Abstract
Background
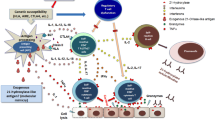
Hashimoto's thyroiditis is the most widespread autoimmune illness targeting a specific organ. "Redox homeostasis" is achieved when the production of Reactive Oxygen Species and their elimination are in balance. Advanced glycation end products (AGEs) are formed when glucose and/or α-oxaloaldehydes react non-enzymatically with the amino groups of lipids, proteins, and DNA. Nowadays, many studies are concerned with AGEs, the polymorphisms of their receptors, and their association with increased risk of HT. However, few studies investigated the role of receptors of advanced glycation end product (RAGE) SNP in Egyptian females.
Objective
The goals of this investigation were to ascertain whether oxidative stress plasma malondialdehyde (MDA) and total antioxidant capacity (TAC) were associated with HT, in addition, to assess the association of RAGE polymorphisms (− 374 T > A and the − 429 T > C and Gly82Ser) with HT.
Subject and methods.
Our case–control study has 80 patients enrolled who have newly been diagnosed with HT and 80 age and sex-matched healthy female controls. Each participant underwent a thorough medical history, physical examination, and laboratory investigations, which included Genotyping of RAGE Gly82Ser, − 374 T > A and − 429 T > C using polymerase chain reaction-restriction fragment length polymorphisms (PCR–RFLP).
Results
Chi-square revealed a significant association regarding the distribution of RAGE (− 374 T < C) genotypes TT and CC between patients and control (P = 0.04). Non-significant associations regarding the distribution of Gly82Ser genotypes Gly/Gly, Gly/Ser, Ser/Ser were found between patients and control (P = 0.5), and non-significant associations related to − 429 T > C gene polymorphism were revealed. In addition, patients with HT had higher MDA and lower TCA compared with controls.
Conclusion
The elevated MDA and decreased TAC as an antioxidant may be one of several risk factors associated with the prevalence of HT in individuals with the − 429 T > C RAGE mutation polymorphism that is associated with an increased risk of HT in Egyptian females.
Similar content being viewed by others
Introduction
Hashimoto's Thyroiditis (HT) is the most frequently occurring autoimmune disease that targets a specific organ [1], which causes dysfunction of the thyroid in variable grades [2]. It is a multifactorial disorder that is characterized by lymphocyte infiltration of the thyroid gland, elevation of serum anti-thyroid antibodies in the form of thyroglobulin autoantibodies (Tg), thyroid peroxidase (TPO), and the thyroid-stimulating hormone receptor (TSH-R), which are all markers of autoimmune thyroid diseases (AITD) [3]. Besides, there is a sign of goitrous or atrophic gland and the gradual destruction of thyroid cells by apoptosis, which determines the outcome [4]. Several years later, the patients who are initially euthyroid may have Hypothyroidism [5].
Reactive Oxygen Species (ROS) and the free radicals developed by the metabolism of the normal cell are well recognized for both beneficial and harmful effects on cells. The presence of low ROS levels is mandatory for various biochemical reactions within the cells. However, excessive ROS produces injury to the cell by its reaction with lipids, proteins, DNA, and inhibition of the normal functions [6, 7]. Normally, there are either enzymatic or non-enzymatic defense systems, called antioxidants, for the prevention of damage. Thus, "redox homeostasis" occurs when ROS generation and elimination are in balance [8]. Meanwhile, oxidative stress occurs by the imbalance between prooxidants and antioxidants, which causes damage to macromolecules, disruption of ions in redox signaling, and alterations of proteins [9]. Many studies found an association between increased oxidative stress and HT [10]. Though, the exact link between them is still debated [11].
Advanced Glycation End products (AGEs) are formed when glucose and/or α-oxaloaldehydes react non-enzymatically with the amino groups of lipids, proteins, and DNA. Those changes cause modification of the structure and function of proteins, and intramolecular and intermolecular cross-link formation. In the vascular endothelium, the binding between the receptor of the advanced glycation end product (RAGE) by AGEs leads to endothelial cells and pericytes changing that are characteristic of many inflammatory diseases such as Hashimoto thyroiditis [12].
