Abstract
Background
The P53 protein has an essential role in several cellular processes, including DNA repair, apoptosis, and cell cycle arrest. The pathophysiology of many cancer types has frequently been linked to polymorphisms in the TP53 locus. Over 200 single nucleotide polymorphisms (SNPs) have been identified in TP53. However, Pro72Arg (rs1042522) at codon 72, shows contradictory results in terms of cancer risk. In this study, we aimed to determine if the Pro72Arg (rs1042522) SNP in the TP53 gene would be linked to breast cancer (BC) risk among Egyptian patients.
Materials and Methods
Genomic DNA was extracted from blood samples of 100 healthy volunteers and 100 breast cancer patients (50 familial and 50 non-familial). TP53 Genotyping was performed using tetra-primer amplification refractory mutation (Tetra-ARMS) PCR. Data were analyzed using SNPstat software.
Results
The prevalence of TP53 (Pro72Arg) rs1042522 genotypes carrying the high-risk allele [Pro/Arg (CG) and Arg/Arg (GG)] were significantly higher in BC patients compared to healthy volunteers and were associated with BC susceptibility (OR 0.2; [95% CI 0.11–0.38]; P = 0.0001). However, there was no statistical significant difference in the prevalence of TP53 (Pro72Arg) rs1042522 genotypes carrying the high-risk allele between familial and non-familial BC patients. In addition, there was no association between the prevalence of TP53 (Pro72Arg) rs1042522 genotypes carrying the high-risk allele and BC patients’ clinical and pathological characteristics including tumor size, tumor grade, lymph node status, presence of lymphovascular invasion, expression of ER, PR and Her-2 in both of familial and non-familial BC patients.
Conclusions
TP53 (Pro72Arg) rs1042522 is more prevalent among BC patients but not associated with disease progression.
Similar content being viewed by others
Introduction
The incidence of Breast Cancer (BC) in the Middle east has substantially increased, especially among young women, and is characterized by late diagnosis [1]. The women diagnosed with BC at earlier stages have a high survival rate, when compared to later stages women [2]. BC mortality rates are increasing in developing countries including Egypt which represents 32% of cancer cases [3] it is ranked fifth as a cause of death in women in less developed regions (324,000 deaths, 14.3% of total) it is now the second leading cause of cancer death in more developed regions (198,000 deaths, 15.4% of total) [3, 4]. Like other cancers, genetic variations have been shown to have a crucial role in BC development [5, 6]. Most BC predisposing genes are tumor suppressor genes such as; Breast Cancer gene 1 and 2 (BRCA1/BRCA2), Tumor Protein 53 (TP53), Phosphatase and TENsin homolog (PTEN) and Checkpoint Kinase 2 (CHK2) that are involved in DNA damage repair pathways and cell cycle control are reported to be associated with the progression of BC [7]. TP53 is the most frequently mutated tumor-suppressor gene in BC and previous epidemiological studies have revealed that mutations in TP53 occur in approximately 30% of BC cases [8, 9]. The role of TP53 mutations in BC survival is confounded by different studies that revealed that TP53 mutations are associated with negative or positive disease outcomes [10,11,12,13].
TP53 gene is located on chromosome 17p13.1 with a 20 kb gene size [14, 15] constituting 13 exons and 11 introns [16]. TP53 codes for the transcription factor P53 [17], which is responsible for initiating the transcription process of several genes involved in cellular processes, such as cell cycle arrest, apoptosis, metabolism, and DNA repair [14, 18]. More than 90% of mutations occur in the TP53 gene encode for a missense mutant protein that extends along 190 different codons localized in the DNA binding domain of the gene [19]. About 10% of the previously stated mutations were reported to have a loss of protein function either through deletion or frameshift mutations [19]. Al Qasem and colleagues indicated that TP53 mutation prevalence in Arab BC patients is found to be the highest in the world representing more than 40% of all BC cases [20]. More than 200 genetic polymorphisms have been detected in TP53 [21]. Three single nucleotide polymorphisms (SNPs) in TP53 gene were associated with tumorigenesis [22]. The first SNP (rs1042522 C > G; CGC-CCC) located at codon 72 of exon 4 of TP53, which results in substitution of proline (Pro) to arginine (Arg) which have the capability to alter the P53 function and has been reported to be associated with BC progression [23, 24]. The second polymorphism is a 16-bp insertion repeat in the third intron region of TP53 gene [25, 26] and the third polymorphism occurs at the MSP I restriction site of TP53 gene in the sixth intron [27]. TP53 rs1042522 is most important and extensively studied polymorphism among these three polymorphisms [28]. Many previous studies have shown an association between these three polymorphisms and the genetic susceptibility of many tumors [26, 29, 30]. In particular, the TP53 rs1042522 SNP is associated with susceptibility to several malignancies including breast, lung, and cervical cancers [28, 31,32,33], suggesting an important role of this part of the TP53 gene in the development of cancer. In addition, the rs1042522 has an important role in the P53-mediated apoptosis [34].
