Abstract
Background
Hepatocellular carcinoma (HCC) is a common, serious malignancy with a dismal prognosis. As HCC is frequently missed in its early stages, non-invasive early detection is urgently needed. The purpose of this study was to evaluate the possible utility of circulating miRNA-29b1, matrix metalloproteinases (MMPs)-2 and -9 mRNAs, and proteins as diagnostic and predictive biomarkers for HCC.
Subjects and methods
This study included 92 subjects, including 52 patients with HCC at various stages and grades and 40 healthy subjects as controls. RT-PCR was used to detect circulating miRNA-29b1, MMPs-2, and 9 mRNAs, while ELISA was used to detect AFP, MMPs-2, and 9 proteins in the participants’ blood.
Results
When HCC patients were compared to controls, there were significant increases in the levels of MMPs-2, 9 mRNAs, and proteins, and a significant drop in the levels of miRNA-29b1. There were no significant variations in the levels of miRNA-29b1, mRNAs, and MMP-2 and -9 proteins in advanced HCC. There were negative associations between miRNA-29b1 and MMPs-2, 9 mRNAs, and proteins, implying overlapping molecular microRNA-mediated mechanisms that control MMPs that should be investigated further in the future. The levels of miRNA-29b1, MMPs-2, 9 mRNAs, and proteins indicated significant sensitivity and specificity in the early identification of HCC.
Conclusion
MMP-2, 9 mRNAs, and proteins may be employed as diagnostic but not prognostic biomarkers in HCC. miRNA-29b1 may play a protective role in HCC. An overlapping molecular microRNA-29b1-mediated pathway that may control MMPs-2 and 9 requires further experimental investigation in the future.
Similar content being viewed by others
Introduction
Hepatocellular carcinoma (HCC) comprises 75–85% of cases of primary liver cancer worldwide, with [1] high morbidity and mortality, so it is one of the most severe cancers [2]. Over 700,000 deaths/year are due to HCC worldwide [3]. With a very poor prognosis, HCC cannot be detected at an early stage. Early diagnosis of HCC can significantly improve the curative outcome, alleviating the patients' suffering [2]. Clinical methods such as histopathology and imaging can detect HCC at a late stage [4]. Thus, it is urgently needed to detect new non-invasive methods for the early detection of HCC.
Alpha-fetoprotein (AFP) was first discovered as a serum biomarker of HCC in 1960 [5] and remains the most frequent HCC biomarker utilized globally. Even though AFP has a sixty percent sensitivity for diagnosing HCC, its specificity is still poor [6]. Additionally, in 15–30 percent of patients with advanced HCC, serum AFP is normal [7]. The AFP rise has also occurred in benign hepatic diseases such as cirrhosis and hepatitis [8]. As a result, the Practice Guidelines Committee of the American Association for the Study of Liver Diseases (AASLD) no longer recommends AFP for the early identification of HCC [9].
Matrix metalloproteinases (MMPs) are zinc-dependent proteases, classified according to their activity into gelatinases, collagenases, matrilysins, and stromelysins [10]. MMP-2, a collagenase IV with a molecular mass of 72 kDa, is involved in tissue remodeling and embryonic development [11], whereas MMP-9, a collagenase IV with a molecular mass of 92 kDa, is involved in developing malignancies and enhances tumor angiogenesis, progression, invasion, and metastasis [11, 12]. MMP-9 activation occurs mainly via MMP-2. Some studies reported a common mechanism of trans-activation for both [13, 14]. Diminished MMP-9/-2 enzymatic activity is associated with enhanced chemotherapy's anti-proliferation and pro-apoptotic activity in human HCC [15]. The critical role of MMPs in cancer is implicated in the degradation of the extracellular matrix (ECM), enhancing cell invasion and metastasis [10, 16, 17]. An accumulating body of publications revealed a potent correlation between the disrupted expressions of MMPs at the RNA or protein level in many cancers and the poor prognosis of the malignancy [12, 15]. MMP-2 and -9 were directly related to downregulated miRNAs [18]. Phosphatidylinositide-3 (PI3) kinase, MMPs-2 and -9, and other genes associated with cell proliferation and migration were discovered to be downregulated in cancer cells, while other microRNAs were found to be upregulated in the same cancer cells, pointing to the microRNA's indirect regulatory role in modulating MMPs-2 and -9 [19].
