Abstract
The coronavirus disease 2019 (COVID-19) pandemic has caused human tragedy through the global spread of the viral pathogen SARS-CoV-2. Although the underlying factors for the severity of COVID-19 in different people are still unknown, several gene variants can be used as predictors of disease severity, particularly variations in viral receptor genes such as angiotensin-converting enzyme 2 (ACE2) or major histocompatibility complex (MHC) genes. The reaction of the immune system, as the most important defense strategy in the case of viruses, plays a decisive role. The innate immune system is important both as a primary line of defense and as a trigger of the acquired immune response. The HLA-mediated acquired immune response is linked to the acquired immune system. In various diseases, it has been shown that genetic alterations in components of the immune system can play a crucial role in how the body responds to pathogens, especially viruses. One of the most important host genetic factors is the human leukocyte antigen (HLA) profile, which includes HLA classes I and II and may be symbolic of the diversity of immune response and genetic predisposition in disease progression. COVID-19 will have direct contact with the acquired immune system as an intracellular pathogen after exposure to the proteasome and its components through class I HLA. Therefore, it is assumed that in different genotypes of the HLA-I class, an undesirable supply causes an insufficient activation of the immune system. Insufficient binding of antigen delivered by class I HLA to host lymphocytes results in uncertain identification and insufficient activation of the acquired immune system. The absence of secretion of immune cytokines such as interferons, which play an important role in controlling viral infection in the early stages, is a complication of this event. Understanding the allelic diversity of HLA in people infected with coronavirus compared with uninfected people of one race not only allows identification of people with HLA susceptible to COVID-19 but also provides better insight into the behavior of the virus, which helps to take effective preventive and curative measures earlier.
Similar content being viewed by others
Introduction
A new coronavirus known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first reported in Wuhan, China, in December 2019. The rapid spread of this viral pathogen around the world has resulted in 619 million confirmed cases of infection and more than 6,537,636 deaths by October 2022 [1,2,3]. This virus is similar to a number of previous beta coronaviruses such as SARS and middle east respiratory syndrome (MERS), which can cause lower respiratory tract infections and even death [4, 5]. Of note, the mortality rate for the new coronaviruses is 3.78%, which is lower than two previous pandemics, SARS-CoV (15%) and MERS (37%) [6, 7]. Reportedly, the severity of infection of SARS-COV-2 varies from mild symptoms [8] to severe illness and even death. The incubation period (the time between exposure and the onset of symptoms) can be up to two weeks [9]. Fever, fatigue, and dry cough are the most common symptoms of COVID-19. Various other symptoms may also occur, such as pain and bruising, stuffy nose, runny nose, sore throat, or diarrhea. To relieve the symptoms, infected people should receive supportive treatment [6]. Although the world is fighting the epidemic COVID-19 by implementing quarantine measures and protocols as well as vaccinations [10], in reality, these efforts have been thwarted by many problems such as the confiscation of vaccines and medical supplies [11, 12].
Host immunity against SARS-Cov-2
Viral infections can be effectively controlled by appropriate activation of cytotoxic T cells in response to antigen-presenting cells [13]. In this context, cluster of differentiation 4 (CD4) T cells (TCD4) play an important role in host immunity against SARS and MERS by stimulating the production of virus-specific antibodies via B cells. Moreover, cytotoxic TCD8 cells can kill infected cells by recognizing MHC on the cells [14], highlighting the important role of T cells in controlling the pathogenesis of these viral infections. On the other hand, T helper cells control infection by producing inflammatory cytokines [15]. However, the abnormal release of cytokines such as some interleukins (IL 6, IL 1, IL 2, IL 10), tumor necrosis factor-alpha (TNFα), and interferon-gamma (IFN-γ) causes an uncontrolled response that leads to the destruction of lung tissue and even death [16,17,18,19], phenomenon known as the cytokine storm.
