Abstract
Background
To evaluate the effect of adding letrozole to the antagonist ovarian stimulation protocol (COS) on in-vitro fertilization/intracytoplasmic sperm injection (IVF/ICSI) outcome in endometriosis patients.
Methods
This randomized clinical trial was carried out in the department of infertility treatment at Arash Women’s Hospital from May 2019 to May 2021. The eligible women with normal ovarian reserve tests who had endometriosis diagnosis and underwent IVF/ICSI cycles were evaluated. A flexible regimen of GnRH-antagonist protocol was used for COS. In the experimental (n = 34), the patients received 5 mg letrozole daily for the first 5 days in combination with 150 IU of recombinant follicle-stimulating hormone (rFSH). In the control group (n = 30), the patients received only the same dose of rFSH. The treatment cycle was compared between groups.
Results
Analysis of demographic characteristics, severity of endometriosis, and baseline hormonal tests of patients showed that the two groups were similar and comparable. The means of total used gonadotropins ampoules and serum E2 level on oocyte trigger day in the letrozole group were significantly lower than those of in the control group (P = 0.03 and P = 0.004, respectively). No statistically significant difference in terms of the total number of retrieved and MII oocytes as well as the total numbers of obtained and top-quality embryos, and cryopreserved embryos was found.
Conclusion
The co-treatment of letrozole with gonadotropins during the antagonist protocol was associated with a reduction in the total dose of gonadotropins, although it had no effect on the oocyte or embryo yield in patients with endometriosis.
Trial registration
The study was registered in the Iranian Registry of Clinical Trials on 2018 -07-13 (IRCT20150310021420N4 at www.irct.ir, registered while recruiting).
Similar content being viewed by others
Background
In recent years, one of the most important goals of assisted reproductive cycles (ART) in women is to focus on optimizing the method of ovarian stimulation with the least destructive effect on the endometrium. Individualization of ovarian stimulation protocol based on the patient’s age, ovarian reserve tests, the cause of infertility, and the previous treatment history can allow for a safer and more effective ART practice [1]. One of the causes of infertility that has been reported to have negative effects on both the ovaries and the endometrium is the diagnosis of endometriosis. It can be associated with ovulation disorders and decreased oocyte quality due to adverse changes in the process of folliculogenesis and steroidogenesis of granulosa cells as well as low-quality embryos, decreased implantation rate, sperm phagocytosis, and toxic environment for fetus due to pelvic adhesions in advanced stages [2,3,4,5]. It is important to select an appropriate ovarian stimulation protocol for these patients, which in turn improves endometrial receptivity.
A type of hormonal manipulation involves the use of aromatase inhibitors (AIs) along with COH standard protocol [6]. The aromatase p450 is a key enzyme in the biosynthesis of estradiol (E2) by ovarian granulosa cells in premenopausal time, whose expression in both eutopic and ectopic endometrial tissue in patients with endometriosis is significantly higher than in non-endometriosis women [7]. Abnormal expression of aromatase leads to the production of estrogen locally at the site of implantation of ectopic endometrial cells. Since endometriosis is an estrogen-dependent disease, AIs appear to be good candidates for the treatment of endometriosis [7,8,9,10]. The third generation of Als, mainly letrozole (LZ) is a selective and non-steroidal AI which is superior to clomiphene citrate for ovulation induction in patients with polycystic ovary syndrome [11]. Lu et al. in vivo study showed that LZ significantly reduces E2 production and aromatase p450 gene expression in luteinized granulosa cells belonging to women with advanced stages of endometriosis [12].
Recently, the beneficial effects of adjuvant therapy with LZ in GnRH antagonist-controlled ovarian stimulation (COS) protocol have been reported in patients with poor ovarian response [13, 14]. Some studies have shown favorable effects of AIs in the treatment and prevention of recurrence of pain and other complications of endometriosis [15]. Furthermore, Miller et al. proposed this hypothesis that lack of endometrial ανβ3 integrin expression is associated with a poor prognosis for IVF in endometriosis patients that might be improved with LZ co-treatment [16]. Only one retrospective study has evaluated the use of LZ in combination with gonadotropin on IVF outcomes in endometriosis patients and concluded that the combination therapy with LZ and gonadotropin produces similar oocyte and embryo yield to the conventional IVF protocol in women with endometriosis [16].
Since the use of AIs for improvement of infertility treatment in women with endometriosis is an interesting subject and clinical trial studies are still necessary for this area, The researchers designed a randomized clinical trial to evaluate the effect of adding LZ to the antagonist ovarian stimulation protocol on IVF outcome in endometriosis patients.
