Abstract
Background
Acute myeloid leukemia (AML) is a disorder characterized by a rapid onset of symptoms attributable to bone marrow failure due to clonal proliferation of primitive hematopoietic stem cells or progenitor cells. Epigenetic abnormalities play an important role in the development and progression of acute leukemia. Long non-coding ribonucleic acid (lncRNA) plays an important role in epigenetic regulation. Homeobox (Hox) transcript antisense intergenic RNA (HOTAIR) is a lncRNA which has been determined to be a negative prognostic indicator in various solid-tumor patients. However, its role in hematopoietic tumors as AML is to be assessed. This study aimed at measuring lncRNA HOTAIR expression level on bone marrow (BM) mononuclear cells in newly diagnosed AML patients and correlating its expression with their outcome and different prognostic variables. This provides new prospective for a novel marker involved in development and progression of AML which can be used as a diagnostic marker and a target of therapy. The current study included 65 subjects divided into 35 newly diagnosed AML adult patients (before initiation of chemotherapy) and 30 non-leukemic adult patients who are candidates for BM aspiration for causes other than hematological malignancies as immune thrombocytopenic purpura and hypersplenism as controls. HOTAIR expression was measured on BM mononuclear cells by quantitative reverse transcription polymerase chain reaction (qRT-PCR).
Results
HOTAIR expression was found to be significantly upregulated in AML patients (probability (p) value = 0.000) and it can be used as a diagnostic biomarker of AML as confirmed by a significant difference between cases and controls using receiver operating characteristic curve (ROC) analysis. However, it was not significantly correlated with event free survival (EFS) or prognostic variables.
Conclusion
This study showed that the expression of HOTAIR is upregulated in de novo AML patients and can be used as a diagnostic marker. However, highly expressed HOTAIR is not associated with poor prognosis.
Similar content being viewed by others
Background
AML is characterized by abnormal proliferation of undifferentiated and non-functional hematopoietic cells (the leukemic blasts) in the bone marrow. AML develops by the accumulation of multiple genetic and epigenetic alterations in hematopoietic progenitors that are subjected to clonal evolution, with the result of considerable inter- and intra-individual heterogeneity of AML clones [1].
AML exemplifies the impact of epigenetic disruption in cancer, as while AML typically exhibits a low mutational burden, these alterations frequently directly or indirectly target epigenetic modulators. Comprehensive cataloging of the AML genome has revealed a high frequency of epigenetic changes that are frequently linked to treatment resistance and poor patient outcome [2].
Epigenetics is defined as a stably heritable phenotype resulting from changes in a chromosome without alterations in the deoxyribonucleic acid (DNA) sequence [3]. The epigenetic machinery is composed principally of three interconnected components: DNA methylation, histone post-translational modifications, and non-coding RNAs (ncRNAs). Based on their size, ncRNAs can be divided into two main groups: short-chain ncRNAs and lncRNAs [4]. LncRNAs are a type of functional RNAs with a transcript of more than 200 nucleotides in length, lacking the protein-encoding ability, but can regulate the protein-coding genes at different levels [5].
HOTAIR, a recently discovered lncRNA, is a polyadenylated RNA with 2158 nucleotides and 6 exons which is transcribed from the antisense strand of the Homeobox C (HOXC) gene cluster located between Homeobox C11 (HoxC11) and Homeobox C12 (HoxC12) on chromosome 12q13.13. It plays a critical role in various areas of cancer, such as proliferation, survival, migration, drug resistance, and genomic stability [6]. HOTAIR has been shown to exert oncogenic and metastatic potential in several solid tumors and it has been shown to be a significant predictor for worse prognosis as in urological cancers, head and neck neoplasms, cancers of the digestive system, and several female cancers, for example, cervical, ovarian, and endometrial cancers [7].
HOTAIR plays a role in the myelopoiesis through modulation of gene expression in the HOXA cluster, so it may also function in malignant hematopoiesis [8].
Upregulated expression of HOTAIR was found to facilitate the growth of lymphoma cells in lymphoma patients, and it was demonstrated that patients with higher expression levels of HOTAIR possessed lower survival probabilities than those with lower expression levels of HOTAIR. In addition, it was found that HOTAIR knockdown inhibited cell viability, induced cell apoptosis, and suppressed cell cycle progression, implying that HOTAIR might also act as an oncogene in lymphoma [9]. Upregulated HOTAIR expression is also incriminated in chronic myeloid leukemia (CML) progression and may play a role in resistance to imatinib which is a tyrosine kinase inhibitor used as an oral chemotherapy for treatment of leukemia especially CML [10]. However, there are still few studies about its level of expression and its role as a prognostic marker of leukemia.
Aim of the work
The aim of this study is to measure the expression level of lncRNA HOTAIR expression level on BM mononuclear cells in newly diagnosed AML patients (before initiation of chemotherapy) and to correlate its expression level with different prognostic variables in order to develop a new diagnostic biomarker of AML that may be also used as a target of therapy.
Methods
Study design and setting
Case-control study of patients with AML. The patients were recruited from hematology unit, Clinical Pathology Department during the years 2018-2019.
Subjects
The current study was conducted on 65 subjects (35 newly diagnosed AML adult patients (before initiation of chemotherapy) and 30 adult controls). The diagnosis of AML was based on World Health Organization (WHO), 2016 AML diagnostic criteria [11]: blast cells constitutes > 20% of all BM nucleated cells, morphology, especially presence of Auer rods, immunophenotyping (IPT) to detect lineage specific cluster of differentiation (CD) markers and myeloperoxidase. Classification of AML according to the French-American-British (FAB) classification criteria was done [12].