Recently, many studies have been concerned with the RAGE roles, the polymorphisms of the correlated receptor, and its association with many autoimmune diseases. RAGE is one of the cell surface receptors which belongs to the immunoglobulin superfamily [13], and it is found in high concentrations in different cells and tissues [14]. The RAGE gene is located on chromosome 6p21.3 in the MHC locus III region and it spans a 1–7-kb 50 flanking area and 11 exons [15].
Various RAGE polymorphisms have been linked to the occurrence of cardio-metabolic syndrome besides vascular complications [16]. Furthermore, the most extensively investigated RAGE polymorphisms, − 429 T > C and − 374 T > A, are located in the gene's promoter region. Additionally, within the exons, a frequent variant (Gly82Serine) and three unusual modifications have been identified (Thr18Pro, Gly329Ala, and Ala389Gln). Some studies reported that Gly82Ser polymorphism in the RAGE gene is linked with HT. However, other studies did not support these findings. A meta-analysis study was done by Jun Wang, who found no significant relationships between − 429 T/C polymorphism and risk of myocardial infarction [16].
Numerous studies have explored the possible relation of RAGE polymorphisms with different diseases. A meta-analysis study was performed by Wenjie Xia et al. to investigate the relation between 82G/S, − 374 T/A, and − 429 T/C polymorphisms and the occurrence of cancer. This meta-analysis showed that 82G/S polymorphism is related to a marked rise in cancer incidence, while − 374 T/A polymorphism is related to a decreased occurrence of cancers [17]. The study of Martens et al. reported that − 429 T > C polymorphism is more frequent in systemic lupus erythematosus (SLE) [18]. Nonetheless, Tiszlavicz et al. stated that 374 AA of RAGE gene polymorphism is a protective factor for multiple sclerosis [19, 20]. One study was concerned with RAGE polymorphism and thyroid autoimmunity (HT). To the best of our knowledge, no other studies were concerned with RAGE polymorphism and HT, especially in Egyptian females.
The goal of this study was to define the viable role of oxidative stress levels (plasma MDA and TAC) and RAGE receptor polymorphisms (− 374 T > A, − 429 T > C, and Gly82Ser) in Egyptian females with HT.
Subject and methods
Study population
Our case–control study was conducted on 80 female patients with recently discovered Hashimotoʼs disease attending to internal medicine clinic and Endocrinology Clinic of Zagazig University hospital. Hashimoto's thyroiditis diagnosis is based on anti-thyroid peroxidase antibodies (anti-TPO-AB) ˃60U/L and antithyroglobulin antibodies (anti-Tg-AB) ˃180 IU/ML) in addition to typical thyroid hypoechogenicity on high-resolution sonography [5]. The control group involved 80 healthy female adults of similar age and sex. All patients were euthyroid, non-pregnant, non-breastfeeding, cardiac, hepatic and renal and other autoimmune diseases were excluded.
Ethical consideration
Consents were obtained from both patients and controls. Moreover, the current study was authorized by the Zagazig University Hospital's Ethical Committee.
Methods
The study included 2 ml of whole blood and 3 ml plasma samples of 80 euthyroid Egyptian patients who suffered from newly diagnosed HT(Centrifugation of venous blood was done at 1500 rpm for 10 min, and separation and storage of plasma at − 80 C until analysis). Each patient underwent a comprehensive history taking and clinical assessment. The weight and height of patients and controls were measured and the Body Mass Index (BMI) was calculated. PCR–RFLP was used to detect the RAGE (− 374 T > A, − 429 T > C, and Gly82Ser) polymorphisms in patients and controls.
Biochemical analysis
-
Analyses of serum thyroid hormones thyroxine (T4) total, triiodothyronine(T3) total, free triiodothyronine(T3), free thyroxine (T4), and thyroid stimulated hormone(TSH) total values
Serum total T4, total T3, free T4, free T3, and TSH levels were determined by ELISA. Total T4 and total T3 ELISA kits were purchased from BioVendor (RCD025R).
-
Antibodies against thyroglobulin (anti-Tg-AB)
Anti-Tg-AB was detected in serum using ELISA kits purchased from Aeskue (Hamburg, Germany).
-
Assessment of oxidative status by evaluation of the TAC and plasma MDA.
Serum TAC was calorimetrically measured according to Koracevic et al. [21]. The MDA level was measured by the spectrophotometer via the reaction between MDA and Thiobarbituric acid with the pink pigment production. According to Buege and Aust [22], kits were purchased from (Nanjing Jiancheng Bio-engineering Institute, Nanjing, China).
