Abstract
Background
Hepatocellular carcinoma and hepatitis C are strongly associated. The current work aimed to study the expression levels of microRNA-331-3p and microRNA-23b-3p as propable biomarkers for detecting liver cancer (HCC) at its early stages in patients with HCV-related liver cirrhosis. The current prospective study included two hundred participants, divided into three groups: group I, 100 patients with HCV-related liver cirrhosis; group II, 50 HCC patients at early stages; and group III, 50 apparentlyhealthy controls. All patients had routine laboratory workup and ultrasound hepatic assessment. Values of microRNA-331-3p and microRNA-23b-3p were measured by real-time quantitative PCR.
Results
Levels of miR-331-3p were significantly higher in HCC patients than in cirrhotic patients and controls (p < 0.001), while levels of miR-23b-3p were significantly lower in HCC patients compared to cirrhotics and controls (p < 0.001). ROC curve revealed that miR-23b-3p had 80% sensitivity and 74% specificity, miR-331-3p had 66% sensitivity and 61% specificity, and AFP had 64% sensitivity and 61% specificity of 61% in discrimination between HCC patients from controls.
Conclusion
Serum miR-23b-3p is a more effective predictor than miR-331-3p and AFP for the development of hepatocellular carcinoma in hepatitis C (HCV)-related cirrhotic patients.
Similar content being viewed by others
Background
Hepatocellular carcinoma (HCC) is a universal health problem. HCC represents the sixth most common cancer worldwide [1], and the fourth common cancer in Egypt [2]. HCV promotes cirrhosis, which is found in 80–90% of patients with HCC [3]. Prospective studies have shown a significant increase in the incidence of HCC among HCV-infected cohorts, compared to HCV-negative cohorts. HCV-induced HCC development is a multi-step process that may progress over 20–40 years [4]. HCV is an RNA virus with limited integration of its genetic material into the host’s genome. Therefore, the carcinogenic potential of HCV is generally assumed to be linked to the indirect mechanisms [5].
MicroRNAs (miRNAs) are a class of small 18-23-nucleotides that have a role in different biological functions including proliferation, differentiation, and apoptosis [6]. Abnormal miRNA expression has been implicated in the pathogenesis of various cancers [7]. Discovery that microRNAs are detected in patients’ sera raised the assumption that these serum biomarkers can be used as a less invasive screening or diagnostic tools [8]. miR-331-3p is one of the most upregulated miRNAs that promote proliferation, migration, and invasion of the tumor cells, including HCC [9]. miR-23b-3p was identified as a tumor suppressor that downregulated in different classes of human malignant tumors [10].
microRNA-331-3p and microRNA-23b-3p expression in hepatic carcinoma was examined in some studies [11, 12].
The current work aimed to determine the potentiality of serum miR-331-3p and miR-23b-3p as screening biomarkers for early stages of liver cancer in patients with HCV-related hepatic cirrhosis.
Methods
The current prospective study involved 150 patients out of 2140 patients who attended the tropical medicine, hepatology, oncology, and general medicine outpatient clinics in the Alexandria University Hospital (Alexandria, Egypt) in the period from December 2017 till January 2020. The control group involved 50 apparently healthy volunteers.
The proposal was explained to all participants, and they signed a written consent. The proposal was accepted by the committee of ethics (Faculty of medicine, University of Alexandria).
Exclusion criteria included patients with coinfections with hepatitis B virus (HBV) or HIV, organ transplantation, autoimmune disease or use of immunosuppressant or antiviral drugs, diabetes mellitus, Schistosoma, and other malignant comorbidities.
All participants were subjected to full history taking, thorough clinical examination, laboratory investigations including complete blood picture, liver transaminases (alanine aminotransferase (ALT), and aspartate aminotransferase (AST)), serum albumin, total bilirubin, and alpha-fetoprotein, and abdominal ultrasonography.
Two hundred participants were divided into three groups: group I, 100 patients with HCV-related liver cirrhosis; group II, 50 patients with early-stage hepatocellular carcinoma (HCC); and group III, 50 healthy controls.
Ultrasound examinations were performed by experienced ultra-sonographers in the Radiology Department of the main Alexandria University Hospital. Items to be observed in ultrasound examination were determination of the liver size, nodularity of the liver surface, the coarseness of the parenchyma, size of lymph nodes around the hepatic artery, patency and flow of veins and arteries, and probable hepatocellular carcinoma.
