, Vance DE, Jacobs RL (2017) The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochim Biophys Acta BBA Biomembr 1859(9):1558–1572. https://doi.org/10.1016/j.bbamem.2017.04.006
Cahlin E, Nilsson S, Scherstén T (1972) Synthesis of phospholipids and triglycerides in human liver slices III. Influence of bile acids, choline, and linoleic acid. Scand J Clin Lab Investig 29(1):109–114. https://doi.org/10.3109/00365517209081062
NCBI (2023) PubChem database entry for Lecithin. https://pubchem.ncbi.nlm.nih.gov/compound/Lecithin
USDA (2023) FoodData central. Search: lecithin. https://fdc.nal.usda.gov/fdc-app.html
FDA (2023) Generally recognized as safe (GRAS) notice inventory: lecithin. https://fda.report/media/145167/GRAS-Notice-GRN-939-Sunflower-Lecithin-part-1.pdf
Akutsu H (2020) Structure and dynamics of phospholipids in membranes elucidated by combined use of nmr and vibrational spectroscopies. Biochim Biophys Acta BBA Biomembr 1862(9):183352. https://doi.org/10.1016/j.bbamem.2020.183352
Taylor EJ (ed) (2015) Dorland’s illustrated medicaldictionary, 27th edn. WB Saunders, Philadelphia, p 1283
Whittle MJ, Wilson AI, Whitfield CR, Paton RD, Logan RW (1981) Amniotic fluid phospholipid profile determined by two-dimensional thin-layer chromatography as index of fetal lung maturation. Br Med J (Clin Res Ed) 282(6262):428–430. https://doi.org/10.1136/bmj.282.6262.428
Lemke A, Castillo-Sánchez JC, Prodinger F et al (2017) Human amniotic membrane as newly identified source of amniotic fluid pulmonary surfactant. Sci Rep 7:6406. https://doi.org/10.1038/s41598-017-06402-w
Calkovska A, Uhliarova B, Joskova M, Franova S, Kolomaznik M, Calkovsky V, Smolarova S (2015) Pulmonary surfactant in the airway physiology: a direct relaxing effect on the smooth muscle. Respir Physiol Neurobiol 209:95–105. https://doi.org/10.1016/j.resp.2015.01.004
Mönki J, Holopainen M, Ruhanen H, Karikoski N, Käkelä R, Mykkänen A (2023) Lipid species profiling of bronchoalveolar lavage fluid cells of horses housed on two different bedding materials. Sci Rep 13(1):21778. https://doi.org/10.1038/s41598-023-49032-1
Schmidt RJ, Liang D, Busgang SA, Curtin P, Giulivi C (2021) Maternal plasma metabolic profile demarcates a role for neuroinflammation in non-typical development of children. Metabolites 11:545. https://doi.org/10.3390/metabo11080545
Kobayashi-Hattori K, Mogi A, Matsumoto Y, Takita T (2005) Effect of caffeine on the body fat and lipid metabolism of rats fed on a high-fat diet. Biosci Biotechnol Biochem 69(11):2219–2223. https://doi.org/10.1271/bbb.69.2219
Olmeda B, Martínez-Calle M, Pérez-Gil J (2017) Pulmonary surfactant metabolism in the alveolar airspace: biogenesis, extracellular conversions, recycling. Ann Anat Anat Anz 209:78–92. https://doi.org/10.1016/j.aanat.2016.09.008
Zhao F, Li R, Liu Y, Chen H (2023) Perspectives on lecithin from egg yolk: extraction, physicochemical properties, modification, and applications. Front Nutr 9:1082671. https://doi.org/10.3389/fnut.2022.1082671
Fennema OR (2017) Food chemistry, 5th edn. CRC Press, Boca Raton
Reineccius GA (2005) Flavor chemistry and technology. CRC Press, Boca Raton. https://doi.org/10.1201/9780203485347
Jonas A (1998) Regulation of lecithin cholesterol acyltransferase activity. Prog Lipid Res 37(4):209–234. https://doi.org/10.1016/s0163-7827(98)00007-1
Ahsan L, Ossoli A, Freeman LA, Vaisman B, Amar M, Shamburek RD, Remaley AT (2014) Role of lecithin. In: The HDL handbook, pp 159–194. https://doi.org/10.1016/b978-0-12-407867-3.00007-x
Ramdath DD, Emily MTP, Sarfaraz S, Renwick S, Duncan AM (2017) Beyond the cholesterol-lowering effect of soy protein: a review of the effects of dietary soy and its constituents on risk factors for cardiovascular disease. Nutrients 9(4):324. https://doi.org/10.3390/nu9040324