Steps of testing RAGE (− 374 T > A, − 429 T > C, and Gly82Ser) polymorphisms using PCR–RFLP
Genomic DNA was isolated from whole blood using the commercially available G-spin TM Total DNA Extraction Kit (iNtRON Biotechnology, Seongnam, Korea). The purity and concentration of DNA were determined using a spectrophotometer set at 260 and 280 nm. Then DNA was stored at − 20 °C until the following usage. The following primers were used for RAGE (− 374 T > A and − 429 T > C) gene polymorphisms forward primer 5′GGGGGCAGTTCTCTCCTC-3′ and reverse primer 5′-TCAGAGCCCCCGATCCTATTT-3′. Moreover, for Gly82Ser, we used forward primer 5′-CACTGTTTAGGCCCTGCTTC-3′ and reverse primer 5′-GGAATTCTTACGGTAGACACGG3′ (BiosourceEurope SA, Belgium, Netherlands, Germany) according to Maria Giannakou et al., 0.2017[20]. Amplification was carried out using the polymerase chain reaction (PCR) in a 25 µL volume containing 25 pmol of each primer. Cycling conditions were initial denaturation of 94 °C for 4 min followed by 40 cycles of 94º C for 30 s.58 º C for 30 s, and the final extension occurred at 72 °C for 30 s. Final extension of 72 º C for 10 min using DNA thermal cycler 480 PERKIN ELMER (Norwalk. CT 06,856, USA, Serial No.P16462). Restriction analysis was done overnight at 37 °C on the PCR amplified mix using AluI for the − 429 T > C and MfeI for the − 374 T > A polymorphisms and AluI (Arthrobacter luteus) (LifeTechnologies) was used for 16 h at 37 °C for Gly82Ser. Separation of the restriction products by 3% agarose electrophoresis (Maxicell, EC 360 M-E-C apparatus Cooperation. St Petersburg Florida USA) and visualization in the ultraviolet (UV) light after staining with ethidium bromide.
Digestion with AluI revealed fragments 344 bp for the − 429 T allele (wild type) and 215, 129 bp for the − 429C allele. Digestion with MfeI revealed fragments 256, 88 bp for the wildtype allele − 374 T and 344 bp for the mutated allele -374A [23]. The polymorphism is discovered to be homozygous for both the CC/TC and AA/TA genotypes (429 CC/TC, 374 AA/TA). Regarding Gly82Ser, a wild type for Gly82 allele (82Gly/Gly) 122, 67, 49 bp bands denote homozygosity for the Ser82 allele (82Ser/Ser). Bands of 189, 122, 67, 49 bp represent a heterozygote state for the Gly82 and Ser82 alleles (82Gly/Ser).
Statistics analysis
Mean ± standard deviation was used to express all demographic and clinical parameters. To compare quantitative data, Student's t-test was utilized. The Hardy & Weinberg equilibrium was verified using the Chi-square test. The strength of genetic risk was determined using an odds ratio with a 95% confidence interval between the case and control groups. To compare genotype groups, the unpaired Student's t-test was used. Statistical significance was well-defined as a two-tailed p-value of (p < 0.05).
Results
The RAGE gene polymorphism was genotyped in 80 female patients with HT and 80 healthy control females. Standard clinical and demographic attributes of HT patients are summarized in Table 1. Age, BMI, blood glucose level, FT3, and FT4 didn’t significantly differ between patients with HT and the control group. There is a statistically measurable difference in TSH levels between HT and the control group (p < 0.001). As expected, anti-Tg-AB (IU/L) was considerably increased in HT compared to control (p < 0.001). Additionally, although TAC levels were lower in HT patients than in controls, MDA levels were higher in HT patients than in controls, as shown in Table 2.
The genotype frequencies for RAGE − 429 T > C were consistent with the HWE in controls (P = 0.96) and patients (P = 0.89) respectively. In the patient group, the frequencies of TT, TC, and CC genotypes were 82.5, 12.5, and 5%, respectively. While in the control group, the frequencies were 92.5, 7.5, and 0%, respectively. Chi-square revealed a significant difference regarding the distribution of RAGE (− 429 T < C) genotypes TT and CC between patients and control (X2 = 7.49, P = 0.04). In terms of risks of developing HT, a higher frequency of the CC genotype was significantly related to an increased risk of developing HT (OR = 5.5 (0.259–120.8), 95% confidence interval CI, and P = 0.04). The increased C allele frequencies were significantly correlated with increased risk HT (OR = 3.25 (0.84–12.94) (Table 3).