HCV antibody testing was done by using a commercial recombinant immunoblot assay and confirmed by real-time quantitative HCV RNA PCR (more than 15 IU/ml).
All the HCC patients were diagnosed by abdominal ultrasound and alpha-fetoprotein [13]. Triphasic CT scan examination and Barcelona clinic liver cancers (BCLC) [25] staging was done for the selection of group II patients. Based on BCLC; patients at a very early stage (stage 0) and early stage (stage A) were selected as candidates for study with single tumors less than 2 cm or multinodular tumor less than 3 cm in size, with the absence of clinically relevant portal hypertension.
Real-time quantitative PCR for miR-331-3p and miR-23b expression levels
Total RNA including microRNA was immediately isolated from plasma samples using miRNeasy Mini Kit (Qiagen, Maryland, USA) according to manufacturers’ instructions. The concentration and purity of the extracted total RNA were assisted using NanoDrop2000 Spectrophotometer (Thermo Scientific, USA). Single-stranded cDNA was synthesized from purified RNA samples using the Taqman miRNA reverse transcription Kit (Applied Biosystems, USA) for RNA reverse transcription according to the manufacturer’s protocol. The real-time amplification was performed using TaqMan MicroRNA assays for miR-331-3p, miR-23-3p, and TaqMan Fast Advanced Mater Mix (Applied Biosystems, USA) on the RotorGene Q Real-Time PCR System (Qiagen, Germany). RNU6 was used as endogenous references; its expression was stable in all the samples and independent of the analyzed variables. The relative expression levels were determined using the 2−ΔΔCT method.
Statistical analysis
Data were fed to the computer and analyzed using IBM SPSS software package version 20.0. (Armonk, NY: IBM Corp). The Kolmogorov- Smirnov was used to verify the normality of distribution of variables. Comparisons between groups for categorical variables were assessed using the Chi-square test. ANOVA was used for comparing the four studied groups and followed by post hoc test (Tukey) for pairwise comparison. While Kruskal-Wallis test was used to compare different groups for abnormally distributed quantitative variables and followed by post hoc test (Dunn’s for multiple comparisons test) for pairwise comparison. The significance of the obtained results was judged at the 5% level.
Results
The current study involved 200 participants divided into the following groups: group I, 100 patients with HCV-related liver cirrhosis; group II, 50 HCC patients at an early stage (16 patients (32%) were BCLC stage 0 and 34 patients (68%) were BCLC stage A); and group III, 50 apparently healthy controls.
The mean age of group I was 47.4 ± 9.7years, group II was 50.6 ± 5.2years, and of group III was 47.5 ± 9.3years with no significant difference between the three groups (p = 0.086) (Table 1).
Group I comprised of 79 males (79%) and 21 females (21%), group II comprised of 40 males (80%) and 10 females (20%), and group III comprised of 35 males (70%) and 15 females (30%), with no statistically significant difference between the three studied groups (p = 0.394) (Table 1).
As regards results of peripheral blood picture, levels of serum transaminases, total bilirubin levels, and serum albumin levels, all were summarized in Table 1.
The mean of AFP was significantly higher in group II than in groups I and III. Furthermore, it was significantly higher in group II than group III (P ˂ 0.001 and P ˂ 0.001, respectively) (Table 1).
Regarding miR-331-3p expression, plasma levels were significantly higher in group II compared to group I and III (p ˂ 0.001 and P ˂ 0.001, respectively). Also, a high statistically significant difference was observed between groups (II and III, p < 0.001) (Table 1).
On the contrary, miR-23b-3p expression levels were significantly lower in group II compared to group I, and both were significantly lower than the controls (p < 0.001) (Table 1).
Further analysis of plasma levels of miRNAs in group II revealed that the mean of miR-331-3p in stage 0 HCC was 8.1 ± 10.3, while it was 7.3 ± 10.1 in patients with stage A, with no statistical difference could be detected between both groups (p = 0.967). Regarding miR-23b-3p, its mean in patients with stage 0 HCC was 0.21 ± 0.22, while it was 0.31 ± 0.37, with no statistical difference between both groups (p = 0.371) (Table 2).