Methods
This randomized clinical trial (RCT) was carried out in the department of infertility treatment in Arash Women’s Hospital from May 2018 to May 2021. The women in the age range of 18 to 42 years old with endometriosis diagnosis who underwent in vitro fertilization/intracytoplasmic sperm injection (IVF/ICSI) cycles were evaluated. Endometriosis diagnosis was defined according to sonographic evaluation by two experienced sonographers or the pathologic result of the previous laparoscopy. Endometriosis staging is done according to Enzian classification by sonographic features (TVS and endo-anal ultrasound) done by 2 expert radiologists) [17, 18].
The patients with body mass index > 25 kg/m2, diminished ovarian reserve (i.e., antral follicle count (AFC) < 5 follicles or anti-Mȕllerian hormone (AMH) < 1.1 ng/ml), donor/recipient or surrogacy treatments, metabolic, or endocrine disorders including (diabetes, hypo/hyperthyroidism, hyperprolactinemia, hypothalamic amenorrhea, etc.), immunologic diseases (lupus, rheumatoid arthritis, antiphospholipid syndrome, cardiovascular, liver and kidney disease), congenital uterine anomalies and endometrial cavity disorders (Asherman syndrome, myoma, polyps, etc.), recurrent IVF failures (more than three consecutive failures and azoospermic male partner were not included in the study.
The eligible patients on 2nd or 3rd day of the menstrual cycle were allocated into two groups randomly by stratified (based on the polycystic ovary syndrome (PCOS) diagnosis) block randomization method. The random allocation list for patients was solely available to the epidemiologist and the number of blocks was considered 6. The type of study group was written on 72 cards, respectively, and then placed inside sealed envelopes. When the physician announced the eligibility of a patient, the methodologist provided the doctor with the envelope. The random allocation process and type of intervention were concealed from the assessor of the final outcome and also the data analyzer.
The same controlled ovarian stimulation protocol (a flexible regimen of GnRH-antagonist) was used for all study populations. The ovarian quiescence was confirmed by documenting the absence of ovarian cyst or lead follicle > 10 mm and the serum E2 concentrations < 50 pg/mL through baseline ultrasounds and hormonal assessment which were performed on the 2nd or 3rd day of the menstrual cycle. In the experimental (LZ group), the patients received 5 mg LZ (Letrofem®; Iran hormone, Tehran, Iran) for the first 5 days of ovarian stimulation with 150 IU of recombinant human FSH (Cinnal-f, Cinagen), In the control group, the patients received only 150 IU of rFSH. The follicular maturation monitoring was done by serial vaginal ultrasound (sonographic device: Phillips, affinity 70) assessments. According to the ovarian response in each patient, the dosage of gonadotropins was adjusted. The administration of GnRH antagonist (Cetrotide ®, Serono International, Geneva, Switzerland) (0.25 mg/day subcutaneously) was initiated when follicle(s) ≥ 13 mm in average diameter were observed, and it was continued until the day of final oocyte triggering. When at least two follicles measuring ≥ 18 mm in diameter were observed, the final stage of oocyte maturation was induced by two doses of recombinant hCG (250 μg) (Ovitrelle; Merck Serono). Transvaginal ultrasound-guided oocyte retrieval was performed 34–36 h after final oocyte triggering. The serum levels of estradiol and progesterone were measured on the day of oocyte trigger and if the amount of progesterone was more than 1 ng/ml, the plan for freezing all the embryos was made.
In vitro fertilization and/or intracytoplasmic sperm injection (IVF/ICSI) was performed with ejaculated sperm to metaphase II (MII) oocytes through standard procedure. The obtained Embryos were cultured in a commercially available culture medium until the day of transfer. Embryo quality was determined according to the number and regularity of blastomeres and the degree of embryonic fragmentation. Two or 3 days after oocyte retrieval, a maximum of two good-quality embryos at the cleavage stage were transferred under ultrasound scan guidance by a catheter (Guardia™, Access ET Catheter, Cook Medical). The luteal phase was supported by 400 mg vaginal progesterone suppository twice daily (Cyclogest, Actavis, Barnstaple, UK) starting on the evening of the oocyte retrieval and it was continued for 10 weeks in cases with a positive pregnancy test. A serum ß-hCG analysis was done 14 days after the ET, and the clinical pregnancy (presence of gestational sac with heartbeat) was documented by ultrasound scan four weeks later. Ongoing pregnancy was considered when the pregnancy was continued over 20 weeks of gestation.