Latif NHA, Taher M, Jaffri JM, Amri MS, Kudos MBA, Sulaiman WMAW, Susanti D (2018) Selected natural agents used for cholesterol controls. Nutr Food Sci 48(2):301–317. https://doi.org/10.1108/nfs-06-2017-0128
Stone NJ, Robinson JG, Lichtenstein AH, Bhatt D, Ginsberg HN, Witztum JL (2021) 2020 ACC/AHA cholesterol management guidelines for the prevention, detection, and treatment of atherosclerotic cardiovascular disease: highlights for clinicians. J Am Coll Cardiol 78(5):369–397
Jomard A, Osto E (2020) High density lipoproteins: metabolism, function, and therapeutic potential. Front Cardiovasc Med 7:39. https://doi.org/10.3389/fcvm.2020.00039
Ouimet M, Barrett TJ, Fisher EA (2019) HDL and reverse cholesterol transport: basic mechanisms and their roles in vascular health and disease. Circ Res 124(10):1505–1518
Xiang F, Ding C, Wang M, Hu H, Ma X, Xu X, Abubakar BZ, Pignitter M, Wei K-N, Shi A-M, Wang Q (2024) Vegetable oils: classification, quality analysis, nutritional value and lipidomics applications. Food Chem 439:138059. https://doi.org/10.1016/j.foodchem.2023.138059
Musakhanian J, Rodier JD, Dave M (2022) Oxidative stability in lipid formulations: a review of the mechanisms, drivers, and inhibitors of oxidation. AAPS PharmSciTech 23:151. https://doi.org/10.1208/s12249-022-02282-0
World Health Organization (2023) Cardiovascular diseases. https://www.who.int/health-topics/cardiovascular-diseases
Biswas I, Khan GA (2020) Endothelial dysfunction in cardiovascular diseases. In: Basic and clinical understanding of microcirculation. IntechOpen. https://doi.org/10.5772/intechopen.89365
Xu S, Liu Z, Liu P (2013) HDL cholesterol in cardiovascular diseases: the good, the bad, and the ugly? Int J Cardiol. https://doi.org/10.1016/j.ijcard.2013.07.210
Robinson JG, Williams KJ, Gidding SS, Borén J, Tabas I, Fisher EA, Packard C, Pencina M, Fayad ZA, Mani V, Rye KA, Nordestgaard BG, Tybjærg-Hansen A, Douglas PS, Nicholls SJ, Pagidipati N, Sniderman AD (2018) Eradicating the burden of atherosclerotic cardiovascular disease by lowering apolipoprotein b lipoproteins earlier in life. J Am Heart Assoc. https://doi.org/10.1161/jaha.118.009778
Daskalopoulos EP, Hermans KC, Delft Lv, Altara R, Blankesteijn M (2015) The role of inflammation in myocardial infarction. Inflamm Heart Fail. https://doi.org/10.1016/b978-0-12-800039-7.00003-7
World Heart Federation (2022) Cardiovascular disease statistics—Africa. https://world-heart-federation.org/resource/cardiovascular-diseases-cvds-global-facts-figures/
Bischoff A, Ekoe T, Perone N, Slama S, Loutan L (2009) Chronic disease management in sub-Saharan Africa: whose business is it? Int J Environ Res Public Health 6(8):2258–2270. https://doi.org/10.3390/ijerph6082258
Institute of Medicine (US) Committee on Preventing the Global Epidemic of Cardiovascular Disease: Meeting the Challenges in Developing Countries; Fuster V, Kelly BB, editors (2010) Promoting cardiovascular health in the developing world: a critical challenge to achieve global health. National Academies Press (US), Washington, DC. 5, Reducing the burden of cardiovascular disease: intervention approaches. https://www.ncbi.nlm.nih.gov/books/NBK45696/
World Health Organization. (2013) Prevention and control of non-communicable diseases in Africa. https://www.afro.who.int/about-us/programmes-clusters
Alaba OA, Chiwire P, Siya A, Saliu OA, Nhakaniso K, Nzeribe E, Okova D, Lukwa AT (2023) Socio-economic inequalities in the double burden of malnutrition among under-five children: evidence from 10 selected sub-Saharan African countries. Int J Environ Res Public Health 20(8):5489. https://doi.org/10.3390/ijerph20085489
Mtintsilana A, Craig A, Mapanga W, Dlamini SN, Norris SA (2023) Association between socio-economic status and non-communicable disease risk in young adults from Kenya, South Africa, and the United Kingdom. Sci Rep 13(1):728. https://doi.org/10.1038/s41598-023-28013-4
