Abstract
Background
Gorlin syndrome (GS) is a rare genetic disorder inherited in an autosomal dominant manner caused by genetic variants in PTCH1, SUFU, or PTCH2 genes. It is characterized by multiple basal cell carcinomas, odontogenic keratocysts, skeletal abnormalities, and predisposition to neoplasms.
Case presentation
A novel splice-site variant in the PTCH1 gene, c.3549+1G>T classified as pathogenic, was identified in a patient with a phenotype compatible with GS (multiple basal cell carcinomas and skeletal malformations).
Conclusions
This case contributes to expand the spectrum of identified variants in Gorlin syndrome increasing knowledge about molecular bases and the diagnosis approach of this syndrome.
Similar content being viewed by others
Background
Gorlin syndrome (GS), also known as Nevoid basal cell carcinoma syndrome (NBCCS) (OMIM#109400), is a rare genetic disorder characterized by systemic developmental abnormalities and predisposition to neoplasms. It was first described by RJ Gorlin in 1960 [1] by linking different reported cases of patients with multiple nevoid basal cell epitheliomas, jaw cysts, and bifid rib. The estimated prevalence of GS varies from 1/30.827 to 1/256.000 without differences between sex [2,3,4,5]. Currently, this prevalence is probably underestimated; however, it is expected that the real prevalence will be higher once molecular techniques are implemented as part of the definitive diagnostic.
GS is characterized by multiple basal cell carcinomas (BCCs) usually present from the third decade of life and/or odontogenic keratocysts frequently beginning in the second decade of life. Skeletal abnormalities (such as, fusion of vertebrae, wedge-shaped vertebrae, bifid or fused ribs, or kyphoscoliosis), palmoplantar pits, and intracranial ectopic calcifications (particularly in the falx) are also frequent. Some individuals manifest facial dysmorphism characterized by macrocephaly, frontoparietal bossing, hypertelorism, cleft lip/palate, coarse facial features, or facial milia. Ocular anomalies and/or lymphomesenteric cysts are also described [3, 5,6,7,8]. A predisposition to a wide spectrum of malignant or benign tumours such as medulloblastoma (majority the desmoplastic subtype) [9], meningioma, or cardiac and ovarian fibroma is observed [10].
Genetic variants in PTCH1 (OMIM*601309) [11, 12], SUFU (OMIM*607035) [13], and PTCH2 (OMIM*603673) [14, 15] have been defined as the molecular cause of GS. These genes encode regulatory proteins of the Hedgehog (Hh) signalling pathway involved in embryonic development, cell proliferation, and differentiation and tumorigenesis control. In adults, Hh pathway is inactive except for its function in tissue repair and maintenance. Mutations in these regulatory proteins lead to constitutive activation of the signalling pathway and development of GS and others sporadic cancers and developmental anomalies [16, 17].
Pathogenic variants in the PTCH1 gene are the main molecular defects associated with GS. The detection rate of PTCH1 variants in GS patients varies considerably between studies, ranging from 40 to 85% [18,19,20,21], with the highest rates corresponding to the most current studies. Pathogenic variants in SUFU are the second cause of GS and are associated with an increased risk of developing medulloblastoma, meningioma, or ovarian fibroma, but a lower risk of jaw cysts [20, 21]. Variants in the PTCH2 gene have only been found in sporadic cases [14, 15].
GS is inherited in an autosomal dominant manner with complete penetrance and variable expressivity [22]. Approximately 70–80% of the individuals diagnosed with GS have an affected parent and only 20–30% of the cases present de novo pathogenic variants [22].
The diagnosis of GS is established based on clinical criteria differentiating major and minor criteria [22,23,24] (Table 1). The diagnosis is established when two major criteria or one major and two minor criteria are fulfilled [23].
Despite compliance diagnostic criteria, molecular studies should be performed to establish a definitive diagnosis. Sequence analysis is recommended as a first-line technique since it allows to establish genetic diagnosis in about 60% of the patients with clinical features of GS [20, 21]. However, approximately 6–16% of the patients with GS compatible phenotype present large deletions/duplications in PTCH1 [20, 21, 25] and 1% in SUFU [20]. Therefore, it is recommended to establish a deletion/duplication analysis technique, such as multiplex ligation-dependent probe amplification (MLPA), as a second-line strategy. Despite this serial testing strategy, in around 27% of the patients, a genetic diagnosis cannot be established yet [20, 21].