Previous study on Egyptian BC women found this variant to be repeatedly found among their patients and was concluded as well to be associated with drug responsiveness [35]. Since codon 72 in TP53 gene influence the ability of P53 to bind to P73 that has a role to influence the BC patient responsiveness to chemotherapy through modulation of its apoptosis dependent pathway. Their findings were also validated by another study which was done by Cheng and colleagues [36]. According to both studies the mutations in TP53 may influence the BC patient’s response to chemotherapy. Additional studies have reported that patients with these hot spot mutations are associated with poor prognosis where they have also suggested that R72 is associated with resistance to chemotherapy and thereof; can be used as a chemotherapy predictive marker in BC patients [37, 38]. The clinical studies showed controversial results about the predictive and prognostic values of TP53 mutation in codon 72, where none of them was conclusive up to date [24, 39]. Therefore, herein we aimed to explore the prevalence of TP53 rs1042522 among familial and non-familial breast cancer patients and its possible association with pathogenesis of BC.
Patients and methods
Patients' samples
The present study was approved by the Institutional Review Board (IRB) of the Ministry of Health (IORG0005704/IRB0000687). A total of 100 Egyptian BC patients (50 familial and 50 non-familial) and 100 healthy volunteers were enrolled in this study. All BC patients were fully subjected to clinical investigations such as the hormonal status (estrogen (ER), progestrogen (PR) and human epidermal receptor-2 (Her-2)), lymph node involvement, menopausal status, tumor size, and tumor grade as well as did not receive neoadjuvant chemotherapy treatment before surgery nor diagnosed with ovarian cancer. Blood samples were recruited from out-patient clinics and Radiodiagnosis Department at El-Demerdash Hospital, National Cancer Institute, and El Matarya Hospital. All enrolled participants signed a consent form for acceptance of the publication of anonymous data.
Genomic DNA isolation and purification
Genomic DNA was isolated from collected blood samples using GeneJET™ genomic DNA purification Kit (Thermo Scientific, MA, USA). The concentration and quality of the genomic DNA were determined using Nanodrop ND2000 Spectrophotometer (Nanodrop Technologies, DE, USA).
Tetra-amplification refractory mutation system PCR
Tetra-amplification refractory mutation system–polymerase chain reaction (T-ARMS-PCR), which is considered a rapid, accurate and simple genotyping technique [40], was conducted for detection of TP53 rs1042522 genotypes as described before [41]. Sequences of all primers are described in Table 1. T-ARMS-PCR reaction was conducted in 25μL total volume containing 1μL of each upstream and downstream primers (10 pmol/μL), 5μL of the DNA containing a maximum 40 ng DNA, 12.5μL of EmeraldAmp® MAX PCR green master mix, and 5.5μL of free RNase water. The thermal profile (AriaMX real-time PCR system, Agilent,USA) included an initial denaturation at 95 °C for 5 min, followed by 35 cycles of 95 °C for 30 s, 56 °C for 1 min, and 72 °C for 1 min, followed by terminal extension at 72 °C for 10 min as described by [41].
Agarose gel electrophoresis
Amplified PCR products were visualized on 2% agarose gels (BioBasic Inc., Canada), stained with ethidium bromide, and photographed by the Gel Doc XR + Gel documentation system (Bio-Rad Laboratories, CA, USA).
Statistical analysis
Data were expressed as mean ± standard deviation (SD). Statistical difference between groups was assessed by Student's t-test and chi square test. P-value < 0.05 were considered to be statistically significant. Hardy–Weinberg equilibrium was used to compare the genotypes prevalence of TP53 rs1042522 among BC patients and healthy volunteers. Logistic regression was used to calculate the odds ratios (OR) and 95% CI to estimate the relative association between BC progression and a particular allele and genotype using SNPstat software [42]. Correlation was assessed by Pearson correlation coefficient using SPSS 22.0 software [41].
Results
Clinical and pathological characterization of familial and non-familial BC patients
Clinical and pathological characterization of familial (n = 50) and non-familial BC patients (n = 50) are described in Table 2. Statistical analysis revealed that there were no significant differences in age, tumor size, tumor grade, lymph node involvement, lymphovascular invasion and status of hormonal receptors among familial and non-familial BC patients.