Adgoy E (2019) Social determinants of non-communicable disease. MOJ Public Health 8(4):149–152
Kodaman N, Aldrich MC, Sobota R, Asselbergs FW, Poku KA, Brown NJ et al (2016) Cardiovascular disease risk factors in Ghana during the rural-to-urban transition: a cross-sectional study. PLoS ONE 11(10):e0162753. https://doi.org/10.1371/journal.pone.0162753
Wekesah FM, Kyobutungi C, Grobbee DE, Klipstein-Grobusch K (2019) Understanding of and perceptions towards cardiovascular diseases and their risk factors: a qualitative study among residents of urban informal settings in Nairobi. BMJ Open 9(6):e026852
Hosseinpoor AR, Bergen N, Mendis S, Harper S, Verdes E, Kunst A, Chatterji S (2012) Socioeconomic inequality in the prevalence of noncommunicable diseases in low-and middle-income countries: results from the World Health Survey. BMC Public Health 12:1–13
Kuuire V, Atuoye K, Bisung E, Braimah JA (2023) A multilevel analysis of neighborhood inequalities and non-communicable disease multimorbidity in Ghana. In: Braimah JA, Bisung E, Kuuire V (eds) Health geography in sub-Saharan Africa. Global perspectives on health geography. Springer, Cham. https://doi.org/10.1007/978-3-031-37565-1_2
Ebrahim S, Smeeth L (2005) Non-communicable diseases in low and middle-income countries: a priority or a distraction? Int J Epidemiol 34(5):961–966
Lamarche B, Tchernof A, Moorjani S, Cantin B, Dagenais GR, Lupien PJ, Després JP (1997) Small, dense low-density lipoprotein particles as a predictor of the risk of ischemic heart disease in men: prospective results from the Québec cardiovascular study. Circulation 95(1):69–75
Ivanova EA, Myasoedova VA, Melnichenko AA, Grechko AV, Orekhov AN (2017) Small dense low-density lipoprotein as biomarker for atherosclerotic diseases. Oxid Med Cell Longev 2017:1273042. https://doi.org/10.1155/2017/1273042
Peelman F, Vandekerckhove J, Rosseneu M (2000) Structure and function of lecithin cholesterol acyl transferase: new insights from structural predictions and animal models. Curr Opin Lipidol 11(2):155–160
Osei-Hwedieh DO, Amar M, Sviridov D, Remaley AT (2011) Apolipoprotein mimetic peptides: mechanisms of action as anti-atherogenic agents. Pharmacol Ther 130(1):83–91
Norata GD, Catapano AL (2007) Molecular mechanisms responsible for the anti-inflammatory and protective effect of high-density lipoprotein on the endothelium. High Blood Press Cardiovasc Prev 14:21–31
Xepapadaki E, Zvintzou E, Kalogeropoulou C, Filou S, Kypreos KE (2020) Τhe antioxidant function of HDL in atherosclerosis. Angiology 71(2):112–121
Rosenson RS, Brewer HB Jr, Davidson WS, Fayad ZA, Fuster V, Goldstein J, Hellerstein M, Jiang X-C, Phillips MC, Rader DJ, Remaley AT, Rothblat GH, Tall AR, Yvan-Charvet L (2012) Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport. Circulation 125(15):1905–1919
Shah PK, Lecis D (2019) Inflammation in atherosclerotic cardiovascular disease. F1000Research 8:1402
Huang Y, Wong J, Zhang W, Chen Y, Luo Y, Wang N (2012) HDL protects against endothelial cell apoptosis through PI3K/Akt/eNOS signaling pathway. Biochem Biophys Res Commun 425(4):705–710
Cho KH, Nam HS, Kang DJ, Park MH, Kim JH (2022) Long-term alcohol consumption caused a significant decrease in serum high-density lipoprotein (HDL)-cholesterol and apolipoprotein AI with the atherogenic changes of HDL in middle-aged Korean women. Int J Mol Sci 23(15):8623
Awan Z, Waili KA, Alrasadi K, Genest J (2008) Treatment of low high-density lipoprotein cholesterol. Can J Cardiol 24:27C-31C
Davignon J, Ganz P (2004) Role of endothelial dysfunction in atherosclerosis. Circulation 109(Suppl 1):III-27–III−32