The genotype frequencies for RAGE − 374 T > A were consistent with the HWE in patients (P = 0.89) and controls (P = 0.96), respectively. The frequencies of the TT, TA, and AA genotypes in the patient group were "52.5, 25, and 22.5"%, respectively, and were 27.5, 47.5, and 25%%, respectively, in the control group. Chi-square revealed a non-significant difference regarding the distribution of RAGE (− 374 T < C) genotypes TT and AA between patients and control (P = 0.2). In terms of risks of developing HT, the increased AA genotype frequency was non-significantly correlated with an increased risk of HT (OR = 0.47 (0.147–1.5)). The increased A allele frequencies were non-significantly correlated with increased risk HT (OR = 0.57 (0.3–1.06).
The genotype frequencies in Hashimoto′s patients for Gly82Ser (Gly/Gly, Gly/Ser, Ser/Ser) genotypes were 90%, 5%, and 5%, respectively, while in the control group, the frequencies were 95, 5, and 0%, respectively. Chi-square revealed a non-significant difference regarding the distribution of Gly82Ser genotypes Gly/Gly, Gly/Ser, Ser/Ser between patients and control (P = 0.5). In terms of risks of developing HT, the increased Ser/Ser genotype frequency was non-significantly correlated with an increased risk of HT (OR = 0.5 (0.009–4.2)) (Table 4).
Discussion
The main purpose of the current work was to correlate oxidative stress plasma malondialdehyde (MDA) and total antioxidant capacity (TAC) with HT, in addition, to assess the association of RAGE polymorphisms (− 374 T > A and the − 429 T > C and Gly82Ser) with HT.
Oxidative stress is a condition that results from either an excess of free radicals production or a deficit of antioxidant defense systems. The pathogenesis of a range of conditions, especially autoimmune diseases, is affected by oxidative damage to molecules [23]. It was suggested that increased oxidative stress is associated with HT. Therefore, in the current investigation, plasma MDA is studied in newly diagnosed HT Egyptian females as a biomarker of oxidative stress and tissue injury. Additionally, TAC measurement is considered the most reliable indicator of antioxidant defense, as plasma TAC evaluation is more useful than individual antioxidant measurement [24].
MDA was significantly higher while TAC was significantly lower in the HT group compared with the control, indicating increased oxidative stress in recently diagnosed HT females. Our results agreed with previous researchers Gerenova J, Erdamar H, and Lassoued et al.[24, 25, 27], who reported the same results; their argument was reinforced by the possibility that HT is a precancerous lesion, as the incidence of carcinoma is increased in HT patients. In addition, HT was found to have proliferating nodules and cytological changes close to papillary thyroid carcinoma [25,26,27]. Moreover, several studies have discovered significantly higher malondialdehyde, nitrite, and myeloperoxidase levels in patients with overt hypothyroidism caused by HT. Additionally, other researchers discovered that HT patients have much lower glutathione (GSH) levels than healthy controls [28]. To the best of our knowledge, there are few studies evaluating the changes in oxidative balance in a large series of euthyroid HT patients. The majority of current research has focused on oxidative stress in people with Hyperthyroidism who also have thyroid dysfunction as both hypothyroidism and hyperthyroidism have been linked to an increase in oxidative stress [29,30,31]. From that finding, supplementations of antioxidants have been suggested as a therapeutic agent in Graves' hyperthyroidism and orbitopathy [32]. However, their therapeutic role in HT is debated [34,35,36 and 37]. Supported by our results, we highly recommend testing the supplementation of antioxidants as therapeutic agents in Egyptian HT euthyroid females.
Recently, we have made considerable advances in our understanding of the genetic risk factors for human autoimmune illnesses. Genetic variations, such as single nucleotide polymorphisms may contribute to the development of HT. Additionally, genome-wide association (GWAS) studies are becoming increasingly prevalent and extensive to create more precise diagnostic and therapeutic solutions for a variety of human illnesses [37].