Prevalence of TP53 rs1042522 genotypes carrying the high-risk allele [Pro/Arg (CG) and Arg/Arg (GG)] was higher in BC patients compared to healthy volunteers
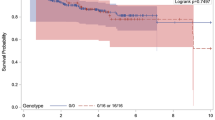
The genotypes and alleles distributions for the TP53 rs1042522 polymorphism are presented in Table 3. The genotype distribution of both studied groups’ fits the Hardy–Weinberg equilibrium (P > 0.05). The prevalence of TP53 rs1042522 genotypes carrying the high-risk allele [Pro/Arg (CG) and Arg/Arg (GG)] was significantly higher in BC patients in comparison with seemingly healthy volunteers (81% and 46% respectively) and were also associated with BC susceptibility (OR 0.2; [95% CI 0.11–0.38]; P = 0.0001). Moreover, Presence of TP53 rs1042522 genotypes carrying the high-risk allele [Pro/Arg (CG) and Arg/Arg (GG)] was not significant between familial and non-familial BC patients (50% and 66% respectively) P = 0.14.
A Prevalence of TP53 rs1042522 genotypes carrying the high-risk allele [Pro/Arg (CG) and Arg/Arg (GG)] with no association for BC disease progression
There was no observed association between the prevalence of TP53 rs1042522 genotypes carrying the high-risk allele [Pro/Arg (CG) and Arg/Arg (GG)] and BC patients with regards to clinical and pathological characters including tumor size, tumor grade, lymph node status, presence of lymphovascular invasion, expression of ER, PR and Her-2 in each of familial and non-familial BC patient groups as shown in Table 4 and 5.
Discussion
TP53, also referred to as the guardian of the genome, is one of the most studied tumor suppressor genes, with key roles in the inhibition of angiogenesis, invasion as well as cell cycle control and apoptosis as a key transcription factor [18, 35]. According to Mutations in Cancer (COSMIC) database it was found to be the second most frequent mutated gene representing about 40–60% of all breast cancer patients [17, 20, 43]. Prevalence of TP53 mutation among Arab patients showed to be the highest in the world (40%) [20].
Previous studies have proposed that the TP53 gene Pro72Arg polymorphism consequently produces a differently functioning protein as a result of transition from CGC to CCC which may be associated with different types of cancer types such as colorectal [41], lung and breast cancer[44], yet the results among different populations are conflicting.
Herein, we evaluated the role of TP53 gene Pro72Arg (rs1042522) polymorphism in BC among familial and non-familial Egyptian patients, where, we have found that the prevalence of TP53 rs1042522 heterogenous genotypes carrying the high-risk allele [Pro/Arg (CG) and Arg/Arg (GG)] were significantly higher in BC patients in comparison with healthy volunteers and were also associated with BC susceptibility which goes in accordance with study among Iranian population who were found to have this heterogenous population among BC patients and control with 75.55% and 62%, respectively. Other studies as well have supported the evidence for the Pro72Arg (rs1042522) polymorphism to be of significant risk for lung and breast cancer among different populations [45, 46] which goes in accordance with our results, where we have found that Pro72Arg (rs1042522) polymorphism was more prevalent in BC patients than healthy women.
Yet, our results contradicts with a study by Al Qasem et al., in Saudi Arabia who reported that this heterogenous genotypes was found higher among the healthy women more than those with BC (60.19% and 25% respectively) [47]. Moreover, our results contradict with the meta-analysis reported by GonÇalves et al., as well as Habyarimana et al., who suggested that the TP53 gene Pro72Arg polymorphism among Rwandese population could not be assessed as a risk factor for BC. Since Pro/Arg (CG) and Arg/Arg (GG) heterogenous genotype predominated in both; healthy and BC patients with no significant association [24, 45].
Additionally, our results did not show significant difference among the familial and non-familial BC patients nor with clinical and pathological data which goes in accordance with Habyarimana et al., [24] and contradicts with study by Tommiska et al., in Finland where they have found that BC patients with TP53 gene Pro72Arg (rs1042522) polymorphism were characterize with grade I tumors [48].
In conclusion, this is the first report designed among the Egyptian BC population which assessed the risk of TP53 gene Pro72Arg polymorphism among familial and non-familial BC patients. Our preliminary results suggest that there is no association between TP53 gene Pro72Arg (rs1042522) and breast cancer and thereof this polymorphism cannot be considered as a risk factor for the predisposition of BC in Egypt. However, further studies to investigate other genetic mutations affecting the activity of TP53 in Egyptian BC patients using larger sample size are needed in order to investigate its association with disease development.