Estruch R, Ros E (2020) The role of the Mediterranean diet on weight loss and obesity-related diseases. Rev Endocr Metab Disord 21(3):315–327
Kwiterovich PO (1997) The effect of dietary fat, antioxidants, and pro-oxidants on blood lipids, lipoproteins, and atherosclerosis. J Am Diet Assoc 97(7):S31–S41
García MS (2013) Emerging role of natural antioxidants in chronic disease prevention with an emphasis on vitamin E and selenium. In: Oxidative Stress and Chronic Degenerative Diseases-a role for antioxidants, p 419
Singh U, Devaraj S (2007) Vitamin E: inflammation and atherosclerosis. Vitam Horm 76:519–549
Moreno JJ, Mitjavila MT (2003) The degree of unsaturation of dietary fatty acids and the development of atherosclerosis. J Nutr Biochem 14(4):182–195
Holló J, Perédi J, Ruzics A, Jeránek M, Erdélyi A (1993) Sunflower lecithin and possibilities for utilization. J Am Oil Chem Soc 70:997–1001
Abumweis S, Barake R, Jones P (2008) Plant sterols/stanols as cholesterol lowering agents: a meta-analysis of randomized controlled trials. Food Nutr Res 52(1):1811
Enkhmaa B, Surampudi P, Anuurad E, Berglund L (2018) Lifestyle changes: effect of diet, exercise, functional food, and obesity treatment on lipids and lipoproteins. Endotext [Internet]
Estruch R, Ros E, Salas-Salvado J, Jordà E, Gasol E, Corella D (2013) Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 368(24):2373–2383
Rahmani P, Melekoglu E, Tavakoli S, Malekpour Alamdari N, Rohani P, Sohouli MH (2023) Impact of red yeast rice supplementation on lipid profile: a systematic review and meta-analysis of randomized-controlled trials. Expert Rev Clin Pharmacol 16(1):73–81
Kong W, Wei J, Abidi P, Lin M, Inaba S, Li C, Wang Y, Wang Z, Si S, Pan H, Wang S, Wu J, Wang Y, Li Z, Liu J, Jiang JD (2004) Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat Med 10(12):1344–1351
Kris-Etherton PM, Miller VT, Appel LJ, Bazan RC, Dicklin K, Dreon DM, Grillo JA (2013) 2012 American Heart Association/American College of Cardiology practice guideline on cholesterol management in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 127(25 Suppl 2):S1–S45
Mabuchi H, Nohara A, Kobayashi J, Kawashiri MA, Katsuda S, Inazu A, Koizumi J, Hokuriku Lipid Research Group (2007) Effects of CoQ10 supplementation on plasma lipoprotein lipid, CoQ10 and liver and muscle enzyme levels in hypercholesterolemic patients treated with atorvastatin: a randomized double-blind study. Atherosclerosis 195(2):e182–e189
Cao J, Devaraj S (2019) Recent AHA/ACC guidelines on cholesterol management expands the role of the clinical laboratory. Clin Chim Acta 495:82–84
Powell-Wiley TM, Poirier P, Burke LE, Després JP, Gordon-Larsen P, Lavie CJ, Lear SA, Ndumele CE, Neeland IJ, Sanders P, St-Onge M-P, American Heart Association Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Epidemiology and Prevention; and Stroke Council (2021) Obesity and cardiovascular disease: a scientific statement from the American Heart Association. Circulation 143(21):e984–e1010
Steptoe A, Kivimäki M (2013) Stress and cardiovascular disease: an update on current knowledge. Annu Rev Public Health 34:337–354
Araujo JA, Nel AE (2009) Particulate matter and atherosclerosis: role of particle size, composition and oxidative stress. Part Fibre Toxicol 6:24. https://doi.org/10.1186/1743-8977-6-24
Behbodikhah J, Ahmed S, Elyasi A, Kasselman LJ, De Leon J, Glass AD, Reiss AB (2021) Apolipoprotein B and cardiovascular disease: biomarker and potential therapeutic target. Metabolites 11(10):690. https://doi.org/10.3390/metabo11100690
Varbo A, Nordestgaard BG (2016) Remnant cholesterol and triglyceride-rich lipoproteins in atherosclerosis progression and cardiovascular disease. Arterioscler Thromb Vasc Biol 36(11):2133–2135. https://doi.org/10.1161/ATVBAHA.116.308305