A cytokine storm describes how the immune system contributes to an uncontrolled and widespread inflammatory response [20]. A cytokine storm is a major contributor to a more severe clinical course, as higher levels of CXCL10, CCL2, and TNFα were found in patients with COVID-19 who required admission to the ICU than in those who did not [21]. The immune system's "attack" on the body immediately follows the cytokine storm, and in the most severe cases of COVID-19 infection, the result is death [4]. It has been reported that among the interleukins, IL-6 plays a more important role in the cytokine storm caused by coronavirus due to its involvement in the regulation of the acute phase response [22]. However, much less is known about the biochemical and clinical implications of this immune system hyperactivity.
The presentation of viral fragments on the surface of host cells could be via MHC molecules, often referred to as human leukocyte antigen (HLA). The MHC, located on chromosome 6, plays an important role in the development of the immune response to protein antigens [2]. MHCs are classified into three classes based on their tissue distribution and function. Epidemiological studies have shown an association between various diseases and certain HLA alleles, including those caused by ribonucleic acids (RNA) viruses, such as SARS, influenza, human immunodeficiency virus (HIV), hepatitis C, rabies, and other [23]. Compared with HLA class II, HLA class I plays a crucial role in viral infection by presenting viral antigens to CD8+ T cells on the surface of infected cells, followed by recognition and destruction of the cells [24]. The likely effect of T cells in SARS-CoV-2 infection could be illustrated by the response of 40–60% of T cells in unexposed individuals to viral proteins due to a cross-immune reaction with other coronaviruses in previous colds [25].
Pathogenesis of SARS-CoV-2
Understanding the susceptibility or resistance to a particular disease associated with the presence of specific alleles can be useful in drug manufacturing and development and in identifying at-risk populations [26]. In this regard, COVID-19 can be prevented by lifelong immunological memory using a vaccine based on natural protective immunity to SARS-CoV-2 infection [27, 28]. Therefore, it is important to find the reasons for the different clinical responses of infected individuals. In this context, reference can be made to polymorphisms in various genes, especially in viral receptor genes (ACE2) or genes involved in the diversity of immune responses (such as MHCs). Angiotensin-converting enzyme 2 (ACE2) is a protein with multiple functions, including catalytic, amino acid transporter, and viral receptor [29]. The host receptor of SARS-CoV-2, ACE2, binds to cell membranes and acts as a transporter of the new virus [14]. Coronavirus entry is mediated by the spike S glycoprotein [30]. Following viral binding and membrane fusion, ACE2 is internalized, and its activity is downregulated on the target cell surface, leading to COVID-19 infection [1].
MHC class I/II may be a symbol of the diversity of a person's immune response and genetic predisposition to disease progression and immunity [31]. Detection of differences in HLA response to SARS-CoV-2 peptides in infected patients could be a potential factor for developing a personalized treatment based on individual risk. HLA polymorphism in different populations could have an impact on the susceptibility and severity of COVID-19. In this context, a common specific allele may be more prevalent in a given population than others. In contrast, various HLA alleles in different populations might have similar binding sites for viral peptides [32, 33].
Antigen presentation in SARS-CoV-2 infection
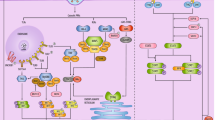
Specifically, MHC-I presents viral antigens via the direct involvement of endoplasmic reticulum aminopeptidase (ERAP) isoforms, ERAP1 and ERAP2 proteins, which in turn can contribute to the recognition of the infected cell by CD8 + cytotoxic T lymphocyte (CTL) clones and trigger a protective immune response [34]. As shown in Fig. 1, during replication of SARS-CoV-2 in host cells, viral antigens are processed by host proteasomes, and the resulting peptides are transported to the endoplasmic reticulum (ER) by the molecular transporter associated with antigen processing (TAP) [35]. In the endoplasmic reticulum, viral peptides are influenced by ERAP1 and ERAP2 to be presented in the clefts of MHC class I molecules. Finally, MHC I enables the monitoring of ongoing infections by CD8 + T cells [36].