Nowadays, sequence analysis are typically carried out by next-generation sequence (NGS) technology employing panels or whole-exome sequencing (WES) targeted to GS-related genes. These analyses detect single nucleotide variants (SNVs), short deletions/insertions, and copy number variations (CNVs) in coding regions and adjacent intronic sequences. Implementation of this technology has extended the spectrum of known genetic variants, increasing the diagnostic yield of molecular studies in GS.
Currently, there is no specific treatment for GS and the management of these patients is based on multidisciplinary follow-up focused on treating the different clinical manifestations. However, the definitive genetic diagnosis allows the implementation of certain surveillance recommendations for the prevention of the most common manifestations (avoid UV exposure, minimize diagnostic X-ray, avoid radiation therapy if possible, perform regular BCC screening, perform periodic orthopantomograms to detect keratocysts, or monitor brain circumference in children for early detection of medulloblastoma) [22, 23]. Increasingly, these recommendations are based on the patient's genotype, recommending, for example, regular brain MRI in patients with SUFU variants due to their increased risk of developing medulloblastoma [26]. Genetic diagnosis also allows family and reproductive genetic counselling. Furthermore, the identification of the patient's genotype may allow the application of possible gene therapies in the future.
We present a case of a 50-year-old male with multiple BCCs, jaw keratocysts, and skeletal malformations in whom a novel variant in PTCH1 gene was identified throughout a WES targeted to GS-related genes (PTCH1, SUFU, and PTCH2). This case contributes to expanding the knowledge about molecular basis and diagnosis approach of GS.
Case presentation and genetic findings
We report the clinical case of a 50-year-old male studied by a dermatologist for multiple BCCs located mainly on the face, back, and thoracic region. The biopsy and anatomopathological study of three of them classified two as nodular BCC and one as superficial BCC. The patient is currently under dynamic phototherapy.
As clinical history, the patient described odontogenic cysts in his childhood that required different surgical interventions. X-ray imaging revealed multiple skeletal anomalies including posterior fusion of the third and fourth left costal arches, dorsal butterfly vertebrae (from C7 to D4), mild thoracolumbar scoliosis with slight increase in physiological lumbar lordosis, generalized widening of middle phalanges of feet, and a fifth toe of the left foot with two phalanges. Cranial X-ray also disclosed calcification of the falx. Phenotypically, the patient showed macrocephaly, with frontoparietal protuberance and coarse facial features.
All these clinical findings focused the diagnostic on Gorlin syndrome but a genetic study was requested for a final diagnosis. WES targeted to PTCH1, SUFU, and PTCH2 was conducted using NextSeq™ 500 System (Illumina).
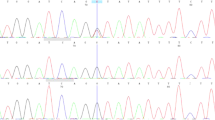
This analysis revealed a heterozygous splice-donor variant (c.3549+1G>T) in PTCH1 gene (NM_000264.5) (Fig. 1). This variant located in intron 21 of PTCH1 gene produces a nucleotide change that affects protein splicing mechanism. Expression studies on PTCH1 mRNA revealed exon 21 skipping that is predicted to result in an absent or disrupted protein product.
Detail of the GeneSystem analysis platform in which the tertiary analysis and prioritization of variants was performed. a Variants identified in PTCH1 gene applying criteria of coverage > 10X and allectic frequency > 20%. b Summary of the characteristics of the identified variant. c IGV detail showing the region of the chromosome 9 gene where the identified nucleotide change variant G > T (complementary sequence C > A shown) is found in all analysed fragments, indicating good horizontal coverage
This variant has not been previously described in databases (ClinVar, dbSNP, HGMD) nor in the reviewed literature related to GS nor in population databases (gnomAD, NHLBI Exome Sequencing Project, 1000 Genomes Project). Nevertheless, in silico prediction studies (BayesDel, EIGEN, FATHMM-MKL or Mutation Taster) indicate impaired protein functionality and similar variants, NM_000264.5(PTCH1):c.3549+1G>A (ClinVar accession: VCV000985872.1) and NM_000264.5(PTCH1):c.3549+2T>G (ClinVar accession: VCV000844500.5), have been classified as probably pathogenic and pathogenic, respectively.