Jacobson TA, Ito MK, Maki KC, Orringer CE, Bays HE, Jones PH, McKenney JM, Grundy SM, Gill EA, Wild RA, Wilson DP, Brown WV (2015) National lipid association recommendations for patient-centered management of dyslipidemia: part 1—full report. J Clin Lipidol 9(2):129–169
Parthasarathy S, Raghavamenon A, Garelnabi MO, Santanam N (2010) Oxidized low-density lipoprotein. In: Free radicals and antioxidant protocols, pp 403–417
Allan GM, Lindblad AJ, Comeau A, Coppola J, Hudson B, Mannarino M, McMinis C, Padwal R, Schelstraete C, Zarnke K, Garrison S, Cotton C, Korownyk C, McCormack J, Nickel S, Kolber MR (2015) Simplified lipid guidelines: Prevention and management of cardiovascular disease in primary care. Can Fam Physician 61(10):857–867
Norata GD, Tsimikas S, Pirillo A, Catapano AL (2015) (2015) Apolipoprotein C-III: from pathophysiology to pharmacology. Trends Pharmacol Sci 36(10):675–687. https://doi.org/10.1016/j.tips.2015.07.001
Ossoli A, Simonelli S, Vitali C, Franceschini G, Calabresi L (2016) Role of LCAT in atherosclerosis. J Atheroscler Thromb 23(2):119–127. https://doi.org/10.5551/jat.32854
Calabresi L, Baldassarre D, Castelnuovo S, Conca P, Bocchi L, Candini C et al (2009) Functional lecithin: cholesterol acyltransferase is not required for efficient atheroprotection in humans. Circulation 120(7):628–635
Dullaart RP, Perton F, van der Klauw MM, Hillege HL, Sluiter WJ (2010) High plasma lecithin:cholesterol acyltransferase activity does not predict low incidence of cardiovascular events: possible attenuation of cardioprotection associated with high HDL cholesterol. Atherosclerosis 208(2):537–542
Haase CL, Tybjaerg-Hansen A, Qayyum AA, Schou J, Nordestgaard BG, FrikkeSchmidt R (2012) LCAT, HDL cholesterol and ischemic cardiovascular disease: a Mendelian randomization study of HDL cholesterol in 54,500 individuals. J Clin Endocrinol Metab 97(2):E248–E256
Oldoni F, Baldassarre D, Castelnuovo S, Ossoli A, Amato M, van Capelleveen J, Hovingh GK, De Groot E, Bochem A, Simonelli S, Barbieri S, Veglia F, Franceschini G, Kuivenhoven JA, Holleboom AG, Calabresi L (2018) Complete and partial lecithin:cholesterol acyltransferase deficiency is differentially associated with atherosclerosis. Circulation 138(10):1000–1007. https://doi.org/10.1161/circulationaha.118.034706
Ayyobi AF, McGladdery SH, John CS, Mancini GB, Hill JS, Frohlich JJ (2004) Lecithin: cholesterol acyltransferase (LCAT) deficiency and risk of vascular disease: 25 year follow-up. Atherosclerosis 177(2):361–366
Hovingh GK, Hutten BA, Holleboom AG, Petersen W, Rol P, Stalenhoef A et al (2005) Compromised LCAT function is associated with increased atherosclerosis. Circulation 112(6):879–884
Scarpioni R, Paties C, Bergonzi G (2008) Dramatic atherosclerotic vascular burden in a patient with familial lecithin-cholesterol acyltransferase (LCAT) deficiency. Nephrol Dial Transplant 23(3):1074
Sethi AA, Sampson M, Warnick R, Muniz N, Vaisman B, Nordestgaard BG et al (2010) High pre-beta1 HDL concentrations and low lecithin: cholesterol acyltransferase activities are strong positive risk markers for ischemic heart disease and independent of HDL cholesterol. Clin Chem 56(7):1128–1137
Duivenvoorden R, Holleboom AG, van den Bogaard B, Nederveen AJ, de Groot E, Hutten BA et al (2011) Carriers of lecithin cholesterol acyltransferase gene mutations have accelerated atherogenesis as assessed by carotid 3.0-T magnetic resonance imaging [corrected]. J Am Coll Cardiol 58(24):2481–2487
van den Bogaard B, Holleboom AG, Duivenvoorden R, Hutten BA, Kastelein JJ, Hovingh GK et al (2012) Patients with low HDL-cholesterol caused by mutations in LCAT have increased arterial stiffness. Atherosclerosis 225(2):481–485