Several micro-RNAs (miRNAs) have been related to cancer [20]. The results suggest that miRNA’s role in cancer is enhanced by the presence of about 50% of miRNA genes in genomic regions associated with cancer [21]. Many miRNAs have been involved in the regulation of the MMPs by disrupting the distinct functions of MMPs, such as remodeling of the ECM, angiogenesis, and the transition of epithelial to mesenchymal tissues [22]. The microRNA processing impairment increased the expression of MMPs-2 and -9, enhancing the proliferation and cell invasion of cancer cells [23]. In the human miRNA-29 (mir-29) family, the precursors' locations are chromosome region 7q32 for miR-29a/miR-29b-1 and chromosome region 1q32 for miR-29b-2/miR-29c, but both miR-29b-1 and miR-29b-2 form identical mature miR-29b [24]. MiR-29b directly inhibits MMP-2 expression in vitro, impairing Vascular Endothelial Growth Factor receptor-2 (VEGFR-2) signaling in endothelial cells. Its upregulation suppresses angiogenesis and carcinogenesis in vivo. It decreased the ability of HCC cells to enhance the formation of capillary tube endothelial cells [25]. MiR-29b was reported to be downregulated in many cancers, such as breast cancer, endometrial carcinoma, pancreatic ductal adenocarcinoma, and HCC [26,27,28]. miR-29b administration in mouse xenograft tumor models inhibited cell proliferation, tumor vascularization, invasion, and migration [29]. Furthermore, the induced upregulation of miR-29b-1 in human osteosarcoma cells was associated with a decrease in cell invasion and proliferation [30]. On the contrary, it was reported that miR-29b-knockdown blocked the growth of the bladder cancer cell line [31]. Furthermore, miRNA-29 was considered a crucial gene implicated in the progression of breast cancer (BC). It was reported to be a possible non-invasive biomarker for the detection of BC [32].
This work aimed to compare circulating miRNA-29b1, MMPs-2, and -9 mRNAs and proteins to AFP, the most common biomarker in HCC, and to assess their potential utility as diagnostic and prognostic biomarkers of HCC. To our knowledge, no previous studies have assessed these circulating parameters in HCC.
Subjects and methods
Ethical approval
According to Helsinki Declaration guidelines [33], the study was carried out. Before registration for the study, written consents were obtained from all subjects. The research was approved by the Research Ethical Committee, Faculty of Medicine, Beni-Suef University, with approval number FMBSUREC/12022023/Ammar.
Study design
This case–control study was conducted from February 2023 to June 2023 in the biochemistry and molecular biology department, Faculty of Medicine, Beni-Suef University. Whole blood samples were collected from 92 subjects, including 52 patients with HCC who were attendants of the Tropical Hepato-Gastroenterology Department and Oncology Department at Beni-Suef University Hospital and 40 healthy subjects that served as controls.
Patients’ selection
According to the American Association for the Study of Liver Diseases radiographic criteria for HCC [34], all HCC patients were diagnosed by findings of HCC criteria at least 2 radiological techniques, including abdominal ultrasound, magnetic resonance imaging, contrast-enhanced dynamic computed tomography, hepatic angiography, and/or alpha-fetoprotein (AFP) ≥ 200 μg/mL. Specific features of HCC, such as the number of nodules and size, stage, grade, and metastasis, were obtained to assess their relations and correlations with the studied markers. The exclusion criteria for patients who had secondary or recurrent tumors, a history of HCV infection, HBV infection, other malignant tumors, or were being treated with chemotherapy or radiotherapy were excluded from this study.
Samples collection
There are two aliquots: whole blood for mRNA and serum for circulating miRNA and ELISA. Three milliliters of whole blood were collected in an EDETA tube from each subject for mRNA extraction. Another three milliliters of whole blood were collected in a plain tube from each subject. After coagulation, they were centrifuged for 10 min at 4000 rpm, then 100 μL of the fresh serum was used for miRNA extraction, and the rest was preserved at − 80 °C to measure AFP, MMPs-2, and -9 proteins by ELISA.
Assay of serum protein levels of AFP, MMP-2, and MMP-9
AFP, MMP-2, and MMP-9 proteins were assessed in the serum according to the manufacturer's recommendations of the commercial ELISA kits [Human MMP-2 ELISA Kit (Catalogue No. 201-12-0905, Sunred, China) and Human MMP-9 ELISA Kit (Catalogue No. 201-12-0937B, Sunred, China)].
Isolation of mRNA
According to the manufacturer’s recommendations, FavorPrep Blood/Cultured Cell, Total RNA Mini Kit (Cat. No.: FABRK 001-1, FAVORGEN, Taiwan) was used to isolate mRNAs from whole blood samples.
Isolation of miRNA
According to the manufacturer’s recommendations, isolated total RNA, including miRNA from the serum samples, was purified using the miRNeasy Mini Kit (cat. no. 217004, QIAGEN, Germany). By spectrophotometry (JENWAY, USA), the extracted RNA was quantified at 260 nm.
Reverse transcription of isolated mRNA
According to the manufacturer's instructions, the isolated mRNA was reverse transcribed into cDNA, using TOPscript™ RT DryMIX (dT18/dN6 plus) kit (Cat. No.: RT220, economics, Life Technologies, India) by adding 100 ng of isolated mRNA to the dissolved pellet of the strips, and then the mixture was incubated at 42 °C for 5 min.
Reverse transcription of isolated total RNA, including miRNA
The isolated total RNA, including miRNA, was reverse transcribed into cDNA using the miScript II RT Kit (cat. no. 218161, Qiagen, Valencia, CA). Twenty microliter RT reactions were prepared after adding 1 μg of total RNA. According to the manufacturer's instructions, incubation was at 37 °C for 60 min and at 95 °C for 5 min.