Our current findings demonstrate a significant correlation between the RAGE system and HT Egyptian patients only regarding the distribution of the − 429 T > C polymorphism, not the − 374 T > A polymorphism. Our results did not support a role for the RAGE − 374 T > A gene polymorphism in the formation and intensity of HT. However, the RAGE − 429 T > C polymorphism was significantly more prevalent in HT Egyptian female groups than in controls. These findings are in agreement with some studies conducted on patients with SLE [38]. Relationships between RAGE polymorphisms and HT patients may suggest that the RAGE system's − 429 T > C polymorphism is associated with increased inflammation associated with autoimmune thyroid disease. In another study, a meta-analysis conducted by Jun Wang, showed no significant relation between − 429 T/C polymorphism and risk of myocardial infarction [39].
Our results reported that the Gly82Ser polymorphism was not associated with HT's susceptibility. Secondly, we found no significant differences regarding genotyping frequencies or allele frequencies of Gly82Ser amongst HT patients and control. Thus, we could suggest that the Gly82Ser polymorphism is not associated with HT development. The effect of the Gly82Ser polymorphism on receptor function at a supposed N-linked glycosylation site and the AGEs binding site in the same immunoglobulin variable domain are explained by these findings.
To the best of our knowledge, the current study is the first to investigate the association between Gly82Ser polymorphism and HT. Our results are consistent with Yousri M Hussein et al. 2017, who reported that Gly82Ser polymorphism in the RAGE gene is not associated with type 2 diabetes susceptibility or diabetic retinopathy (DR) development in type 2 diabetic subjects. In addition, Gao J X found no association between Gly82Ser polymorphism of the RAGE gene and type 2 diabetes in Chinese patients [40]. However, our work has some limitations because only three polymorphisms were genotyped and linkage disequilibrium analysis was not possible. In terms of genetic variability research, the sample size is quite tiny.
Conclusions
Increased MDA and decreased TAC as an antioxidant may be among the risk factors for HT in individuals carrying the − 429 T > C RAGE polymorphism. Additionally, further research is necessary to establish the validity of the existing findings. We recommend that elevated serum MDA and decreased TAC levels in individuals with HT can be a guide for the usage of antioxidant treatment for thyroid function improvement.
Availability of data and materials
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- HT:
-
Hashimoto's thyroiditis
- AGE:
-
Advanced glycation end product
- RAGE:
-
Receptor of Advanced glycation end product
- MDA:
-
Malondialdehyde
- TAC:
-
Total antioxidant capacity
- RFLP:
-
Restriction fragment length polymorphism
- TPO:
-
Thyroid peroxidase
- Tg:
-
Thyroglobulin
- AITD:
-
Autoimmune thyroid diseases
- ROS:
-
Reactive Oxygen Species
- TSH-R:
-
Thyroid-stimulating hormone receptor
- PCR:
-
Polymerase chain reaction
References
Burek CL, Rose NR (2008) Autoimmune thyroiditis and ROS. Autoimmun Rev 7(7):530–537
D Shah, N Mahajan, S Sah, SK Nath, B Paudyal. (2014) Oxidative stress and its biomarkers in systemic lupus erythematosus. J Biomed Sci. 21
Michels AW, Eisenbarth GS (2010) Immunologic endocrine disorders. J AllergyClin Immunol 125:S226eS237
Fountoulakis S, Tsatsoulis A (2004) On the pathogenesis of autoimmune thyroid disease a unifying hypothesis. Clin Endocrinol 60(4):397–409
Brown RS (2013) Autoimmune thyroiditis in childhood. J Clin Res Pediatr Endocrinol 5(Suppl 1):45–49
Wang SH, Baker JR (2007) The role of apoptosis in thyroid autoimmunity. Thyroid 17:975–979
Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M (2007) Telser J Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39:44–84
McCord JM (1993) Human disease, free radicals, and the oxidant/antioxidant balance. Clin Biochem 26:351–357
Sies H (2015) Oxidative stress a concept in redox biology and medicine. Redox Biol 4:180e183