SARS-CoV-2 antigen presentation pathway through MHC-I molecules. Cells infected with SARS-CoV-2 produce various isoforms of endoplasmic reticulum aminopeptidase 2 (ERAP2) genes dimerized with either ERAP2-wild type (wt) or ERAP1-wt which can be presented on cells and recognized by specific CD8 + cytotoxic T lymphocytes (CTL). ER: endoplasmic reticulum; TAP: transporter associated with antigen processing [36]. Created with BioRender.com
Discussion
HLA genotypes and SARS-CoV-2
As shown in Table 1, many studies have been performed on the HLA types involved in the susceptibility or severity of COVID-19. In describing these studies, we first discuss the significant relationship between viral infections with a similar pathogenicity mechanism as SARS-CoV-2 and the HLA system. Then, we will evaluate SARS-CoV-2 in this regard. For example, Nguyen et al. identified HLA-B*15:03 and found that individuals with this allele were more able to deliver viral peptides to the cell surface. In contrast, they predicted that the HLA-B*46:01 allele was the least able to bind to viral peptides, suggesting that individuals with this allele may have a weaker immune response and more severe symptoms [26]. In HIV-1 infection, which has a similar pathogenic mechanism to COVID-19, the presence of HLA-A*02:05 results in relative resistance to the disease. However, in the Thai population, some HLA alleles, including HLA-A*02:07 and HLA-B*51, have been associated with increased disease severity [37, 38].
Notably, HLA-Cw1502, DR0301, HLA-Cw*1502, DRB1*0301, HLA-B*15:02, A*02:06, A*68:01, A*02:22, and A*24:03 confer resistance to SARS-CoV-1 infection [39, 40]. It has also been reported that the MHC class I haplotype (HLA-B*-4601, HLA-B*-0703, HLA-Cw* 0801, HLA-A*11:01, B*51:01, C* 14:02) and the MHC class II haplotype, HLA-DRB1*1202, are associated with increased susceptibility to SARS-CoV-1 [40,41,42]. Because the homology and pathogenicity mechanism of SARS-Co-V are very similar to COVID-19, the HLAs likely mentioned in various studies, such as HLA-A*02:01, HLA-A*02:06, HLA-A*24:02, HLA-B*15:03, HLA-B*44, C*01, HLA-A*25, and HLA-B*08, also play a vital role in susceptibility or resistance to COVID-19 [39, 43, 44].
To better understand the relationship between HLA alleles and SARS-CoV-2 outcomes, we will describe additional studies in this section. The first study examined HLA-A*24:02 in a small group of the Wuhan population. Warren et al. found this allele in four of five individuals and described it as an allele associated with susceptibility to SARS-Cov-2 [45]. After the publication of the current article, the results of the study by Tomita et al. showed that individuals carrying the alleles HLA-A*24:02 and HLA-A*11:01 have a relatively higher ability to present SARS-CoV-2 antigens than individuals carrying HLA-A*02:01. In other words, they mentioned HLA-A*02:01 as a susceptible allele for COVID-19 [46]. In another study, 190 patients infected with SARS-CoV-2 in Hong Kong were found to have an association between serotype HLA-B*22 and an increased risk of COVID-19 [47].
The association between mortality rate and frequency of HLA allele haplotypes was found in 28 countries. In addition, the HLA-A*11:01 allele was found to be associated with a lower mortality rate [48]. In addition, three studies have been conducted in different regions of Italy to investigate the HLA allele haplotype and the incidence and mortality of COVID-19. The first study found that HLA-B*44 and HLA-C*01 allele groups were associated with an increased incidence of COVID-19 [43]. In the second study, the authors found that HLA-A*01:01, B*08:01, C*07:01, and DRB1*030:1 were associated with increased incidence and mortality of COVID-19, whereas the HLA-A*02:01-B*18:01-C*07:01-BRB1*11:04 haplotype was associated with lower incidence and mortality [49]. In the third study, 99 Italian patients infected with the severe form of COVID-19 were compared with a control group of 1017 uninfected individuals. The results showed that the following HLA alleles were susceptible to SARS-CoV-2: HLA-B*27:07 from MHC class I and HLA-DRB1*15:01 and HLA-DQB1*06:02 alleles from MHC class II [50].