Real-time quantitative PCR using SYBR Green
According to the manufacturer's recommendations, cDNA prepared from mRNA in a reverse transcription reaction, primers of MMPs-2 and MMPs-9, and GAPDH (a housekeeping gene expression) were added to the contents of the Maxima SYBR Green/ROX qPCR Master Mix (2X) kit (Catalog number: K0221, Thermo Scientific™, Lithuania), while cDNA prepared from miRNA in a reverse transcription reaction using miScript HiFlex Buffer served as the template for real-time PCR analysis, which was done using a miScript Precursor Assay which consists of a specific forward and reverse primers for the precursor-miRNA targeting its stem-loop sequence and then added to the contents of the miScript SYBR Green PCR Kit (Cat. No. 218073, Qiagen) with the RNU6, which was used as the reference miRNA.
All primer sequences were designed from GenBank RNA sequences with Tm: 60–65 °C as shown in Table 1, and they were provided by Qiagen, Germany. Before assay preparation, cDNA was diluted 1:5 with nuclease-free H2O in a net volume of 25 μL. Samples were analyzed by the Applied Biosystems StepOne™ Real-Time PCR System (software V.2.0.1) with optimum conditions: a 15-min initial phase at 95 °C, then three stages of 40 cycles for 15 s. at 94 °C, 30 s. at 55 °C, and 30 s. at 70 °C.
Calculation of relative quantification (RQ) (relative expression):
The relative expression levels of mRNA and miRNA were measured by the comparative cycle threshold (Ct) method relative to GAPDH and RNU6 snRNA expressions. mRNA and miRNA expression fold changes were calculated using the 2−ΔΔCT method [35].
Statistical analysis
Statistical analysis was done using SPSS version 23 (SPSS Inc., Chicago, IL, USA). The description of the data was expressed as the Mean ± SD. Post hoc testing was done with the Tukey test to compare the groups' differences. Linear relations between the studied genes were obtained using simple linear correlation (Pearson correlation coefficient test) (r). A P value < 0.05 is considered significant. Receiver operating characteristics curves (ROC curves) were utilized to assess the diagnostic performance of all studied parameters and AFP.
Results
Demographic and clinicopathological data of studied groups
Demographic and clinicopathological data of the studied groups, as shown in Table 2, did not show significant differences between the studied groups or significant correlations with the studied parameters.
The studied parameters in the control and HCC groups
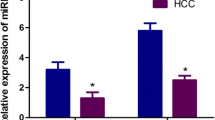
Figure 1a reveals high significant (P < 0.001) increases in the mean levels of circulating MMP-2 protein (366.5 ± 101.5), MMP-9 protein (336 ± 79.5), and AFP (683 ± 1404), whereas Fig. 1b demonstrates high significant (P <0.001) increases in the mean levels of MMP-2 gene expression (2.2 ± 0.46), and MMP-9 gene expression (1.3 ± 0.25) in HCC patients than their corresponding levels in the healthy subjects (231.8 ± 41, 168.9 ± 43.5, 33 ± 12, 1.18 ± 0.42, and 0.94 ± 0.05, respectively). On the contrary, the mean levels of circulating miRNA-29b1 were significantly (P < 0.001) lower in the HCC patient’s serum (0.78 ± 0.05) than in healthy subjects’ serum (0.93 ± 0.040), as shown in Fig. 1b.
The relations between the studied parameters and the signs of cancer progression
As shown in Table 3, the mean levels of miRNA-29b1, AFP, MMPs-2, and 9 gene expressions and proteins showed no significant differences in different grades, stages, and metastasis degrees of HCC.
The correlations between the studied parameters
As shown in Table 4, there were significant (P < 0.001) positive correlations between MMPs-2, 9 gene expressions, and proteins. On the contrary, there were significant (P < 0.001) negative correlations between miRNA-29b1, MMPs-2, and 9 gene expressions and proteins. Furthermore, Table 4 shows a significant (P < 0.001) positive correlation between AFP levels and MMP-2 protein.
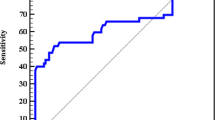
ROC curve analysis of the studied parameters in the prediction of the presence of HCC
As shown in Fig. 2a, MMP-2 protein levels showed AUC = 0.927, 82.7% sensitivity, and 87.5% specificity at the optimum cutoff value ≥ 282.5, MMP-9 protein levels showed AUC = 0.962, 92.3% sensitivity, and 97.5% specificity at the optimum cutoff value ≥ 231, MMP-2 gene expression showed AUC = 0.938, 94.2% sensitivity, and 85% specificity at the optimum cutoff value ≥ 1.43, MMP-9 gene expression showed AUC = 0.983, 90.2% sensitivity, and 100% specificity at the optimum cutoff value ≥ 1.05, and AFP showed AUC = 0.858, 82.7% sensitivity, and 82.5% specificity at the optimum cutoff value ≥ 45. Also, Fig. 2a indicates that MMP-9 gene expression showed the highest AUC and specificity among other biomarkers. According to Fig. 2b, miRNA 29b1 showed AUC = 0.962, 90.4% sensitivity, and 97.5% specificity at the optimum cutoff value ≤ 0.855.