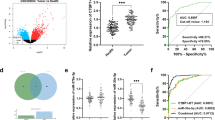
Using ROC curves to determine the diagnostic accuracy of AFP, miR-331-3p, and miR-23-3p in differentiating patients with early stages of hepatic cancer from patients with HCV-related liver cirrhosis revealed that values of AFP more than 148 ng/ml had a 64% sensitivity and 61% specificity to differentiate early carcinoma patients from cirrhotic ones (Fig. 1). Values of miR-331-3p more than 2.18 had a 66% sensitivity and 61% specificity to differentiate patients with early stages of HCC (Fig. 2). Regarding miR-23b-3p, values less than or equal to 0.36 had an 80% sensitivity and 74% specificity to diagnose HCC at its early stages (Fig. 3). Combining AFP (ng/ml) and miR-331-3p (Fig. 4) gave sensitivity of 76% and specificity of 65%. Combining AFP (ng/ml) & miR-23b-3p (Fig. 5) gave sensitivity of 82% and specificity of 69%. While the sensitivity and specificity of combining the three parameters (Fig. 6) were 86% and 64%, respectively (Table 3).
Discussion
Hepatitis C virus (HCV) is one of the main carcinogens for hepatocellular carcinoma (HCC) [14]. It infects around 71 million people around the world [15]. The HCV infects the liver leading to HCC [16]. And while around 399,000 patients died from HCV in 2016, only around 19% knew about their infection status [16]. And although AFP has been always used to diagnose HCC, it is neither specific nor sensitive [12]. Therefore, it is crucial to look for new predictive markers of HCC as early as possible to improve the patients’ prognosis.
miRNAs are small non-coding RNA molecules that are composed of 21–23 nucleotides that affect target gene expression by negatively controlling post-transcriptional gene expression [16, 17]. They are highly tissue-specific and can withstand severe conditions like extreme temperature and low pH, making them highly stable in the serum or plasma samples and, therefore, qualifying them as perfect noninvasive markers for early HCC diagnosis [12].
Several studies identified a microRNA panel to distinguish HCV-related HCC from liver cirrhosis [18,19,20]. In a recent study, it was discovered that there are four miRNAs, including MiR-331-3p that significantly increased in HCC and two miRNAs including miR-23b-3p that significantly decreased in HCC patients compared to liver cirrhosis. Those six miRNAs have been found in the studies to effectively discriminate the HCC from liver cirrhosis and healthy controls [21].
In our study, miR-331-3p was shown to significantly increase in patients with early stages HCC compared to cirrhotic patients. Similar results were also reported by a previous study on HCC tissues that detected overexpression of miR-331-3p in the HCC cells and proved that it helped proliferation and spread of HCC via suppressing leucine-rich repeat protein phosphatase mediated dephosphorylation of protein kinase B [22]. These results were consistent with Chen et al. [23] results who found that serum miR-331-3p significantly increased in HCC compared to patients with benign hepatic tumors and was associated with poor HCC prognosis. In the current study, comparison between patients with stage 0 HCC and stage A regarding levels of miR331-3p did not show significant difference.
miR-23b-3p showed a significant decrease in the plasma of patients with early HCC compared to cirrhotic patients. These results are consistent with those results reported previously by Cao et al. who stated that miR-23b-3p was downregulated in HCC tissue cells [24]. Despite the miR-23b-3p in tissues has been identified as a diagnostic molecular marker and a possible therapeutic target for patients with HCC [13], its serum concentrations have not been thoroughly researched in many studies before.
On further analysis, the diagnostic sensitivities and specificities of both miR-331-3p and mi-23b-3p had been calculated using the ROC curves. According to the ROC curves data, it was shown that mi-23b-3p had the best sensitivity (80%) and specificity (74%). In a study by Sun et al., miR-23b-3p had better sensitivity compared to miR-331-3p, but contrary to our results miR-331-3p showed the highest specificity [12].
Conclusion
Serum miR-23b-3p is a more effective predictor than miR-331-3p and AFP for the development of hepatocellular carcinoma in cirrhotic HCV patients. The usual marker used in the clinical practice to predict HCC is AFP, but our results revealed that miR-23b-3p has a greater sensitivity and specificity than AFP. The cost-effectiveness as well as the benefits depends on the price of the kit and the number of the samples to be done.