Statistical analysis
The primary outcomes in the present study were the number of oocytes retrieved, the number of MII oocytes, total number and quality of obtained embryos. The secondary outcomes were clinical pregnancy and live birth rates. The Statistical Package for the Social Sciences, version 22, SPSS Inc, Chicago, IL, USA (SPSS) was used for the statistical analysis. The Kolmogorov–Smirnov test was applied to detect the normality of quantitative variables and it was determined that all of these variables had normal distribution. The independent Student t-test and chi-square test were used for the comparison of the quantitative and qualitative variables between groups respectively. The descriptive data were presented as mean ± standard deviation (SD) or number (percent). The statistical significance level was considered as p value < 0.05. The sample size was estimated based on Kim et al. (a retrospective study) using NCSS-PASS software (version 2007; NCSS Inc., Kaysville, UT, USA) and it was determined that 70 subjects were needed in each study group considering α = 0.05, and 80% power. However, in the sampling process, we found that the number of patients with a diagnosis of endometriosis who have a normal ovarian reserve and consented to participate in the study was very limited. Due to the long duration of the project, it was decided to end the study; since this study is one of the first RCTs in patients diagnosed with endometriosis, it can be reported as a pilot with a minimum sample size according to Julious’s study [19].
Results
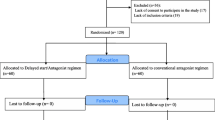
Among 124 women who were screened during the study period, 70 were eligible and enrolled in the study after obtaining their informed consent (35 patients in each group), after follow-up finally the result of treatment cycles were compared between groups (Fig. 1). The baseline characteristics and hormonal profiles of the patients are illustrated in Table 1. The analysis showed that there was no significant difference in terms of women’s age and BMI, duration and type of infertility, PCOS diagnosis, and AFC as well as basal serum levels of LH and FSH, serum AMH and, TSH between groups. There was no significant difference in the type and severity of endometriosis between groups (P = 0.528 and P = 0.405).
The outcomes of the ovarian stimulation cycle are compared between groups in Table 2. The means of total used gonadotropins ampoules and serum E2 level on oocyte trigger day in the LZ group were significantly lower than that of the control group (P = 0.03 and P = 0.004, respectively); however, the duration of ovarian stimulation was similar in two groups (P = 0.58). The analysis indicated that the two groups had no statistically significant difference in terms of the number of follicles with 14–17 mm in diameter at trigger, total number of retrieved and MII oocytes as well as total number of obtained embryos, number of top-quality and total number of cryopreserved embryos.
In the following, the number of cases with fresh and frozen embryo transfers was comparable between groups. It is worth noting that 10 (29.4%) cases of the patients in the intervention group and 9 (30%) patients in the control group had not been referred for the frozen embryo transfer until the manuscript preparation time.
Discussion
The use of LZ as an adjunct in GnRH-antagonist ovarian stimulation protocol for infertile women with endometriosis was associated with a significantly lower dosage of administrated gonadotropins compared with the standard GnRH-antagonist protocol. The trend toward improvement in the total number of retrieved and metaphase II oocytes as well as the total number of obtained embryos was observed in the LZ group; however, it was not statistically significant. Interestingly, in the follow-up after ET, the clinical pregnancy rate was significantly higher than the control group.