Discussion
HCC is a vascularized tumor with increased active angiogenesis and metastasis responsible for HCC recurrence and poor prognosis [36].
Dysregulation of miRNAs was known to be involved in uncontrolled cell proliferation and anti-apoptosis-enhancing cancer development [20, 37].
Matrix metalloproteinases (MMPs) can degrade the ECM and enhance endothelial cell migration; MMPs are also involved in angiogenesis, tumor growth, invasion, and metastasis [10, 38].
The present study demonstrated significant increases in the mean levels of gene expression and protein levels of both MMP-2 and MMP-9 in HCC patients compared to their corresponding levels in the healthy subjects.
These findings concurred with Yeh et al. [39], who observed higher MMP-9/MMP-2 ratios in patients with HCC than those with chronic liver diseases and controls. Kuyvenhoven et al. [40] agreed with the current results regarding MMP-2 levels in patients with HCC which were higher than controls but comparable with chronic liver disease without HCC. However, they disagreed with the present findings regarding MMP-9 levels in patients with HCC, which were reported to have no significant differences between patients with or without HCC and controls.
The current findings corroborated with those of Murawaki et al. [41], who reported that serum MMP-2 was significantly increased in HCC patients compared to controls.
The present results showed that the mean levels of AFP, miRNA-29b1, MMPs-2, 9 mRNAs, and proteins did not significantly change between the various grades, stages, and degrees of metastasis in HCC.
However, Yeh et al. [39] agreed with the current findings concerning AFP levels, as they reported that they were comparable between early and advanced HCC. In contrast, MMP-9/MMP-2 ratios were higher in advanced HCC than in those in naive HCC.
The current findings concurred with Hayasaka et al. [42], who found that plasma MMP-9 levels in HCC patients were considerably higher than in controls. Still, they disputed the current findings, declaring that plasma MMP-9 levels were higher in HCC with metastasis than in situ.
In contrast to the current results, Arii et al. [43] and Sakamoto et al. [44] demonstrated positive correlations between both MMP-9 gene expressions and protein levels and HCC progression signs.
On the other hand, Kuyvenhoven et al. [40] found that MMP-2 and, to a lesser extent, MMP-9 correlated with the severity of liver disease. However, they concluded that both could not be used as diagnostic biomarkers for HCC due to the significant overlap in patients with chronic liver disease with or without HCC.
The current findings revealed significant positive correlations between MMPs-2, 9 gene expressions, and proteins and between AFP and MMP-2, suggesting their complementary roles as biomarkers in HCC. However, further experimental research is required to fully explain these associations.
Coinciding with the current findings, Chung et al. [45] demonstrated an association between AFP and the MMP-9/MMP-2 ratio in HCC. Also, Meng et al. [46] reported that silencing of AFP expression led to decreased expression of MMP-2 and MMP-9, suggesting AFP's regulatory role in angiogenesis and cell invasion in HCC.
The present ROC curve analysis of MMPs-2 and -9 gene expressions and proteins showed higher significant sensitivity and specificity in predicting the presence of HCC than AFP.
The current results were consistent with those published by Chen et al. [47], who found MMP-9 superior to MMP-2 in predicting tumor recurrence and survival in HCC.
Hayasaka et al. [42] performed similar work. They concluded that the Roc curve analysis of plasma MMP-9 levels had a sensitivity of 53% and a specificity of 89% for detecting HCC at a cutoff value of 60 ng/mL.
This study found that serum miRNA-29b1 levels in HCC patients were significantly lower than in healthy subjects. Furthermore, the current ROC curve analysis of miRNA-29b1 demonstrated higher significant sensitivity and specificity than AFP in predicting the presence of HCC. Still, no significant associations were found between miRNA-29b1 and the severity of HCC. These findings pointed to miRNA-29b1’s protective role in HCC and its probable use as a diagnostic rather than a prognostic biomarker in HCC.
Except for Fang et al. [25], who explored the role of miRNA-29b in HCC tissues, no previous research has evaluated the circulating miRNA-29b1 in HCC. They concurred with the current results suggesting that miR-29b had an anti-oncogenic role in HCC using mouse model.
Also, miR-29b was considered a tumor suppressor in other malignancies like AML [48], multiple myeloma (MM) [49], and lung cancer [50]. Furthermore, it has been demonstrated that the miR-29b epigenetic effects contributed to its role in tumors [51].
Moreover, some studies evaluated other members of the miRNA 29 family in HCC, such as Xiong et al. 2010 [37]. Braconi et al. 2011 [52] reported similar results as they concluded downregulation of miR-29a in HCC cells compared to non-tumorous-tissues. On the contrary, Zhou et al. [53], Lin et al. [54], and Zhu et al. [55] identified miR-29a, miR-29c as HCC diagnostic markers increased in the circulation of HBV-positive HCC patients. Moreover, Kong et al. [56] demonstrated that miR-29a was upregulated in HCC, while miRNA-29c was reported as a tumor suppressor of HCC [57].