According to the American College of Medical Genetics and Genomics (ACMG) variant classification guidelines [27], the variant is classified as pathogenic, since it meets the following criteria: it is a splice-site variant that can lead to loss of function in a gene where loss of function is a known mechanism of disease considered a very strong criteria of pathogenicity (PVS1), it is absent from controls in NHLBI Exome Sequencing Project, 1000 Genomes Project, or gnomAD considered a moderate criteria of pathogenicity (PM2), and it is predicted to be deleterious in 6 out of 6 in silico prediction systems (BayesDel addAF, BayesDel noAF, EIGEN, EIGEN PC, FATHMM-MKL and Mutation Taster) considered a supporting criteria of pathogenicity (PP3). This deleterious effect on the gene is also predicted by PTCH1 mRNA expression studies.
The presence of pathogenic variants in PTCH1 is associated with Gorlin syndrome (OMIM#109400) and/or Holoprosencephaly type 7 (OMIM#610828). The high compatibility of patient's clinical features with GS and given that the mode of inheritance of the disease is autosomal dominant, the presence of this single heterozygous variant (c.3549+1G>T) in PTCH1 allows genetic diagnosis of Gorlin syndrome. Therefore, we can consider this novel variant (PTCH1 (NM_000264.5):c.3549+1G>T) as a cause of GS.
Discussion
GS is a rare genetic disorder with an autosomal dominant inheritance caused by genetic variants in PTCH1, SUFU, or PTCH2 genes [11, 13, 28]. In this work, we present a new splice-site variant in PTCH1 associated with GS.
GS should be suspected in individuals presenting multiple basal cell carcinomas, ondontogenic keratocysts, and/or skeletal malformations. GS patients can also show facial dysmorphisms like macrocephaly, frontoparietal bossing, hypertelorism, cleft lip/palate, or coarse facial features [3, 5,6,7, 22].
The patient described above presented multiple basal cell carcinomas, odontogenic keratocysts during his childhood, and different skeletal abnormalities (butterfly vertebrae, fused costal arches, calcification of the falx, thoracolumbar scoliosis, and foot malformations). In the morphological examination, macrocephaly, frontoparietal protuberance, and coarse facial features were the most characteristic aspects. Other neoplasms, palmoplantar pits, or ocular anomalies previously described as clinical characteristics [3, 5, 7, 10] were not found.
Diagnosis of GS is based on specific clinical criteria. However, molecular studies are indispensable for the definitive diagnostic.
In this case, WES targeting the PTCH1, SUFU, and PTCH2 genes was performed to confirm the clinical suspicion of GS. The genetic study revealed a novel splice-donor variant (c.3549+1G>T) in intron 21 of PTCH1 gene (NM_000264.5). This variant had not been described previously neither in databases nor in literature. According to the ACMG variant classification guidelines, this variant should be classified as pathogenic.
Most cases of GS are caused by genetic variants in PTCH1. PTCH1, the human homolog of the Drosophila patched-1 gene (NCBI Reference Sequence: NM_000264.5) is located on chromosome 9q22.32. It encodes the protein Patched 1 (PTCH1), a 1447-amino acid transmembrane glycoprotein, which is a component of the Hedgehog (Hh) signalling pathway [16] (Fig. 2).
Simplified model for Hh signalling pathway. a In absence of the Hh ligands, PTCH1 inhibits SMO signalling and GLI transcription factors remain sequestered by SUFU. The Hh signalling pathway is inactive. b In presence of Hh ligands, PTCH1 suppression of SMO is abrogated and SMO signal SUFU to release GLI transcription factors which will activate the Hh target genes
PTCH1 loss of function results in constitutive activation of the Hedgehog pathway and is the main cause of GS development. Different genetic variants in PTCH1 gene are currently known. ClinVar Database [29] lists 2677 unique PTCH1 variants of which 280 have a pathogenic or likely pathogenic clinical significance associated to GS clinical phenotype (date of revision: November 27, 2022) (Fig. 3). 73% of these pathogenic or likely pathogenic variants lead to premature protein truncation by either nonsense or frameshift variants, becoming the main cause of GS following by splice-site variants (17%). These results, in agreement with those previously published by other authors [30, 31], suggest the haploinsufficiency of PTCH1 as main cause of GS development.
The analysis of variants close to the study variant, described in ClinVar [29], LOVD [32], and Simple ClinVar [33] databases (Table 2), shows that most of the variants classified as pathogenic or likely pathogenic produce a premature stop codon or a splicing alteration involving exon 21. This same mechanism produces the study variant and supports its deleterious effect.