Cai TT, Wang X, Muhali FS, Song R, Shi XH, Jiang WJ, Xiao L, Li FD, JA (2014) Zhang: Lack of association between polymorphisms in the UBASH3A gene and autoimmune thyroid disease: a case-control study. Arq Bras Endocrinol Metab 58(6):640e645
Rostami R, Aghasi MR, Mohammadi A, Nourooz-Zadeh J (2013) Enhanced oxidative stress in Hashimoto’s thyroiditis: inter-relationships to biomarkers of thyroid function. Clin Biochem 46:308e312
Gao J, Teng J, Liu H, Han X, Chen B, Xie A (2014) Association of RAGE gene polymorphisms with sporadic Parkinson’s disease in Chinese han population. Neurosci Lett 559:158–162
Neeper M, Schmidt AM, Brett J, Yan SD, Wang F, Pan YC et al (1992) Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J Biol Chem 267:14998–15004
Brett J, Schmidt AM, Yan SD, Zou YS, Weidman E, Pinsky D et al (1993) Survey of the distribution of a newly characterized receptor for advanced glycation end products in tissues. Am J Pathol 143:1699–1712
Sugaya K, Fukagawa T, Matsumoto K, Mita K, Takahashi E, Ando A et al (1994) Three genes in the human MHC class III region near the junction with the class II gene for a receptor of advanced glycosylation end products, PBX2 homeobox gene and a notch homolog, human counterpart of mouse mammary tumor gene. Genomics 23:408–419
Jang Y, Kim JY, Kang SM, Kim JS, Chae JS, Kim OY et al (2007) Association of the Gly82Ser polymorphism in the receptor for advanced glycation end products (RAGE) gene with circulating levels of soluble RAGE and inflammatory markers in nondiabetic and nonobese Koreans. Metab Clin Exp 56(2):199–205
Wenjie Xia, Youtao Xu, Qixing Mao, Gaochao Dong, Run Shi, Jie Wang, YanYan Zheng, Lin Xu and Feng Jiang (2012) Association of RAGE polymorphisms and cancer risk: a metaanalysisof 27 studies
Martens HA, Nienhuis HL, Gross S, van der Steege G, Brouwer E, Berden JH et al (2012) Receptor for advanced glycation end products (RAGE) polymorphisms are associated with systemic lupus erythematosus and disease severity in lupus nephritis. Lupus 21:959–968
Tiszlavicz Z, Gyulai Z, Bencsik K, Szolnoki Z, Kocsis AK, Somogyvari F et al (2009) RAGE gene polymorphisms in patients with multiple sclerosis. J Mol Neurosci 39:360–365
Giannakou M, Saltiki K, Mantzou E, Loukari E, Philippou G et al (2017) RAGE polymorphisms and oxidative stress levels in Hashimoto’s thyroiditis. Eur J Clin Investig 47(5):341–347. https://doi.org/10.1111/eci.12739
Koracevic D, Koracevic G, Djordjevic V et al (2001) Method for the measurement of antioxidant activity in human fluids. J Clin Pathol 54:356–361
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Methods by adding the BHT solution to prevent further lipid peroxidation during boiling. Methods Enzymol 52:302–310
Hudson BI, Stickland MH, Futers TS, Grant PJ (2001) Effects of novel polymorphisms in the RAGE gene on transcriptional regulation and their association with diabetic retinopathy. Diabetes 50:1505–1511
Gerenova J, Gadjeva V (2007) Oxidative stress and antioxidant enzyme activities in patients with Hashimoto’s thyroiditis. Comp Clin Pathol 16(4):259–264
Lassoued S, Mseddi M, Mnif F et al (2010) A comparative study of the oxidative profile in Graves’ disease, Hashimoto’s thyroiditis, and papillary thyroid cancer. Biol Trace Elem Res 138(1–3):107–115
Hultqvist M, Olsson LM, Gelderman KA et al (2009) The protective role of ROS in autoimmune disease. Trends Immunol 30(5):201–208
Erdamar H, Demirci H, Yaman H et al (2008) The effect of hypothyroidism, hyperthyroidism, and their treatment on parameters of oxidative stress and antioxidant status. Clin Chem Lab Med 46:1004–1010
Rostami R, Aghasi MR, Mohammadi A et al (2013) Enhanced oxidative stress in Hashimoto’s thyroiditis: inter-relationships to biomarkers of thyroid function. Clin Biochem 46(4–5):308–312
Abalovich M, Llesuy S, Gutierrez S, Repetto M (2003) Peripheral parameters of oxidative stress in Graves’ disease: the effects of methimazole and 131 iodine treatments. Clin Endocrinol (Oxf) 59:321–327