Other research contradicted the current findings, revealing that miR-29b had oncogenic effects in other cancers. Cheng et al. [58] considered miR-29b an oncogene in malignant hematopoiesis. In addition, Xu et al. [31] reported that miR-29b1 levels were upregulated in bladder cancer more than controls. Moreover, they indicated that miR-29b1 knockdown inhibited bladder cancer growth, suggesting miR-29b1 oncogenicity. Furthermore, Wang et al. [59] observed that miR-29b was upregulated more in breast cancer with metastasis than in breast cancer with low metastasis, suggesting miR-29b as a prognostic biomarker.
The present study demonstrated significant negative correlations between miRNA-29b1, MMP-2, and -9 mRNAs and proteins, suggesting miRNA-29b1-mediated MMP-2 and MMP-9 regulation. Moreover, there were no significant correlations between miRNA-29b1 and the levels of AFP. However, AFP was considered a functional antagonist of miR-29, contributing to epigenetic alterations and a poor prognosis in HCC [60].
This correlation result was consistent with Fang et al. [25], who found that miRNA-29b inhibited angiogenesis in cancer cells by lowering MMP-2 expression. Compared to the current findings, Abdallah et al. [61] found that overexpression of tumor suppressor microRNAs was associated with a decrease in MMP-9 in HCC. Han et al. [23] supported these results, as they found that microRNA processing impairment increased the expression of MMP-2 and MMP-9, accelerating tumor growth. This finding suggested that MMP-2 and MMP-9 could be regulated by microRNAs.
Significant correlations between miRNA-29 b and MMP-2 were reported in other malignancies, as in prostate cancer cells identifying miRNA-29 b as a biomarker with potential implications for invasion and metastasis [62]. These results were also demonstrated in breast cancer [63] and colorectal cancer [64].
Previous research has not investigated the relationship between MMP-9 and miRNA 29b1 in HCC. Still, similar correlations have been found between other microRNAs and MMP-9 regulation in liver cancer cells, where MMP-9 expression decreased in cancer cells transfected with pre-miR-338-3p and increased in cancer cells transfected with anti-miR-338-3p [65].
Moreover, positive correlations were observed between the expression of the MMP-9 protein and an oncogenic miRNA in hepatoma cells, enhancing migration and invasion in cancer cells [66].
Conclusion
The current study concluded that increased levels of circulating mRNAs, proteins of MMP-2, and -9 in HCC patients, as well as the high significant sensitivity and specificity of their measurements in the early detection of the presence of HCC, suggested that they may be used as diagnostic biomarkers in HCC. MiRNA 29b1 levels were lower in the serum of HCC patients, indicating that it may play a protective function in the disease. The significant negative correlations between miRNA 29b1 and both mRNAs, proteins of MMPs-2 and -9 suggested overlapping molecular microRNA-mediated mechanisms that control MMPs for further future experimental evaluation. The significant positive correlations between MMP-2 and AFP suggested their probable synergistic utility in HCC. The insignificant correlations between the studied parameters and the severity of HCC ruled out the possibility of their utility as prognostic biomarkers. The study has some limitations, including the requirement for a larger sample size to assess more parameters to comprehend the molecular mechanisms behind the interactions between miRNA-29b1, MMP-2, and MMP-9.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Dasgupta P, Henshaw C, Youlden DR, Clark PJ, Aitken JF, Baade PD (2020) Global trends in incidence rates of primary adult liver cancers: a systematic review and meta-analysis. Front Oncol 10:171
Gao T, Zhi J, Mu C, Gu S, Xiao J, Yang J et al (2018) One-step detection for two serological biomarker species to improve the diagnostic accuracy of hepatocellular carcinoma. Talanta 178:89–93
Cadier B, Bulsei J, Nahon P, Seror O, Laurent A, Rosa I et al (2017) Early detection and curative treatment of hepatocellular carcinoma: a cost-effectiveness analysis in France and in the United States. Hepatology 65(4):1237–1248
Benson AB 3rd, Abrams TA, Ben-Josef E, Bloomston PM, Botha JF, Clary BM et al (2009) NCCN clinical practice guidelines in oncology: hepatobiliary cancers. J Natl Compr Cancer Netw JNCCN 7(4):350–391