In another case–control study in China, two HLA-I alleles at high risk for SARS-CoV-2 were identified: HLA-C*07:29 and HLA-B*15:27 [44]. A study examining a small number of mild, moderate, and severe forms of COVID-19 patients in Spain also found that the number of SARS-CoV-2 peptides bound to HLA molecules was negatively associated with disease severity. Theoretically, the higher affinity of HLA-I to bind to SARS-CoV-2 peptides was associated with the less severe forms of the disease [51].
Accordingly, the genome-wide association study (GWAS), involving 835 patients with severe forms of COVID-19 from Italy and 775 patients from Spain, did not identify any HLA alleles associated with the development of infection or disease severity. Similar to the previous study, the high number of SARS-COV-2 peptides binding in the cleft of HLA-I molecules was found to be associated with the less severe forms of the disease [52].
It should be noted that information from studies with a large statistical population does not always show a significant association between HLA and COVID-19. For example, in a study of 3886 healthy controls and 72 COVID-19 patients by Lorente et al. the HLAA*32 allele was found to be a protective allele, whereas the frequency of the HLA-C*16 and HLA-B*39 alleles was higher in infected individuals; however, the results were not statistically significant [53].
Sakuraba et al. examined the frequency of HLA class I alleles in 74 countries around the world using the Allele Frequency Net Database and the website www.worldometer.info. The results supported an association between the HLA-C*05 allele and higher mortality after developing COVID-19 [54]. Evaluation of HLA for SARS-CoV-2 severity in the Sardinian population also showed a protective effect of the following HLA haplotypes against SARS-CoV-2: HLA-A*02:05, B*58:01, C*07:01, and DRB1*03:01 [55].
Some studies have also examined the relationship between COVID-19 and HLA in patients with certain diseases, such as cancer. For example, no significant allelic relationship was found in the HLA genotype of lung cancer patients with and without COVID-19 [24].
SARS-CoV-2 mutations not only influence the COVID-19 course of the pandemic but also have a major impact on T-cell immunity, depending on the HLA supertype. Specifically, certain types of mutations in the viral genome trigger a variety of CD8 + T cell targets. Mutational biases also affect epitope presentation in a manner that depends on the HLA supertype. An important point regarding the dependence on HLA supertype in infection with mutant variants of SARS-CoV-2 is the differential modulation of T cell responses in different populations [27]. Based on the genetic landscapes of different populations, a way to predict infections and evaluate the efficacy of vaccines could be paved.
Conclusion
Although some studies have identified a specific HLA allele or allele group significantly associated with COVID-19 severity, the resulting data appear to be controversial. In addition, in the largest study examining more than 1500 patients with severe forms of COVID-19, no allelic association was found. Therefore, further studies should be performed to clarify the relationship between HLA alleles and COVID-19 development. Because of the diversity of HLAs and their various ethnic-genetic and geographic distributions [67–70], it is essential to study the susceptible and protective HLA alleles against SARS-CoV-2 in each race to take effective preventive and curative measures.
Availability of data and materials
Not applicable.
Abbreviations
- ACE2:
-
Angiotensin- converting enzyme 2
- COVID-19:
-
Coronavirus disease 2019
- CD4:
-
Cluster of differentiation 4
- CTL:
-
Cytotoxic T lymphocyte
- ERAP:
-
Endoplasmic reticulum aminopeptidase
- ER:
-
Endoplasmic reticulum
- GWAS:
-
Genome-wide association study
- HLA:
-
Human leukocyte antigen
- HIV:
-
Human immunodeficiency virus
- IL:
-
Interleukins
- IFN-γ:
-
Interferon-gamma
- MHC:
-
Major histocompatibility complex
- MERS:
-
Middle east respiratory syndrome
- RNA:
-
Ribonucleic acids
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- TNFα:
-
Tumor necrosis factor-alpha
- TAP:
-
Transporter associated with antigen processing
References
Jackson CB, Farzan M, Chen B, Choe H (2022) Mechanisms of SARS-CoV-2 entry into cells. Nat Rev Mol Cell Biol 23(1):3–20
Ranga V, Niemelä E, Tamirat MZ, Eriksson JE, Airenne TT, Johnson MS (2020) Immunogenic SARS-CoV-2 epitopes: In silico study towards better understanding of COVID-19 disease—paving the way for vaccine development. Vaccines. 8(3):408
Costela-Ruiz VJI-MR, Puerta-Puerta JM, Ruiz C, Melguizo-Rodríguez L. SARS-CoV-2, 2020. itrociC-dCGFR.
Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C et al (2020) Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 8(4):420–422
Saburi E, Abazari MF, Hassannia H, Mansour RN, Eshaghi-Gorji R, Gheibi M et al (2021) The use of mesenchymal stem cells in the process of treatment and tissue regeneration after recovery in patients with Covid-19. Gene 777:145471
Singh SP, Pritam M, Pandey B, Yadav TP (2020) Microstructure, pathophysiology, and potential therapeutics of COVID-19: a comprehensive review. J Med Virol 5:99
Abdelghany T, Ganash M, Bakri MM, Qanash H, Al-Rajhi AM, Elhussieny NI (2021) SARS-CoV-2, the other face to SARS-CoV and MERS-CoV: future predictions. Biomed J 44(1):86–93
Teymouri M, Mollazadeh S, Mortazavi H, Ghale-Noie ZN, Keyvani V, Aghababaei F et al (2021) Recent advances and challenges of RT-PCR tests for the diagnosis of COVID-19. Pathol-Res Pract 221:153443
Xu X, Chen P, Wang J, Feng J, Zhou H, Li X et al (2020) Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci 63(3):457–460
https://www.hsph.harvard.edu/news/hsph-in-the-news/the-latest-on-the-coronavirus/.
Saha A, Sharma AR, Bhattacharya M, Sharma G, Lee S-S, Chakraborty C (2020) Tocilizumab: a therapeutic option for the treatment of cytokine storm syndrome in COVID-19. Arch Med Res 51(6):595–597
Rosendahl Huber S, van Beek J, de Jonge J, Luytjes W, van Baarle D (2014) T cell responses to viral infections–opportunities for peptide vaccination. Front Immunol 5:171
Shang J, Wan Y, Luo C, Ye G, Geng Q, Auerbach A et al (2020) Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci 117(21):11727–11734
Atabati H, Yazdanpanah E, Mortazavi H, Raoofi A, Esmaeili S-A, Khaledi A et al (2021) Immunoregulatory effects of tolerogenic probiotics in multiple sclerosis. Rev New Drug Targets Age-Related Disord 8:87–105
Coperchini F, Chiovato L, Croce L, Magri F, Rotondi M (2020) The cytokine storm in COVID-19: an overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev 8:64
Costela-Ruiz VJ, Illescas-Montes R, Puerta-Puerta JM, Ruiz C, Melguizo-Rodríguez L (2020) SARS-CoV-2 infection: the role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev 6:87
Saha A, Sharma AR, Bhattacharya M, Sharma G, Lee S-S, Chakraborty C (2020) Tocilizumab: a therapeutic option for the treatment of cytokine storm syndrome in COVID-19. Arch Med Res 6:14
Zangouei AS, Hamidi AA, Rahimi HR, Saburi E, Mojarrad M, Moghbeli M (2021) Chemokines as the critical factors during bladder cancer progression: an overview. Int Rev Immunol 40(5):344–358
Coperchini F, Chiovato L, Croce L, Magri F, Rotondi M (2020) The cytokine storm in COVID-19: an overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev 53:25–32
Zhang C, Wu Z, Li J-W, Zhao H, Wang G-Q (2020) Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents 55(5):105954
Brocker C, Thompson D, Matsumoto A, Nebert DW, Vasiliou V (2010) Evolutionary divergence and functions of the human interleukin (IL) gene family. Hum Genom 5(1):30
Ovsyannikova IG, Haralambieva IH, Crooke SN, Poland GA, Kennedy RB (2020) The role of host genetics in the immune response to SARS-CoV-2 and COVID-19 susceptibility and severity. Immunol Rev 296(1):205–219
Weider T, Richardson SJ, Morgan NG, Paulsen TH, Dahl-Jørgensen K, Hammerstad SS (2021) HLA class I upregulation and antiviral immune responses in graves disease. J Clin Endocrinol Metab 106(4):e1763–e1774