Holleboom AG, Kuivenhoven JA, Vergeer M, Hovingh GK, van Miert JN, Wareham NJ et al (2010) Plasma levels of lecithin:cholesterol acyltransferase and risk of future coronary artery disease in apparently healthy men and women: a prospective case-control analysis nested in the EPIC-Norfolk population study. J Lipid Res 51(2):416–421
Calabresi L, Baldassarre D, Simonelli S, Gomaraschi M, Amato M, Castelnuovo S et al (2011) Plasma lecithin:cholesterol acyltransferase and carotid intima-media thickness in European individuals at high cardiovascular risk. J Lipid Res 52(8):1569–1574
Morales E, Alonso M, Sarmiento B, et al (2018) LCAT deficiency as a cause of proteinuria and corneal opacification. Case Rep 2018: bcr-2017-224129
Colantuono R, Pavanello C, Pietrobattista A, Turri M, Francalanci P, Spada M, Vajro P, Calabresi L, Mandato C (2022) Case report: unusual and extremely severe lipoprotein X-mediated hypercholesterolemia in extrahepatic pediatric cholestasis. Front Pediatr 10:969081
Zhou MFP, Zhang J (2008) Novel small molecule LCAT activators raise HDL levels in rodent models. Arterioscler Thromb Vasc Biol 28:E65–E66
Rousset X, Vaisman B, Auerbach B, Krause BR, Homan R, Stonik J et al (2010) Effect of recombinant human lecithin cholesterol acyltransferase infusion on lipoprotein metabolism in mice. J Pharmacol Exp Ther 335(1):140–148
Tani S, Takahashi A, Nagao K, Hirayama A (2016) Association of lecithin-cholesterol acyltransferase activity measured as a serum cholesterol esterification rate and low-density lipoprotein heterogeneity with cardiovascular risk: a cross-sectional study. Heart Vessels 31(6):831–840. https://doi.org/10.1007/s00380-015-0678-9
Yokoyama K, Tani S, Matsuo R et al (2018) (2018) Association of lecithin-cholesterol acyltransferase activity and low-density lipoprotein heterogeneity with atherosclerotic cardiovascular disease risk: a longitudinal pilot study. BMC Cardiovasc Disord 18:224. https://doi.org/10.1186/s12872-018-0967-1
Saeed A, Feofanova EV, Yu B, Sun W, Virani SS, Nambi V, Coresh J, Guild CS, Boerwinkle E, Ballantyne CM, Hoogeveen RC (2018) Remnant-like particle cholesterol, low-density lipoprotein triglycerides, and incident cardiovascular disease. J Am Coll Cardiol 72(2):156–169. https://doi.org/10.1016/j.jacc.2018.04.050
Calabresi L, Franceschini G (2010) Lecithin:cholesterol acyltransferase, high-density lipoproteins, and atheroprotection in humans. Trends Cardiovasc Med 20(2):50–53. https://doi.org/10.1016/j.tcm.2010.03.007
Albers JJ, Vuletic S, Cheung MC (2012) Role of plasma phospholipid transfer protein in lipid and lipoprotein metabolism. Biochim Biophys Acta BBA Mol Cell Biol Lipids 1821(3):345–357
Singh SA, Andraski AB, Higashi H, Lee LH, Ramsaroop A, Sacks FM, Aikawa M (2021) Metabolism of PLTP, CETP, and LCAT on multiple HDL sizes using the Orbitrap Fusion Lumos. JCI Insight 6(3):e143526. https://doi.org/10.1172/jci.insight.143526
Gebhard C, Rhainds D, He G, Rodés-Cabau J, Lavi S, Spence JD, Title L, Kouz S, L’Allier PL, Grégoire J, Ibrahim R, Cossette M, Guertin MC, Beanlands R, Rhéaume E, Tardif JC (2018) Elevated level of lecithin:cholesterol acyltransferase (LCAT) is associated with reduced coronary atheroma burden. Atherosclerosis 276:131–139. https://doi.org/10.1016/j.atherosclerosis.2018.07.025
Acknowledgements
Not applicable.
Funding
The current was self-sponsored by the authors.
Author information
Authors and Affiliations
Contributions
MCO, ODA, BSO wrote the original draft, OPA and LOO contributed to writing—review and editing, WJA was involved in revised and finalized the manuscript, and AAF helped in conceptualization and supervision.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Onaolapo, M.C., Alabi, O.D., Akano, O.P. et al. Lecithin and cardiovascular health: a comprehensive review. Egypt Heart J 76, 92 (2024). https://doi.org/10.1186/s43044-024-00523-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43044-024-00523-0