The results of the genetic study made it possible to initiate certain surveillance and protection measures to prevent the main manifestations of GS in the patient: increase BCC screening, avoid sun exposure, minimize exposure to ionizing radiation or perform periodic orthopantomogram to detect keratocysts. At the same time, a family study was carried out with the aim of identifying apparently asymptomatic at-risk relatives who could benefit from measures to prevent GS complications.
The patient described in this study was the second of four siblings of non-consanguineous parents and he had no offspring (Fig. 4). None of his relatives presented a GS compatible phenotype. Considering the complete penetrance but the variable expressivity of this disorder, genetic studies were performed on his living first-degree relatives (mother and siblings). The variant presented in the proband was not found in his relatives, suggesting de novo inheritance. A correct family genetic counselling was carried out according to the genetic results.
Improved genetic and molecular techniques are accelerating the identification of new variants in PTCH1 gene, as well as in SUFU and PTCH2 genes. It is expected that in the coming years, new insights into the molecular basis of GS will be revealed, making the diagnosis and genetic counseling of this pathology easier. In this study, we presented a novel splice-site variant of PTCH1 (NM_000264.5(PTCH1):c.3549+1G>T) associated with GS and classified according to the ACMG variants classification guidelines as pathogenic, contributing to expand the spectrum of variants in PTCH1.
Conclusion
Gorlin syndrome is a rare autosomal dominant disorder characterized by multiple basal cell carcinomas, odontogenic keratocysts, skeletal abnormalities, and predisposition to neoplasms. Molecular-genetic studies are the gold standard for its diagnosis. The finding of a heterozygous (c.3549+1G>T) variant in PTCH1 gene in a patient with clinical features consistent with the OMIM phenotype of Gorlin syndrome allowed us to consider this variant as a cause of GS. These findings expand the spectrum of variants in PTCH1 and show the decisive role of genetic studies in the diagnosis of this syndrome.
Availability of data and materials
All data are available upon request.
References
Gorlin RJ, Goltz RW (1960) Multiple nevoid basal-cell epithelioma, jaw cysts and bifid rib. A syndrome. N Engl J Med 262:908–912
Evans DG, Howard E, Giblin C et al (2010) Birth incidence and prevalence of tumor-prone syndromes: estimates from a UK family genetic register service. Am J Med Genet A 152A:327–332
Shanley S, Ratcliffe J, Hockey A et al (1994) Nevoid basal cell carcinoma syndrome: review of 118 affected individuals. Am J Med Genet 50:282–290
Endo M, Fujii K, Sugita K et al (2012) Nationwide survey of nevoid basal cell carcinoma syndrome in Japan revealing the low frequency of basal cell carcinoma. Am J Med Genet A 158A:351–357
Lo Muzio L, Nocini PF, Savoia A et al (1999) Nevoid basal cell carcinoma syndrome. Clinical findings in 37 Italian affected individuals. Clin Genet 55:34–40
Lo Muzio L (2008) Nevoid basal cell carcinoma syndrome (Gorlin syndrome). Orphanet J Rare Dis 3:1–16. https://doi.org/10.1186/1750-1172-3-32
Kimonis VE, Singh KE, Zhong R et al (2013) Clinical and radiological features in young individuals with nevoid basal cell carcinoma syndrome. Genet Med 15:79–83
Fernández LT, Ocampo-Garza SS, Elizondo-Riojas G, et al. Basal cell nevus syndrome: an update on clinical findings. Int J Dermatol [Internet]. 2021 [cited 2022 May 19]. Available from: https://pubmed.ncbi.nlm.nih.gov/34494262/.