Mobini Kesheh M, Shavandi S, Hosseini P, Kakavand-Ghalehnoei R, Keyvani H (2021) Bioinformatic HLA studies in the context of SARS-CoV-2 pandemic and review on association of HLA alleles with preexisting medical conditions. BioMed Res Int 2:21
Nguyen A, David JK, Maden SK, Wood MA, Weeder BR, Nellore A et al (2020) Human leukocyte antigen susceptibility map for SARS-CoV-2. J Virol 6:41
Hamelin DJ, Fournelle D, Grenier J-C, Schockaert J, Kovalchik K, Kubiniok P et al (2021) The mutational landscape of SARS-CoV-2 variants diversifies T cell targets in an HLA supertype-dependent manner. bioRxiv 9:417
Ghale-Noie ZN, Salmaninejad A, Bergquist R, Mollazadeh S, Hoseini B, Sahebkar A (2021) Genetic aspects and immune responses in Covid-19: important organ involvement. Identif Biomarkers New Treat Vaccines COVID-19 5:3–22
Medina-Enríquez MM, Lopez-León S, Carlos-Escalante JA, Aponte-Torres Z, Cuapio A, Wegman-Ostrosky T (2020) ACE2: the molecular doorway to SARS-CoV-2. Cell Biosci 10(1):1–17
Liu PP, Blet A, Smyth D, Li H (2020) The science underlying COVID-19: implications for the cardiovascular system. Circulation 142(1):68–78
Forouzesh M, Rahimi A, Valizadeh R, Dadashzadeh N, Mirzazadeh A (2020) Clinical display, diagnostics and genetic implication of Novel coronavirus (COVID-19) epidemic. Eur Rev Med Pharmacol Sci 24:4607–4615
Patarroyo ME, Patarroyo MA, Alba MP, Pabon L, Rugeles MT, Aguilar-Jimenez W et al (2021) The first chemically-synthesised, highly immunogenic anti-SARS-CoV-2 peptides in DNA genotyped aotus monkeys for human use. Front Immunol 12:724060
Naemi FM, Al-adwani S, Al-khatabi H, Al-nazawi A (2021) Association between the HLA genotype and the severity of COVID-19 infection among South Asians. J Med Virol 93(7):4430–4437
Saulle I, Vicentini C, Clerici M, Biasin M (2021) Antigen presentation in SARS-CoV-2 infection: the role of class I HLA and ERAP polymorphisms. Hum Immunol 82(8):551–560
D’amico S, Tempora P, Lucarini V, Melaiu O, Gaspari S, Algeri M et al (2021) ERAP1 and ERAP2 enzymes: a protective shield for RAS against COVID-19? Int J Mol Sci 22(4):1705
Saulle I, Vicentini C, Clerici M, Biasin M (2021) Antigen presentation in SARS-CoV-2 infection: the role of class I HLA and ERAP polymorphisms. Hum Immunol 8:47
MacDonald KS, Fowke KR, Kimani J, Dunand VA, Nagelkerke NJ, Blake Ball T et al (2000) Influence of HLA supertypes on susceptibility and resistance to human immunodeficiency virus type 1 infection. J Infect Dis 181(5):1581–1589
Stephens H, Klaythong R, Sirikong M, Vaughn D, Green S, Kalayanarooj S et al (2002) HLA-A and-B allele associations with secondary dengue virus infections correlate with disease severity and the infecting viral serotype in ethnic Thais. Tissue Antigens 60(4):309–318
Barquera R, Collen E, Di D, Buhler S, Teixeira J, Llamas B et al (2020) Binding affinities of 438 HLA proteins to complete proteomes of seven pandemic viruses and distributions of strongest and weakest HLA peptide binders in populations worldwide. HLA 8:948
Keicho N, Itoyama S, Kashiwase K, Phi NC, Long HT, Van Ban V et al (2009) Association of human leukocyte antigen class II alleles with severe acute respiratory syndrome in the Vietnamese population. Hum Immunol 70(7):527–531