Until now, some studies have been designed and conducted to investigate the effect of LZ as an adjunctive treatment in the COS protocol in patients with poor ovarian [11, 13, 20,21,22,23,24], normal [25, 26], and high responses [27] as well as in patients with breast cancer for fertility preservation [28]. The reported results in various studies have been conflicting. Bülow et al. in a meta-analysis study concluded that co-administration of LZ in IVF cycles in patients with a poor ovarian response may be associated with improved outcomes; however, studies regarding normal patients or high responders are limited, and further randomized clinical trials are required in this field [29]. The LZ increases ovarian response to stimulation protocol through mediation in reducing serum estrogen levels and temporary rising in intraovarian androgen concentrations that cause prolongation of the follicular phase, enhance the affinity of FSH receptors, preantral and antral follicle growth [30, 31]. Besides, the reduced serum E2 concentration attributed to LZ may justify the negative impact of excessive E2 levels on oocyte quality and endometrial receptivity in ART cycles [21]. In line with the results of the present study, Eftekhar et al., in a clinical trial reported that co-treatment of LZ with gonadotropins reduced the total amount of consumed gonadotropin in patients with normal ovarian reserve; however, it did not improve the pregnancy outcomes [25]. In a similar way, Haas et al. demonstrated that co-treatment with LZ improves the IVF outcome in normal responders in terms of the increased number of obtained blastocysts without changing the pregnancy rate or the risk of OHSS [26]. Elsewhere, et al., reported that total dose of administrated rFSH and risk of OHSS were significantly decreased in patients with male factor infertility who received LZ as an adjunct to gonadotropins. Furthermore, Yang and colleagues in a pilot RCT, concluded that LZ supplementation could not reduce the incidence of the premature rising of progesterone during the late follicular phase in patients with expected high ovarian response to standard stimulated IVF cycles, which was associated with producing a harmful effect on the pregnancy outcome. Finally, Bülow et al., in a multicenter double-blinded RCT evaluated 129 women with expected normal ovarian reserve to this question of whether LZ supplementation during COS with gonadotropins for IVF reduces the proportion of women with premature progesterone levels above 1.5 ng/ml at the time of final oocyte maturation triggering?. The results of their study showed that although the use of LZ has no impact on the proportion of women with a premature rise in progesterone on the day of oocyte triggering, the increased progesterone in the mid-luteal phase due to LZ may contribute to optimizing the luteal phase endocrinology [32]. The effect of LZ on increasing androgens and reducing gonadotropin consumption might be applied in poor responders. It was concluded that the impact of LZ on implantation and pregnancy outcomes should be assessed in a meta-analysis or larger RCT [32].
Based on our knowledge, the studies that are specific to patients diagnosed with endometriosis are limited. Recently, Kim et al., in a retrospective study, compared IVF outcomes of 38 patients who received standard COS protocol along with co-treatment of LZ versus 26 patients with standard COS protocol alone. It was concluded that the combination therapy with LZ and gonadotropin was associated with a significantly lower peak estradiol level and similar oocyte and embryo yield to the conventional IVF protocol in endometriosis patients [16]. In agreement with their study, we also did not find a positive effect of LZ on the number and quality of oocytes and embryos. Although in the follow-up of pregnancies in the LZ group, the clinical pregnancy rate per total embryos transferred cycles was higher than the control group, due to the fact that the majority of embryo transfers were of the frozen type, we could not comment regarding the effect of LZ on the implantation and pregnancy rates. Based on the results obtained from previous studies in patients with different causes of infertility, it is likely that LZ through the mentioned mechanisms can have a positive effect on reducing the duration of stimulation or the total dose of consumed gonadotropin and in some cases has accompanied by increasing the number of MII oocytes and/or high quality of embryos. However, the conclusion about its effect on the pregnancy rate requires RCT studies with larger sample sizes.
The study has a strong point in that it is designed as a randomized clinical trial. The present study had limitations that should be mentioned, considering that the eligible population had a low prevalence, the number of subjects collected in the time frame considered for the study was less than the estimated sample size, so the study was terminated as a pilot trial. On the other hand, the number of cases of all-freeze embryos in both groups was high due to various reasons, including OHSS at risk, the spread of COVID-19 disease, or the personal preference of the patients, so we have no data to report about the effect of letrozole on the implantation rate in fresh embryo transfer cycles. We suggest that future studies be designed with the primary aim of investigating the rates of implantation and clinical pregnancy in patients with endometriosis.
The current pilot study indicated that the co-treatment of letrozole with gonadotropins during the antagonist protocol was associated with a reduction in the total dose of gonadotropins, although it had no effect on the oocyte or embryo yield, more studies are necessary to determine its impact on the rate of implantation in fresh embryo transfer cycles in patients with endometriosis.
Availability of data and materials
The datasets used or analyzed during the current study are available from the corresponding authors on reasonable request.