Limitation of the study
Small sample size and future studies should be done in large number of patients to verify our results.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AFP:
-
Alpha-fetoprotein
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- EMT:
-
Epithelial-mesenchymal transformation
- HBV:
-
Hepatitis B virus
- HCC:
-
Hepatocellular carcinoma
- HCV:
-
Hepatitis C virus
- HIV:
-
Human immunodeficiency virus
- miRNAs:
-
Microribonucleic acid
- Pyk2:
-
Proline-rich tyrosine kinase 2
References
Forner A, Reig M, Bruix J (2018) Hepatocellular carcinoma. Lancet. 391(10127):1301–1314. https://doi.org/10.1016/S0140-6736(18)30010-2
Rashed WM, Kandeil MAM, Mahmoud MO, Ezzat S (2020) Hepatocellular carcinoma (HCC) in Egypt: a comprehensive overview. J Egypt Natl Canc Inst. 32(1):5. https://doi.org/10.1186/s43046-020-0016-x
El-Serag HB (2012) Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 142((6)):1264–73. e1
Goossens N, Hoshida Y (2015) Hepatitis C virus-induced hepatocellular carcinoma. Clin Mol Hepatol. 21(2):105–114. https://doi.org/10.3350/cmh.2015.21.2.105
Billerbeck E, de Jong Y, Dorner M, de la Fuente C, Ploss A (2013) Animal models for hepatitis C. Curr Top Microbiol Immunol. 369:49–86. https://doi.org/10.1007/978-3-642-27340-7_3
Gao B, Ning S, Li J, Liu H, Wei W, Wu F et al (2015) Integrated analysis of differentially expressed mRNAs and miRNAs between hepatocellular carcinoma and their matched adjacent normal liver tissues. Oncol Rep. 34(1):325–333. https://doi.org/10.3892/or.2015.3968
Hironaka-Mitsuhashi A, Matsuzaki J, Takahashi RU, Yoshida M, Nezu Y, Yamamoto Y et al (2017) A tissue microRNA signature that predicts the prognosis of breast cancer in young women. PLoS One. 12(11):e0187638. https://doi.org/10.1371/journal.pone.0187638
Yokoi A, Yoshioka Y, Hirakawa A, Yamamoto Y, Ishikawa M, Ikeda SI,2017 Kato T, Niimi K, Kajiyama H, Kikkawa F, Ochiya T A combination of circulating miRNAs for the early detection of ovarian cancer. Oncotarget. ;8(52):89811-89823, doi: https://doi.org/10.18632/oncotarget.20688.
Papadopoulos EI, Papachristopoulou G, Ardavanis A, Scorilas A (2018) A comprehensive clinicopathological evaluation of the differential expression of microRNA-331 in breast tumors and its diagnostic significance. Clin Biochem. 60:24–32. https://doi.org/10.1016/j.clinbiochem.2018.07.008
Jiang W, Min J, Sui X, Qian Y, Liu Y, Liu Z, Zhou H, Li X, Gong Y (2015) MicroRNA-26a-5p and microRNA-23b-3p up-regulate peroxiredoxin III in acute myeloid leukemia. Leuk Lymphoma. 56(2):460–471. https://doi.org/10.3109/10428194.2014.924115
He RQ, Wu PR, Xiang XL, Yang X, Liang HW, Qiu XH, Yang LH, Peng ZG, Chen G (2018) Downregulated miR-23b-3p expression acts as a predictor of hepatocellular carcinoma progression: a study based on public data and RT-qPCR verification. Int J Mol Med. 41(5):2813–2831. https://doi.org/10.3892/ijmm.2018.3513
Sun Q, Li J, Jin B, Wang T, Gu J (2020) Evaluation of miR-331-3p and miR-23b-3p as serum biomarkers for hepatitis c virus-related hepatocellular carcinoma at early stage. Clin Res Hepatol Gastroenterol. 44(1):21–28. https://doi.org/10.1016/j.clinre.2019.03.011
He RQ, Wu PR, Xiang XL, Yang X, Liang HW, Qiu XH, Yang LH, Peng ZG, Chen G (2018) Downregulated miR-23b-3p expression acts as a predictor of hepatocellular carcinoma progression: a study based on public data and RT-qPCR verification. Int J Mol Med. 41(5):2813–2831. https://doi.org/10.3892/ijmm.2018.3513
Axley P, Ahmed Z, Ravi S, Singal AK (2018) Hepatitis C virus and hepatocellular carcinoma: a narrative review. J Clin Transl Hepatol. 6(1):79–84. https://doi.org/10.14218/JCTH.2017.00067
Mason LM, Duffell E, Veldhuijzen IK, Petriti U, Bunge EM, Tavoschi L (2019) Hepatitis B and C prevalence and incidence in key population groups with multiple risk factors in the EU/EEA: a systematic review, Euro Surveill. 24((30))
Lorini S, Gragnani L, Zignego AL (2020) The relevance of MicroRNAs in the pathogenesis and prognosis of HCV-disease: the emergent role of miR-17-92 in cryoglobulinemic vasculitis. Viruses 12((12))