Lin M, Tseng H-K, Trejaut JA, Lee H-L, Loo J-H, Chu C-C et al (2003) Association of HLA class I with severe acute respiratory syndrome coronavirus infection. BMC Med Genet 4(1):9
Ng MH, Lau K-M, Li L, Cheng S-H, Chan WY, Hui PK et al (2004) Association of human-leukocyte-antigen class I (B* 0703) and class II (DRB1* 0301) genotypes with susceptibility and resistance to the development of severe acute respiratory syndrome. J Infect Dis 190(3):515–518
Correale P, Mutti L, Pentimalli F, Baglio G, Saladino RE, Sileri P et al (2020) HLA-B* 44 and C* 01 prevalence correlates with Covid19 spreading across Italy. Int J Mol Sci 21(15):5205
Wang W, Zhang W, Zhang J, He J, Zhu F (2020) Distribution of HLA allele frequencies in 82 Chinese individuals with coronavirus disease-2019 (COVID-19). Hla 2:78
Warren RL, Birol I (2020) HLA predictions from the bronchoalveolar lavage fluid samples of five patients at the early stage of the Wuhan seafood market COVID-19 outbreak. ArXiv 9:56
Tomita Y, Ikeda T, Sato R, Sakagami T (2020) Association between HLA gene polymorphisms and mortality of COVID-19: an in silico analysis. Immun Inflamm Dis 8(4):684–694
Yung YL, Cheng CK, Chan HY, Xia JT, Lau KM, Wong RS et al (2021) Association of HLA-B22 serotype with SARS-CoV-2 susceptibility in Hong Kong Chinese patients. Hla 97(2):127–132
Toyoshima Y, Nemoto K, Matsumoto S, Nakamura Y, Kiyotani K (2020) SARS-CoV-2 genomic variations associated with mortality rate of COVID-19. J Hum Genet 5:1–8
Pisanti S, Deelen J, Gallina AM, Caputo M, Citro M, Abate M et al (2020) Correlation of the two most frequent HLA haplotypes in the Italian population to the differential regional incidence of Covid-19. J Transl Med 18(1):1–16
Novelli A, Andreani M, Biancolella M, Liberatoscioli L, Passarelli C, Colona VL et al (2020) HLA allele frequencies and susceptibility to COVID-19 in a group of 99 Italian patients. Hla 96(5):610–614
Iturrieta-Zuazo I, Rita CG, García-Soidán A, de Malet P-F, Alonso-Alarcón N, Pariente-Rodríguez R et al (2020) Possible role of HLA class-I genotype in SARS-CoV-2 infection and progression: a pilot study in a cohort of Covid-19 Spanish patients. Clin Immunol 219:108572
Group SC-G (2020) Genomewide association study of severe Covid-19 with respiratory failure. New England J Med 383(16):1522–34
Lorente L, Martín MM, Franco A, Barrios Y, Cáceres JJ, Solé-Violán J et al (2021) HLA genetic polymorphisms and prognosis of patients with COVID-19. Med Intensiva 45(2):96–103
Sakuraba A, Haider H, Sato T (2020) Population difference in allele frequency of HLA-C* 05 and its correlation with COVID-19 mortality. Viruses 12(11):1333
Littera R, Campagna M, Deidda S, Angioni G, Cipri S, Melis M et al (2020) Human leukocyte antigen complex and other immunogenetic and clinical factors influence susceptibility or protection to SARS-CoV-2 infection and severity of the disease course. Sardinian Exp Front Immunol 11:3320
Abdelhafiz AS, Ali A, Fouda MA, Sayed DM, Kamel MM, Kamal LM et al (2022) HLA-B* 15 predicts survival in Egyptian patients with COVID-19. Hum Immunol 83(1):10–16
Shkurnikov M, Nersisyan S, Jankevic T, Galatenko A, Gordeev I, Vechorko V et al (2021) Association of HLA class I genotypes with severity of coronavirus disease-19. Front Immunol 12:641900