Johnson P (1999) Role of alpha-fetoprotein in the diagnosis and management of hepatocellular carcinoma. J Gastroenterol Hepatol 14(5s):S32–S36
Trevisani F, D’Intino PE, Morselli-Labate AM, Mazzella G, Accogli E, Caraceni P et al (2001) Serum α-fetoprotein for diagnosis of hepatocellular carcinoma in patients with chronic liver disease: influence of HBsAg and anti-HCV status. J Hepatol 34(4):570–575
Han L-L, Lv Y, Guo H, Ruan Z-P, Nan K-J (2014) Implications of biomarkers in human hepatocellular carcinoma pathogenesis and therapy. World J Gastroenterol WJG 20(30):10249
Chen S, Chen H, Gao S, Qiu S, Zhou H, Yu M et al (2017) Differential expression of plasma microRNA-125b in hepatitis B virus-related liver diseases and diagnostic potential for hepatitis B virus-induced hepatocellular carcinoma. Hepatol Res 47(4):312–320
Bruix J, Sherman M (2011) Management of hepatocellular carcinoma: an update. Hepatology (Baltimore, MD) 53(3):1020
Egeblad M, Werb Z (2002) New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2(3):161–174
Zítka O, Kukacka J, Krizkov S, Húska D, Adam V, Masarik M et al (2010) Matrix metalloproteinases. Curr Med Chem 17(31):3751–3768
Groblewska M, Siewko M, Mroczko B, Szmitkowski M (2012) The role of matrix metalloproteinases (MMPs) and their inhibitors (TIMPs) in the development of esophageal cancer. Folia Histochem Cytobiol 50(1):12–19
Maolood N, Hardin-Pouzet H, Grange-Messent V (2008) Matrix metalloproteinases MMP2 and MMP9 are upregulated by noradrenaline in the mouse neuroendocrine hypothalamus. Eur J Neurosci 27(5):1143–1152
McCawley LJ, Li S, Wattenberg EV, Hudson LG (1999) Sustained activation of the mitogen-activated protein kinase pathway: a mechanism underlying receptor tyrosine kinase specificity for matrix metalloproteinase-9 induction and cell migration. J Biol Chem 274(7):4347–4353
Kong L, Zhu J, Han W, Jiang X, Xu M, Zhao Y et al (2011) Significance of serum microRNAs in pre-diabetes and newly diagnosed type 2 diabetes: a clinical study. Acta Diabetol 48:61–69
Sternlicht MD, Werb Z (2001) How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 17(1):463–516
Kwon MJ (2023) Matrix metalloproteinases as therapeutic targets in breast cancer. Front Oncol 12:1108695
Hong EH, Hwang M, Yu H, Park H-H, Cho H, Koh S-H et al (2022) Differential expression of aqueous humor microRNAs in central retinal vein occlusion and its association with matrix metalloproteinases: a pilot study. Sci Rep 12(1):16429
Mo M, Peng F, Wang L, Peng L, Lan G, Yu S (2013) Roles of mitochondrial transcription factor A and microRNA-590-3p in the development of bladder cancer. Oncol Lett 6(2):617–623
Di Leva G, Garofalo M, Croce CM (2014) MicroRNAs in cancer. Annu Rev Pathol 9:287–314
Calin GA, Sevignani C, Dumitru CD, Hyslop T, Noch E, Yendamuri S et al (2004) Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci 101(9):2999–3004
Abba M, Patil N, Allgayer H (2014) MicroRNAs in the regulation of MMPs and metastasis. Cancers 6(2):625–645
Han L, Zhang A, Zhou X, Xu P, Wang G-X, Pu P-Y et al (2010) Downregulation of Dicer enhances tumor cell proliferation and invasion. Int J Oncol 37(2):299–305
Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T (2001) Identification of novel genes coding for small expressed RNAs. Science 294(5543):853–858
Fang JH, Zhou HC, Zeng C, Yang J, Liu Y, Huang X et al (2011) MicroRNA-29b suppresses tumor angiogenesis, invasion, and metastasis by regulating matrix metalloproteinase 2 expression. Hepatology 54(5):1729–1740
Chen H-X, Xu X-X, Zhang Z, Tan B-Z, Zhou X-D (2017) MicroRNA-29b inhibits angiogenesis by targeting VEGFA through the MAPK/ERK and PI3K/Akt signaling pathways in endometrial carcinoma. Cell Physiol Biochem 41(3):933–946
Li Y, Cai B, Shen L, Dong Y, Lu Q, Sun S et al (2017) MiRNA-29b suppresses tumor growth through simultaneously inhibiting angiogenesis and tumorigenesis by targeting Akt3. Cancer Lett 397:111–119
Zhao X, Liu Y, Li Z, Zheng S, Wang Z, Li W et al (2018) Linc00511 acts as a competing endogenous RNA to regulate VEGFA expression through sponging hsa-miR-29b-3p in pancreatic ductal adenocarcinoma. J Cell Mol Med 22(1):655–667
Poudyal D, Cui X, Le PM, Hofseth AB, Windust A, Nagarkatti M et al (2013) A key role of microRNA-29b for the suppression of colon cancer cell migration by American ginseng. PLoS ONE 8(10):e75034