Inui M, Martello G, Piccolo S (2010) MicroRNA control of signal transduction. Nat Rev Mol Cell Biol. 11(4):252–263. https://doi.org/10.1038/nrm2868
Elemeery MN, Badr AN, Mohamed MA, Ghareeb DA (2017) Validation of a serum microRNA panel as biomarkers for early diagnosis of hepatocellular carcinoma post-hepatitis C infection in Egyptian patients. World J Gastroenterol. 23(21):3864–3875. https://doi.org/10.3748/wjg.v23.i21.3864
Zekri AN, Youssef AS, El-Desouky ED, Ahmed OS, Lotfy MM, Nassar AA et al (2016) Serum microRNA panels as potential biomarkers for early detection of hepatocellular carcinoma on top of HCV infection. Tumour Biol. 37(9):12273–12286. https://doi.org/10.1007/s13277-016-5097-8
El-Abd NE, Fawzy NA, El-Sheikh SM, Soliman ME (2015) Circulating miRNA-122, miRNA-199a, and miRNA-16 as biomarkers for early detection of hepatocellular carcinoma in Egyptian patients with chronic hepatitis C virus infection. Mol Diagn Ther. 19(4):213–220. https://doi.org/10.1007/s40291-015-0148-1
Li J, Jin B, Wang T, Li W, Wang Z, Zhang H, Song Y, Li N (2019) Serum microRNA expression profiling identifies serum biomarkers for HCV-related hepatocellular carcinoma. Cancer Biomark. 26(4):501–512. https://doi.org/10.3233/CBM-181970
Chang RM, Yang H, Fang F, Xu JF, Yang LY (2014) MicroRNA-331-3p promotes proliferation and metastasis of hepatocellular carcinoma by targeting PH domain and leucine-rich repeat protein phosphatase. Hepatology. 60(4):1251–1263. https://doi.org/10.1002/hep.27221
Chen L, Chu F, Cao Y, Shao J, Wang F (2015) Serum miR-182 and miR-331-3p as diagnostic and prognostic markers in patients with hepatocellular carcinoma. Tumour Biol. 36(10):7439–7447. https://doi.org/10.1007/s13277-015-3430-2
Cao J, Liu J, Long J, Fu J, Huang L, Li J et al (2017) microRNA-23b suppresses epithelial-mesenchymal transition (EMT) and metastasis in hepatocellular carcinoma via targeting Pyk2. Biomed Pharmacother 89:642–650
Acknowledgements
We sincerely thank all patients and healthy volunteers for their participation in the current study. We also thank Mr. Amgad Hamza for revising the statistical analysis of the results.
Funding
No funding resources.
Author information
Authors and Affiliations
Contributions
All authors have contributed to the current work. R.A. and M.G. had performed laboratory investigations of the participants, analysis, and interpretation of the data. W.E. contributed substantially to the conception and design of the study, acquisition of data, and analyzed and interpreted the data. M.S. contributed to the acquisition of data, drafted the manuscript, and critical revision of the manuscript. All the authors approved the final version submitted for publication and take responsibility for the statements made in the published article.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The proposal of the current study was approved by the ethical committee of the Faculty of Medicine—Alexandria University (the approval number is not available). Informed written consent was signed by all participants or their caregivers before the study. The committee’s reference number is not available. The current study is original and has not been published elsewhere in any form or language (partially or in full). Results of the current study were presented, honestly, and without fabrication, falsification, or inappropriate data manipulation. No data, text, or theories by others are presented as if they were the author’s own (“plagiarism”).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aboelwafa, R.A., Ellakany, W.I., Gamaleldin, M.A. et al. The expression of microRNA-331-3p and microRNA-23b3 in Egyptian patients with early-stage hepatocellular carcinoma in hepatitis C-related liver cirrhosis. Egypt Liver Journal 11, 49 (2021). https://doi.org/10.1186/s43066-021-00122-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43066-021-00122-7