Anzurez A, Naka I, Miki S, Nakayama-Hosoya K, Isshiki M, Watanabe Y et al (2021) Association of HLA-DRB1* 09: 01 with severe COVID-19. Hla 98(1):37–42
Weiner J 3rd, Suwalski P, Holtgrewe M, Rakitko A, Thibeault C, Müller M et al (2021) Increased risk of severe clinical course of COVID-19 in carriers of HLA-C* 04: 01. EClinicalMedicine 40:101099
Langton DJ, Bourke SC, Lie BA, Reiff G, Natu S, Darlay R et al (2021) The influence of HLA genotype on the severity of COVID-19 infection. Hla 98(1):14–22
Romero-Lopez JP, Carnalla-Cortes M, Pacheco-Olvera DL, Ocampo-Godinez JM, Oliva-Ramirez J, Moreno-Manjon J et al (2021) A bioinformatic prediction of antigen presentation from SARS-CoV-2 spike protein revealed a theoretical correlation of HLA-DRB1* 01 with COVID-19 fatality in Mexican population: an ecological approach. J Med Virol 93(4):2029–2038
Warren RL, Birol I (2021) HLA alleles measured from COVID-19 patient transcriptomes reveal associations with disease prognosis in a New York cohort. PeerJ 9:e12368
Wang WZW, Zhang J, He J, Zhu F (2020) Distribution of HLA allele frequencies in 82 Chinese individuals with coronavirus disease-2019 (COVID-19). Hla 8:42
Lorente L, Martín M, Franco A, Barrios Y, Cáceres J, Solé-Violán J et al (2021) HLA genetic polymorphisms and prognosis of patients with COVID-19. Med Intensiva 45(2):96–103
Correale P, Mutti L, Pentimalli F, Baglio G, Saladino RE, Sileri P et al (2020) HLA-B*44 and C*01 prevalence correlates with Covid19 spreading across Italy. Int J Mol Sci 21(15):87
Littera R, Campagna M, Deidda S, Angioni G, Cipri S, Melis M et al (2020) Human leukocyte antigen complex and other immunogenetic and clinical factors influence susceptibility or protection to SARS-CoV-2 infection and severity of the disease course. Sardinian Exper Front Immunol 11:605688
Esmaeili A, Rabe SZT, Mahmoudi M, Rastin M (2017) Frequencies of HLA-A, B and DRB1 alleles in a large normal population living in the city of Mashhad, Northeastern Iran. Iran J Basic Med Sci. 2017;20(8):940–943. https://doi.org/10.22038/IJBMS.2017.9117.
Mobasheri L, Nasirpour MH, Masoumi E, Azarnaminy AF, Jafari M, Esmaeili SA (2022) SARS-CoV-2 triggering autoimmune diseases. Cytokine. 2022;154:155873. https://doi.org/10.1016/j.cyto.2022.155873.
Saburi E, Abazari MF, Hassannia H, Mansour RN, Eshaghi-Gorji R, Gheibi M, Rahmati M, Enderami SE (2021) The use of mesenchymal stem cells in the process of treatment and tissue regeneration after recovery in patients with Covid-19. Gene. 2021;777:145471. https://doi.org/10.1016/j.gene.2021.145471.
Arab F, Jafari Rad M, Esmaeili S, Mirhosseini A, Moharreri M, Saburi E (2022) Investigation of polymorphisms of ACEII gene in people with coronavirus with severe and mild symptoms or asymptomatic. Int J Travel Med Global Health 10(3):122–126. https://doi.org/10.34172/ijtmgh.2022.22.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
FA & MM & FG & SM & ES was involved in search strategy and drafting. MM & ES supervised the project, and revised and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Arab, F., Mollazadeh, S., Ghayourbabaei, F. et al. The role of HLA genotypes in understanding the pathogenesis of severe COVID-19. Egypt J Med Hum Genet 24, 14 (2023). https://doi.org/10.1186/s43042-023-00392-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43042-023-00392-3