Abbreviations
- ART:
-
Assisted reproduction technologies
- AMH:
-
Anti-müllerian hormone
- AIs:
-
Aromatase inhibitors
- COS:
-
Ovarian stimulation protocol
- E2: :
-
Estradiol
- FSH:
-
Follicle stimulating hormone
- GnRH:
-
Gonadotropin-releasing hormone
- hCG:
-
Human chorionic gonadotropin
- IVF:
-
In-vitro fertilization
- ICSI:
-
Intracytoplasmic sperm injection
- LZ:
-
Letrozole
- RCT:
-
Randomized clinical trial
- LH:
-
Luteinizing hormone
- SD:
-
Standard deviation
References
La Marca A, Sunkara SK (2014) Individualization of controlled ovarian stimulation in IVF using ovarian reserve markers: from theory to practice. Hum Reprod Update 20(1):124–140
Gupta S, Goldberg JM, Aziz N, Goldberg E, Krajcir N, Agarwal A (2008) Pathogenic mechanisms in endometriosis-associated infertility. Fertil Steril 90(2):247–257
Xu B, Guo N, Zhang XM, Shi W, Tong XH, Iqbal F et al (2015) Oocyte quality is decreased in women with minimal or mild endometriosis. Sci Rep 5(1):1–8
Sanchez AM, Viganò P, Quattrone F, Pagliardini L, Papaleo E, Candiani M et al (2014) The WNT/β-catenin signaling pathway and expression of survival promoting genes in luteinized granulosa cells: endometriosis as a paradigm for a dysregulated apoptosis pathway. Fertil Steril 101(6):1688–1696
Lessey BA, Kim JJ (2017) Endometrial receptivity in the eutopic endometrium of women with endometriosis: it is affected, and let me show you why. Fertil Steril 108(1):19–27
Lee K-H, Kim C-H, Suk H-J, Lee Y-J, Kwon S-K, Kim S-H et al (2014) The effect of aromatase inhibitor letrozole incorporated in gonadotrophin-releasing hormone antagonist multiple dose protocol in poor responders undergoing in vitro fertilization. Obstet Gynecol Sci 57(3):216–222
Noble LS, Simpson ER, Johns A, Bulun SE (1996) Aromatase expression in endometriosis. J Clin Endocrinol Metab 81(1):174–179
Bulun SE, Zeitoun KM, Takayama K, Sasano H (2000) Molecular basis for treating endometriosis with aromatase inhibitors. Hum Reprod Update 6(5):413–418
Attar E, Bulun SE (2006) Aromatase inhibitors: the next generation of therapeutics for endometriosis? Fertil Steril 85(5):1307–1318
Hu S, Yu Q, Wang Y, Wang M, Xia W, Zhu C (2018) Letrozole versus clomiphene citrate in polycystic ovary syndrome: a meta-analysis of randomized controlled trials. Arch Gynecol Obstet 297(5):1081–1088
Goswami S, Das T, Chattopadhyay R, Sawhney V, Kumar J, Chaudhury K et al (2004) A randomized single-blind controlled trial of letrozole as a low-cost IVF protocol in women with poor ovarian response: a preliminary report. Hum Reprod 19(9):2031–2035
Lu X, Wu Y, Gao X-H, Wang Y-W, Wang L, Sun X-X (2012) Effect of letrozole on estradiol production and P450 aromatase messenger RNA expression of cultured luteinized granulosa cells from women with and without endometriosis. Fertil Steril 98(1):131–135
Moini A, Lavasani Z, Kashani L, Mojtahedi MF, Yamini N (2019) Letrozole as co-treatment agent in ovarian stimulation antagonist protocol in poor responders: a double-blind randomized clinical trial. Int J Reprod BioMed 17(9):653
Ferrero S, Gillott DJ, Venturini PL, Remorgida V (2011) Use of aromatase inhibitors to treat endometriosis-related pain symptoms: a systematic review. Reprod Biol Endocrinol 9(1):1–10
Miller PB, Parnell BA, Bushnell G, Tallman N, Forstein DA, Higdon HL III et al (2012) Endometrial receptivity defects during IVF cycles with and without letrozole. Hum Reprod 27(3):881–888
Kim SJ, Choo CW, Kim SK, Lee JR, Jee BC, Suh CS et al (2020) The effects of letrozole on women with endometriosis undergoing ovarian stimulation for in vitro fertilization. Gynecol Endocrinol 36(3):257–260
Keckstein J, Hudelist G (2021) Classification of deep endometriosis (DE) including bowel endometriosis: From r-ASRM to# Enzian-classification. Best Pract Res Clin Obstet Gynaecol 71:27–37
Keckstein J, Saridogan E, Ulrich UA, Sillem M, Oppelt P, Schweppe KW et al (2021) The Enzian classification: a comprehensive non-invasive and surgical description system for endometriosis. Acta Obstet Gynecol Scand 100(7):1165–1175
Julious SA (2005) Sample size of 12 per group rule of thumb for a pilot study. Pharm Stat J Appl Stat Pharm Ind 4(4):287–291