Amlashi SFA, Riffaud L, Brassier G et al (2003) Nevoid basal cell carcinoma syndrome: relation with desmoplastic medulloblastoma in infancy. A population-based study and review of the literature. Cancer 98:618–624
Guerrini-Rousseau L, Smith MJ, Kratz CP et al (2021) Current recommendations for cancer surveillance in Gorlin syndrome: a report from the SIOPE host genome working group (SIOPE HGWG). Fam Cancer 20:317–325
Johnson RL, Rothman AL, Xie J et al (1996) Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science 272:1668–1671
Hahn H, Wicking C, Zaphiropoulos PG et al (1996) Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell 85:841–851
Pastorino L, Ghiorzo P, Nasti S et al (2009) Identification of a SUFU germline mutation in a family with Gorlin syndrome. Am J Med Genet A 149A:1539–1543
Fan Z, Li J, Du J et al (2008) A missense mutation in PTCH2 underlies dominantly inherited NBCCS in a Chinese family. J Med Genet 45:303–308
Fujii K, Ohashi H, Suzuki M et al (2013) Frameshift mutation in the PTCH2 gene can cause nevoid basal cell carcinoma syndrome. Fam Cancer 12:611–614
Skoda AM, Simovic D, Karin V et al (2018) The role of the Hedgehog signaling pathway in cancer: a comprehensive review. Bosn J basic Med Sci 18:8–20
Otsuka A, Levesque MP, Dummer R et al (2015) Hedgehog signaling in basal cell carcinoma. J Dermatol Sci 78:95–100
Marsh A, Wicking C, Wainwright B et al (2005) DHPLC analysis of patients with Nevoid Basal Cell Carcinoma Syndrome reveals novel PTCH missense mutations in the sterol-sensing domain. Hum Mutat 26:283
Kimonis VE, Mehta SG, DiGiovanna JJ et al (2004) Radiological features in 82 patients with nevoid basal cell carcinoma (NBCC or Gorlin) syndrome. Genet Med 6:495–502
Smith MJ, Beetz C, Williams SG et al (2014) Germline mutations in SUFU cause Gorlin syndrome-associated childhood medulloblastoma and redefine the risk associated with PTCH1 mutations. J Clin Oncol 32:4155–4161
Evans DG, Oudit D, Smith MJ et al (2017) First evidence of genotype-phenotype correlations in Gorlin syndrome. J Med Genet 54:530–536
Evans DG, Fardon PA. Nevoid basal cell carcinoma syndrome—GeneReviews®—NCBI Bookshelf [Internet]. 2002 [cited 2022 May 19]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1151/.
Bree AF, Shah MR (2011) Consensus statement from the first international colloquium on basal cell nevus syndrome (BCNS). Am J Med Genet Part A 155:2091–2097
Jones EA, Sajid MI, Shenton A et al (2011) Basal cell carcinomas in gorlin syndrome: a review of 202 patients. J Skin Cancer 2011:1–6
Nagao K, Fujii K, Saito K et al (2011) Entire PTCH1 deletion is a common event in point mutation-negative cases with nevoid basal cell carcinoma syndrome in Japan. Clin Genet 79:196–198
Foulkes WD, Kamihara J, Evans DGR et al (2017) Cancer surveillance in Gorlin syndrome and rhabdoid tumor predisposition syndrome. Clin Cancer Res 23:e62
Richards S, Aziz N, Bale S et al (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17:405–424
Smyth I, Narang MA, Evans T et al (1999) Isolation and characterization of human patched 2 (PTCH2), a putative tumour suppressor gene inbasal cell carcinoma and medulloblastoma on chromosome 1p32. Hum Mol Genet. 8:291–297
PTCH1[gene]—ClinVar—NCBI [Internet]. [cited 2022 Jul 31]. Available from: https://www.ncbi.nlm.nih.gov/clinvar/?gr=0.
Onodera S, Nakamura Y, Azuma T (2020) Gorlin syndrome: recent advances in genetic testing and molecular and cellular biological research. Int J Mol Sci 21:7559
Reinders MG, van Hout AF, Cosgun B et al (2018) New mutations and an updated database for the patched-1 (PTCH1) gene. Mol Genet Genomic Med. 6:409–415
The PTCH1 gene homepage—Global Variome shared LOVD [Internet]. [cited 2022 Jul 31]. Available from: https://databases.lovd.nl/shared/genes/PTCH1.
Simple ClinVar [Internet]. [cited 2022 Jul 31]. Available from: https://simple-clinvar.broadinstitute.org/.
Acknowledgements
To the patient.
Funding
No benefits or funds were received in support of this study.
Author information
Authors and Affiliations
Contributions
AJGM was the principal physician in the patient’s case. RGT, with the collaboration of PCR, carried out the genetic diagnosis and genetic counselling. PCR drafted the manuscript with input and critical review from RGT. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for publication.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Conde-Rubio, P., García-Malinis, A.J., Salvador-Rupérez, E. et al. A novel pathogenic splice-site variant in the PTCH1 gene c.3549+1G>T, associated with Gorlin syndrome: a case report. Egypt J Med Hum Genet 24, 83 (2023). https://doi.org/10.1186/s43042-023-00463-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43042-023-00463-5