Salamat JM, Abbott KL, Flannery PC, Ledbetter EL, Pondugula SR (2022) Interplay between the Cannabinoid system and microRNAs in cancer. ACS Omega 7(12):9995–10000
Xu F, Zhang Q, Cheng W, Zhang Z, Wang J, Ge J (2013) Effect of miR-29b-1* and miR-29c knockdown on cell growth of the bladder cancer cell line T24. J Int Med Res 41(6):1803–1810
Shaker O, Ayeldeen G, Abdelhamid A (2021) The impact of single nucleotide polymorphism in the long non-coding MEG3 gene on microRNA-182 and microRNA-29 expression levels in the development of breast cancer in Egyptian women. Front Genet 12:683809
Ashcroft RE (2008) The declaration of Helsinki. In: The Oxford textbook of clinical research ethics, pp 141–8
Kim T-H, Kim SY, Tang A, Lee JM (2019) Comparison of international guidelines for noninvasive diagnosis of hepatocellular carcinoma: 2018 update. Clin Mol Hepatol 25(3):245
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 25(4):402–408
Fernández M, Semela D, Bruix J, Colle I, Pinzani M, Bosch J (2009) Angiogenesis in liver disease. J Hepatol 50(3):604–620
Xiong Y, Fang JH, Yun JP, Yang J, Zhang Y, Jia WH et al (2010) Effects of MicroRNA-29 on apoptosis, tumorigenicity, and prognosis of hepatocellular carcinoma. Hepatology 51(3):836–845
Curran S, Murray GI (2000) Matrix metalloproteinases: molecular aspects of their roles in tumour invasion and metastasis. Eur J Cancer 36(13):1621–1630
Yeh H-C, Lin S-M, Chen M-F, Pan T-L, Wang P-W, Yeh C-T (2010) Evaluation of serum matrix metalloproteinase (MMP)-9 to MMP-2 ratio as a biomarker in hepatocellular carcinoma. Hepatogastroenterology 57(97):98–102
Kuyvenhoven JP, van Hoek B, Blom E, van Duijn W, Hanemaaijer R, Verheijen JH et al (2003) Assessment of the clinical significance of serum matrix metalloproteinases MMP-2 and MMP-9 in patients with various chronic liver diseases and hepatocellular carcinoma. Thromb Haemost 89(04):718–725
Murawaki Y, Yamada S, Ikuta Y, Kawasaki H (1999) Clinical usefulness of serum matrix metalloproteinase-2 concentration in patients with chronic viral liver disease. J Hepatol 30(6):1090–1098
Hayasaka A, Suzuki N, Fujimoto N, Iwama S, Fukuyama E, Kanda Y et al (1996) Elevated plasma levels of matrix metalloproteinase-9 (92-kd type IV collagenase/gelatinase B) in hepatocellular carcinoma. Hepatology 24(5):1058–1062
Arii S, Mise M, Harada T, Furutani M, Ishigami S-I, Niwano M et al (1996) Overexpression of matrix metalloproteinase 9 gene in hepatocellular carcinoma with invasive potential. Hepatology 24(2):316–322
Sakamoto Y, Mafune K, Mori M, Shiraishi T, Imamura H, Takayama T et al (2000) Overexpression of MMP-9 correlates with growth of small hepatocellular carcinoma. Int J Oncol 17(2):237–280
Chung TW, Kim JR, Suh JI, Lee YC, Chang YC, Chung TH et al (2004) Correlation between plasma levels of matrix metalloproteinase (MMP)-9/MMP-2 ratio and α-fetoproteins in chronic hepatitis carrying hepatitis B virus. J Gastroenterol Hepatol 19(5):565–571
Meng W, Li X, Bai Z, Li Y, Yuan J, Liu T et al (2014) Silencing alpha-fetoprotein inhibits VEGF and MMP-2/9 production in human hepatocellular carcinoma cell. PLoS ONE 9(2):e90660
Chen R, Cui J, Xu C, Xue T, Guo K, Gao D et al (2012) The significance of MMP-9 over MMP-2 in HCC invasiveness and recurrence of hepatocellular carcinoma after curative resection. Ann Surg Oncol 19:375–384
Garzon R, Liu S, Fabbri M, Liu Z, Heaphy CE, Callegari E et al (2009) MicroRNA-29b induces global DNA hypomethylation and tumor suppressor gene reexpression in acute myeloid leukemia by targeting directly DNMT3A and 3B and indirectly DNMT1. Blood J Am Soc Hematol 113(25):6411–6418
Amodio N, Leotta M, Bellizzi D, Di Martino MT, D’Aquila P, Lionetti M et al (2012) DNA-demethylating and anti-tumor activity of synthetic miR-29b mimics in multiple myeloma. Oncotarget 3(10):1246
Pandey M, Sultana S, Gupta KP (2014) Involvement of epigenetics and microRNA-29b in the urethane induced inception and establishment of mouse lung tumors. Exp Mol Pathol 96(1):61–70
Mims A, Walker AR, Huang X, Sun J, Wang H, Santhanam R et al (2013) Increased anti-leukemic activity of decitabine via AR-42-induced upregulation of miR-29b: a novel epigenetic-targeting approach in acute myeloid leukemia. Leukemia 27(4):871–878
Braconi C, Kogure T, Valeri N, Huang N, Nuovo G, Costinean S et al (2011) microRNA-29 can regulate expression of the long non-coding RNA gene MEG3 in hepatocellular cancer. Oncogene 30(47):4750–4756