Abdel Mohsen I, Ezz El Din R (2013) Minimal stimulation protocol using letrozole versus microdose flare up GnRH agonist protocol in women with poor ovarian response undergoing ICSI. Gynecol Endocrinol 29(2):105–8
Bastu E, Buyru F, Ozsurmeli M, Demiral I, Dogan M, Yeh J (2016) A randomized, single-blind, prospective trial comparing three different gonadotropin doses with or without addition of letrozole during ovulation stimulation in patients with poor ovarian response. Eur J Obstet Gynecol Reprod Biol 203:30–34
Ozmen B, Sönmezer M, Atabekoglu CS, Olmuş H (2009) Use of aromatase inhibitors in poor-responder patients receiving GnRH antagonist protocols. Reprod Biomed Online 19(4):478–485
Lee VCY, Chan CCW, Ng EHY, Yeung WSB, Ho PC (2011) Sequential use of letrozole and gonadotrophin in women with poor ovarian reserve: a randomized controlled trial. Reprod Biomed Online 23(3):380–388
Ebrahimi M, Akbari-Asbagh F, Ghalandar-Attar M (2017) Letrozole+ GnRH antagonist stimulation protocol in poor ovarian responders undergoing intracytoplasmic sperm injection cycles: an RCT. Int J Reprod BioMed 15(2):101
Eftekhar M, Saeed L (2020) Effect of adding letrozole to gonadotropin on in vitro fertilization outcomes: an RCT. Int J Reprod BioMed 18(4):287
Haas J, Bassil R, Meriano J, Samara N, Barzilay E, Gonen N et al (2017) Does daily co-administration of letrozole and gonadotropins during ovarian stimulation improve IVF outcome? Reprod Biol Endocrinol 15(1):1–5
Yang X, Lin G, Lu G, Gong F (2019) Letrozole supplementation during controlled ovarian stimulation in expected high responders: a pilot randomized controlled study. Reprod Biol Endocrinol 17(1):1–8
Bonardi B, Massarotti C, Bruzzone M, Goldrat O, Mangili G, Anserini P et al (2020) Efficacy and safety of controlled ovarian stimulation with or without letrozole co-administration for fertility preservation: a systematic review and meta-analysis. Front Oncol 10:574669
Bülow NS, Holt MD, Skouby SO, Petersen KB, Englund ALM, Pinborg A, et al (2021) Co-treatment with letrozole during ovarian stimulation for IVF/ICSI–a systematic review and meta-analysis. Reprod BioMed Online 44(4):717–736. https://doi.org/10.1016/j.rbmo.2021.12.006
Garcia-Velasco JA, Moreno L, Pacheco A, Guillén A, Duque L, Requena A et al (2005) The aromatase inhibitor letrozole increases the concentration of intraovarian androgens and improves in vitro fertilization outcome in low responder patients: a pilot study. Fertil Steril 84(1):82–87
Mitwally MF, Casper RF, Diamond MP (2005) The role of aromatase inhibitors in ameliorating deleterious effects of ovarian stimulation on outcome of infertility treatment. Reprod Biol Endocrinol 3(1):1–45
Bülow NS, Skouby SO, Warzecha AK, Udengaard H, Andersen CY, Holt MD et al (2022) Impact of letrozole co-treatment during ovarian stimulation with gonadotrophins for IVF: a multicentre, randomized, double-blinded placebo-controlled trial. Hum Reprod 37(2):309–321
Acknowledgements
We would like to thank all the participants and co-workers at Arash Women’s Hospital for their assistance in this study.
Funding
The study did not have any funding support.
Author information
Authors and Affiliations
Contributions
M.FM. and A.M. designed the research. M.FM and L.K. contributed to patient selection, data collection, interpretation of data, and manuscript editing. A.M. and M.FM wrote the manuscript. T.M. assisted in the analysis of the data. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Institutional Review Boards and the Ethics Committees of Tehran University of Medical Sciences approved this study (approval code: IR.TUMS.MEDICINE.REC.1398.186). All procedures performed in studies involving human participants were in accordance with the ethical standards of the 1964 Helsinki Declaration and its later amendments. The eligible patients signed written informed consent forms prior to participation in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mojtahedi, M.F., Moini, A., Kashani, L. et al. The effect of letrozole as an adjunct in GnRH-antagonist protocol on IVF/ICSI outcome in women with endometriosis: a randomized clinical trial. Middle East Fertil Soc J 28, 26 (2023). https://doi.org/10.1186/s43043-023-00153-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43043-023-00153-7