Bednarek J, Wysocki H, Sowiñski J (2005) Oxidative stress peripheral parameters in Graves’ disease: the effect of methimazole treatment in patients with and without infiltrative ophthalmopathy. Clin Biochem 38(1):13–18. https://doi.org/10.1016/j.clinbiochem.2004.09.015
Resch U, Helsel G, Tatzber F, Sinzinger H (2002) Antioxidant status in thyroid dysfunction. Clin Chem Lab Med 40:1132–1134
Marcocci C, Kahaly GJ, Krassas GE, Bartalena L, Prummel M, Stahl M, Altea MA, Nardi M, Pitz S, Boboridis K, Sivelli P, von Arx G, Mourits MP, Baldeschi L, Bencivelli W, Wiersinga W (2011) The European group on graves’ orbitopathy selenium and the course of mild graves’ orbitopathy. N Engl J Med 364:1920–1931
Duntas LH (2015) The role of iodine and selenium in autoimmune thyroiditis. Horm Metab Res 47:721–726
Duntas LH, Xue H, Li Y, Hou X, Fan C, Zhang H, Wang H, Shan Z, Teng W (2015) Effects of selenium supplementation on spontaneous autoimmune thyroiditis in NOD. -24h mice. Thyroid 25:1137–1144
Ates I, Yilmaz FM, Altay M, Yilmaz N, Berker D (2015) Gu ¨ler S The relationship between oxidative stress and autoimmunity in Hashimoto’s thyroiditis. Eur J Endocrinol 173:791–799
Baser H, Can U, Baser S, Yerlikaya FH, Aslan U (2014) Hidayetoglu BT: Assessment of oxidative status and its association with thyroid autoantibodies in patients with euthyroid autoimmune thyroiditis. Endocrine 48:916–923
Colin IM, Poncin S, Leveque P, Gallez B, Gerard AC (2014) Differential regulation of the production of reactive oxygen species in Th1 cytokine-treated thyroid cells. Thyroid 24:441–452
Marique L, Van Regemorter V, Gerard AC, Craps J, Senou M, Marbaix E, Rahier J, Daumerie C, Mourad M, Lengele B, Colin IM, Many MC (2014) The expression of dual oxidase, thyroid peroxidase, and caveolin-1 differs according to the type of immune response (TH1/TH2) involved in thyroid autoimmune disorders. J Clin Endocrinol Metab 99:1722–1732
Jackson SH, Devadas S, Kwon J, Pinto LA, Williams MS (2004) T cells express a phagocyte-type NADPH oxidase that is activated after T cell receptor stimulation. Nat Immunol 5:818–827
Rabinovitch A, Suarez-Pinzon WL, Strynadka K, Lakey JR, Rajotte RV (1996) Human pancreatic islet b-cell destruction by cytokines involves oxygen free radicals and aldehyde production. J Clin Endocrinol Metab 81:3197–3202
Kurien BT, Scofield RH (2008) Autoimmunity and oxidatively modified autoantigens. Autoimmun Rev 7:567–573
Laddha NC, Dwivedi M, Mansuri MS, Gani AR, Ansarullah M, Ramachandran AV, Dalai S, Vitiligo BR (2013) interplay between oxidative stress and immune system. Exp Dermatol 22:245–250
Berden JH et al (2012) Receptor for advanced glycation end products (RAGE) polymorphisms are associated with systemic lupus erythematosus and disease severity in lupus nephritis. Lupus 21:959–968
Hussein M, Etewa R, Hassan B, Bacala R, Vlassara H (1995) Advanced glycation end products in diabetic renal and vascular disease. Am J Kidney Diseases 26:875–888
Acknowledgements
Not applicable
Funding
No funding was delivered from any institution.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by NMM, and AA. The first draft of the manuscript was written by NMM and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
An informed consent was taken from patients and control. The study was approved by the Ethical Committee of Zagazig University Hospital.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
In the present study, all the testing procedures were performed using non-invasive techniques and adhering to the conditions of the ethical approval committee of the institute. Agreement with written knowledgeable consent was gained from the participant.
Competing interests
The authors have no conflict of interests to report.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mohamed, N.M., elfatah, A.H.A. Receptor of advanced glycation end product (RAGE) polymorphism and oxidative status in Hashimoto’s thyroiditis in Egyptian female patients: case control study. Egypt J Med Hum Genet 23, 98 (2022). https://doi.org/10.1186/s43042-022-00311-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43042-022-00311-y