Availability of data and materials
There are no restrictions on the availability of the presented materials, data, and associated protocols.
References
Hashim MJ et al (2018) Burden of breast cancer in the Arab world: findings from global burden of disease, 2016. J Epidemiol Glob Health 8(1–2):54–58
Brandt J et al (2015) Age at diagnosis in relation to survival following breast cancer: a cohort study. World J Surg Oncol 13:33–33
Loutfy SA et al (2021) Prevalence of MMTV-Like env sequences and its association with BRCA1/2 genes mutations among Egyptian breast cancer patients. Cancer Manag Res 13:2835–2848
Ferlay J et al (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136(5):E359–E386
Shiovitz S, Korde LA (2015) Genetics of breast cancer: a topic in evolution. Ann Oncol 26(7):1291–1299
Feng Y et al (2018) Breast cancer development and progression: Risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes Dis 5(2):77–106
Rasool R et al (2022) Theranostic Interpolation of Genomic Instability in Breast Cancer. Int J Mol Sci 23(3):1861
Comprehensive molecular portraits of human breast tumours. Nature 2012. 490(7418):61–70
Shahbandi A, Nguyen HD, Jackson JG (2020) TP53 mutations and outcomes in breast cancer: reading beyond the headlines. Trends Cancer 6(2):98–110
Berns EM et al (2000) Complete sequencing of TP53 predicts poor response to systemic therapy of advanced breast cancer. Cancer Res 60(8):2155–2162
Bertheau P et al (2008) TP53 status and response to chemotherapy in breast cancer. Pathobiology 75(2):132–139
Silwal-Pandit L et al (2014) TP53 mutation spectrum in breast cancer is subtype specific and has distinct prognostic relevance. Clin Cancer Res 20(13):3569–3580
Chen MB et al (2012) Value of TP53 status for predicting response to neoadjuvant chemotherapy in breast cancer: a meta-analysis. PLoS ONE 7(6):e39655
Peng L et al (2016) Association between BRCA status and P53 status in breast cancer: a meta-analysis. Med Sci Monit 22:1939–1945
Abubakar M et al (2019) Clinicopathological and epidemiological significance of breast cancer subtype reclassification based on p53 immunohistochemical expression. NPJ Breast Cancer 5:20
Berke TP, Slight SH, Hyder SM (2022) Role of reactivating mutant p53 protein in suppressing growth and metastasis of triple-negative breast cancer. Onco Targets Ther 15:23–30
Pollock NC et al (2022) Differences in somatic TP53 mutation type in breast tumors by race and receptor status. Breast Cancer Res Treat 192(3):639–648
Li T et al (2012) Tumor suppression in the absence of p53-mediated cell-cycle arrest, apoptosis, and senescence. Cell 149(6):1269–1283
Baugh EH et al (2018) Why are there hotspot mutations in the TP53 gene in human cancers? Cell Death Differ 25(1):154–160
Al-Qasem AJ et al (2011) TP53 genetic alterations in Arab breast cancer patients: novel mutations, pattern and distribution. Oncol Lett 2(2):363–369
Pietsch EC, Humbey O, Murphy ME (2006) Polymorphisms in the p53 pathway. Oncogene 25(11):1602–1611
Naccarati A et al (2012) Mutations and polymorphisms in TP53 gene–an overview on the role in colorectal cancer. Mutagenesis 27(2):211–218
Pim D, Banks L (2004) p53 polymorphic variants at codon 72 exert different effects on cell cycle progression. Int J Cancer 108(2):196–199
Habyarimana T et al (2018) Association of p53 Codon 72 polymorphism with breast cancer in a Rwandese population. Pathobiology 85(3):186–191
Costa S et al (2008) Importance of TP53 codon 72 and intron 3 duplication 16bp polymorphisms in prediction of susceptibility on breast cancer. BMC Cancer 8:32–32
Eskandari-Nasab E et al (2015) Effect of TP53 16-bp and β-TrCP 9-bp INS/DEL polymorphisms in relation to risk of breast cancer. Gene 568(2):181–185
Tan C et al (2011) Effect of CYP1A1 MSPI polymorphism on the relationship between TP53 mutation and CDKN2A hypermethylation in non-small cell lung cancer. Arch Med Res 42(8):669–676
He J et al (2017) The TP53 gene rs1042522 C>G polymorphism and neuroblastoma risk in Chinese children. Aging (Albany NY) 9(3):852–859
Fernández-Rubio A et al (2008) The TP53 Arg72Pro polymorphism and lung cancer risk in a population of Northern Spain. Lung Cancer 61(3):309–316
Laprano TD et al (2014) Association of TP53 codon 72 and intron 3 16-bp Ins/Del polymorphisms with cervical cancer risk. Tumour Biol 35(8):7435–7440