Zhou J, Yu L, Gao X, Hu J, Wang J, Dai Z et al (2011) Plasma microRNA panel to diagnose hepatitis B virus-related hepatocellular carcinoma. J Clin Oncol 29(36):4781–4788
Lin X-J, Chong Y, Guo Z-W, Xie C, Yang X-J, Zhang Q et al (2015) A serum microRNA classifier for early detection of hepatocellular carcinoma: a multicentre, retrospective, longitudinal biomarker identification study with a nested case–control study. Lancet Oncol 16(7):804–815
Zhu H-T, Hasan AM, Liu R-B, Zhang Z-C, Zhang X, Wang J et al (2016) Serum microRNA profiles as prognostic biomarkers for HBV-positive hepatocellular carcinoma. Oncotarget 7(29):45637
Kong G, Zhang J, Zhang S, Shan C, Ye L, Zhang X (2011) Upregulated microRNA-29a by hepatitis B virus X protein enhances hepatoma cell migration by targeting PTEN in cell culture model. PLoS ONE 6(5):e19518
Ratnasari N, Lestari P, Renovaldi D, Raditya Ningsih J, Qoriansas N, Wardana T et al (2022) Potential plasma biomarkers: miRNA-29c, miRNA-21, and miRNA-155 in clinical progression of Hepatocellular Carcinoma patients. PLoS ONE 17(2):e0263298
Cheng J, Guo S, Chen S, Mastriano SJ, Liu C, D’Alessio AC et al (2013) An extensive network of TET2-targeting MicroRNAs regulates malignant hematopoiesis. Cell Rep 5(2):471–481
Wang C, Bian Z, Wei D, Zhang J-G (2011) miR-29b regulates migration of human breast cancer cells. Mol Cell Biochem 352:197–207
Parpart S, Roessler S, Dong F, Rao V, Takai A, Ji J et al (2014) Modulation of miR-29 expression by alpha-fetoprotein is linked to the hepatocellular carcinoma epigenome. Hepatology 60(3):872–883
Abdallah HM, El Awdan SA, Abdel-Rahman RF, Farrag ARH, Allam RM (2022) 1, 8 Cineole and Ellagic acid inhibit hepatocarcinogenesis via upregulation of MiR-122 and suppression of TGF-β1, FSCN1, Vimentin, VEGF, and MMP-9. PLoS ONE 17(1):e0258998
Steele R, Mott JL, Ray RB (2010) MBP-1 upregulates miR-29b, which represses Mcl-1, collagens, and matrix metalloproteinase-2 in prostate cancer cells. Genes Cancer 1(4):381–387
Ding Q, Chang CJ, Xie X, Xia W, Yang JY, Wang SC et al (2012) Erratum: APOBEC3G promotes liver metastasis in an orthotopic mouse model of colorectal cancer and predicts human hepatic metastasis (Journal of Clinical Investigation (2012) 122, 1 (419. J Clin Investig 122(1):419
Dong CG, Wu WK, Feng SY, Wang XJ, Shao JF, Qiao J (2012) Co-inhibition of microRNA-10b and microRNA-21 exerts synergistic inhibition on the proliferation and invasion of human glioma cells. Int J Oncol 41(3):1005–1012
Huang XH, Chen JS, Wang Q, Chen XL, Wen L, Chen LZ et al (2011) miR-338-3p suppresses invasion of liver cancer cell by targeting smoothened. J Pathol 225(3):463–472
Li Q, Wang G, Shan JL, Yang ZX, Wang HZ, Feng J et al (2010) MicroRNA-224 is upregulated in HepG2 cells and involved in cellular migration and invasion. J Gastroenterol Hepatol 25(1):164–171
Acknowledgements
All authors are appreciated for all residents in gastroenterology and hepatology , clinical pathology, and oncology departments at Beni-Suef University Hospital for their support and cooperation.
Funding
The authors declare that no funds, grants, or other supports were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. SN, SS, and HHM performed material preparation, data collection, and analysis. HHF, MSA-T, and AA.M did laboratory, experimental work, and data analysis. DMK and HAM did statistical analysis and English editing. The first draft of the manuscript was written by MSA-T, and all authors commented on previous versions. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by Research Ethical Committee, Faculty of Medicine, Beni-Suef University, with approval number: FMBSUREC/12022023/Ammar. Informed consent was obtained from all individual participants included in the study.
Consent for publication
Our raw data and manuscript did not contain any individual details, images, or videos. The authors used to number the cases to maintain confidentiality of patient data.
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdel-Tawab, M.S., Fouad, H., Khalil, D.M. et al. The role of miRNA-29b1, MMP-2, MMP-9 mRNAs, and proteins in early diagnosis of HCC. Egypt J Med Hum Genet 24, 57 (2023). https://doi.org/10.1186/s43042-023-00434-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43042-023-00434-w