Sonoyama T et al (2011) TP53 codon 72 polymorphism is associated with pancreatic cancer risk in males, smokers and drinkers. Mol Med Rep 4(3):489–495
Dong Z et al (2018) Association of mRNA expression of TP53 and the TP53 codon 72 Arg/Pro gene polymorphism with colorectal cancer risk in Asian population: a bioinformatics analysis and meta-analysis. Cancer Manag Res 10:1341–1349
Fu W et al (2017) Association between TP53 gene Arg72Pro polymorphism and Wilms’ tumor risk in a Chinese population. Onco Targets Ther 10:1149–1154
Sakamuro D et al (1997) The polyproline region of p53 is required to activate apoptosis but not growth arrest. Oncogene 15(8):887–898
Nassar A et al (2020) Targeted next generation sequencing identifies somatic mutations in a cohort of Egyptian breast cancer patients. J Adv Res 24:149–157
Cheng H et al (2012) Individual and combined effects of MDM2 SNP309 and TP53 Arg72Pro on breast cancer risk: an updated meta-analysis. Mol Biol Rep 39(9):9265–9274
Basu S et al (2018) Mutant p53 controls tumor metabolism and metastasis by regulating PGC-1alpha. Genes Dev 32(3–4):230–243
AbdelHamid SG et al (2021) BRCA1 and BRCA2 truncating mutations and variants of unknown significance in Egyptian female breast cancer patients. Clin Chim Acta 512:66–73
Icen-Taskin I, Irtegun-Kandemir S, Munzuroglu O (2020) TP53 rs1042522 polymorphism and early-onset breast cancer. J Res Med Sci 25:25
Ye S et al (2001) An efficient procedure for genotyping single nucleotide polymorphisms. Nucleic Acids Res 29(17):E88–E98
Asadi M et al (2017) TP53 gene Pro72Arg (rs1042522) single nucleotide polymorphism as not a risk factor for colorectal cancer in the Iranian Azari population. Asian Pac J Cancer Prev 18(12):3423–3427
Solé X et al (2006) SNPStats: a web tool for the analysis of association studies. Bioinformatics 22(15):1928–1929
Walerych D et al (2012) The rebel angel: mutant p53 as the driving oncogene in breast cancer. Carcinogenesis 33(11):2007–2017
Jafrin S et al (2020) Association of TP53 codon 72 Arg>Pro polymorphism with breast and lung cancer risk in the South Asian population: a meta-analysis. Asian Pac J Cancer Prev 21(6):1511–1519
Goncalves ML et al (2014) Association of the TP53 codon 72 polymorphism and breast cancer risk: a meta-analysis. Springerplus 3:749
Henriquez-Hernandez LA et al (2009) Gene polymorphisms in TYMS, MTHFR, p53 and MDR1 as risk factors for breast cancer: a case-control study. Oncol Rep 22(6):1425–1433
Al-Qasem A et al (2012) The p53 codon 72 polymorphism is associated with risk and early onset of breast cancer among Saudi women. Oncol Lett 3(4):875–878
Tommiska J et al (2005) Breast cancer patients with p53 Pro72 homozygous genotype have a poorer survival. Clin Cancer Res 11(14):5098–5103
Acknowledgements
This work was conducted in the National Cancer Institute, Cairo University, Egypt. Special thanks to Dr. Hossam Taha Mohamed, Faculty of Biotechnology at October University for Modern Sciences and Arts, for his help and support during practical experiments and data analysis.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
SA and SAL suggested the idea and designed the research strategy and experimental protocols. Surgeon MMM was responsible for enrolling patients. SA collected patients’ clinical and pathological data. SA conducted all practical experiments of the study. SA and SAL analyzed the data using SNPSTATS online tools and Statistical Package of the Social Sciences and biomedical informatics software. SA and SAL drafted and wrote the manuscript with the input of all co-authors. SAL, AEG, AAE, and GS revised and edited the final draft.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The present study was approved by the Institutional Review Board (IRB) of the Ministry of Health (IORG0005704/IRB0000687). Before participation, all patients signed consent forms.
Consent for publication
Written informed consent for publication of the study results was obtained from all patients before participation.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ahmed, S., Safwat, G., Moneer, M.M. et al. Prevalence of TP53 gene Pro72Arg (rs1042522) single nucleotide polymorphism among Egyptian breast cancer patients. Egypt J Med Hum Genet 24, 24 (2023). https://doi.org/10.1186/s43042-023-00405-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43042-023-00405-1