Follow-up of the patients by BM blast % was done at day 28 and afterwards till either the end of the study or last contact with the patient (at least for 1 year from the start of the study). Accordingly, patients were classified as responders (BM blasts % less than 5% at day 28 of starting chemotherapy), non-responders (BM blasts % more than 5% at day 28 of starting chemotherapy), and relapsed (BM blasts % increased more than 5% during follow up) patients.
Thirty non-leukemic adult patients who are candidates for bone marrow aspiration for causes other than hematological malignancies as immune thrombocytopenic purpura and hypersplenism were studied as controls.
Inclusion criteria
All included patients are adults newly diagnosed AML patients.
Exclusion criteria
Cases of transformation of myelodysplastic syndrome (MDS), acute transformation of CML, relapsed or therapy-related AML, and other types of malignant tumors were excluded from this study.
Ethics approval and consent to participate
The approval of the study was taken from the Institutional Ethics Committee (Ethical Committee’s reference number: 138/2018; 27 May 2018). Written informed consent was taken from all patients who were invited to participate in the research.
All patients were subjected to full history taking, thorough clinical examination, complete blood count (CBC) on Sysmex-XN 1000TM using peripheral blood (PB) samples with examination of Leishman-stained PB films, BM aspiration with examination of Leishman-stained BM smears, IPT carried on BM blasts/blast equivalent cells using a standard panel of monoclonal antibodies using Navios flowcytometer (Coulter, Electronics, Hialeah, FL, USA) and cytogenetic analysis carried on BM samples, if feasible, searching for translocation (t)(15;17), t(8;21), t(9;22), inversion (inv)16, and duplication of X chromosome using Laika. HOTAIR expression level on BM mononuclear cells was done on BM samples by qRT-PCR using (Biometra, Germany) for reverse transcription and (Rotor-Gene, Germany) for real time PCR. This was performed on fresh samples taken from both controls and patients at time of diagnosis before initiation of chemotherapy. Another sets of samples were taken from patients at day 28 after treatment and during follow-up for assessment of BM blasts %. No stored samples were used.
Sampling
Three milliliters (mL) of venous blood were aseptically collected from each patient, dispensed in a K2-ethylene diamine tetraacetic acid (K2-EDTA) tube to be used for CBC and Leishman-stained PB smear. BM aspiration was done for all subjects and several BM smears were spread to be examined by Leishman stain. In total, 1.5 mL of BM aspiration was drawn and dispensed into sterile K2-EDTA vacutainer tube to be used for IPT. A total of 0.5 mL BM samples were drawn and dispensed into heparin anticoagulated tubes for cytogenetic analysis. In total, 1.5 mL of BM aspiration was drawn and dispensed into sterile K2-EDTA vacutainer tube to be used for measurement of HOTAIR expression level on BM mononuclear cells by qRT-PCR.
These samples were taken from both controls and patients at time of diagnosis before initiation of chemotherapy. Another sets of BM aspiration samples were taken from patients at day 28 after treatment and during follow-up for BM blasts % assessment.
HOTAIR expression level on BM mononuclear cells measurement by qRT-PCR
Ribonucleic acid (RNA) extraction
RNA extraction was done using QIAamp RNA Blood Mini Kit, Qiagen (04/2010), catalog (cat.) number (no.): (52304) (http://www.qiagen.com). RNA species longer than 200 bases bind to the QIAamp silica-based membrane using a specialized high-salt buffering system. Erythrocytes are lysed and leukocytes are recovered by centrifugation then lysed using highly denaturing conditions that immediately inactivate ribonucleases (RNases), allowing the isolation of intact RNA. Homogenization of the lysate is done by a brief centrifugation through a QIAshredder spin column. Ethanol is added to adjust binding conditions and the sample is applied to the QIAamp spin column. RNA is bound to the silica membrane during a brief centrifugation step. Contaminants are washed away and total RNA is eluted in 30 microliters (uL) or more of RNase-free water for direct use.
Integrated removal of genomic DNA contamination and complementary DNA (cDNA) synthesis
This was done using QuantiTect® Reverse Transcription, Qiagen (1056039 03/2009), cat. no.: (204654, 204754) (http://www.qiagen.com).
It comprises 2 main steps: elimination of genomic DNA and reverse transcription (RT) [13] (http://www.qiagen.com).
Elimination of genomic DNA
The purified RNA sample is briefly incubated in genomic DNA Wipeout Buffer at 42 °C for 2 min to effectively remove contaminating genomic DNA, the RNA sample is then used directly in reverse transcription.
Reverse transcription
The entire reaction takes place at 42 °C and is then inactivated at 95 °C. Quantiscript reverse transcriptase has a high affinity for RNA and is optimized for efficient and sensitive cDNA synthesis from 10 picograms (pg) to 1 microgram (ug) of RNA. This high RNA affinity, in combination with quantiscript RT buffer, enables high cDNA yields. RT primer mix ensures cDNA synthesis from all regions of RNA transcripts, even from 5′ regions. This allows high yields of cDNA template for real-time PCR analysis regardless of where the target region is located on the transcript.
qRT-PCR
HOTAIR TaqManTM Gene Expression Assay, ThermoFisher, cat. no.: (4448892) (Fig. 1) (https://www.thermofisher.com/taqman-gene-expression/) with primer sequence (Forward 5′-GCA GTA GAA AAA TAG ACA TAG GAGA-3′, Reverse 5′-AAT GAT AGG GAC ACA TCG GGG AAC T-3) (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4774541/) was used.
HOTAIR genomic map (https://www.thermofisher.com/taqman-gene-expression) [14]
TaqManTM Gene Expression Master Mix, ThermoFisher (Part No: 4371135 Rev. C, 07/2010) (https://www.thermofisher.com), was used for qRT-PCR which contains AmpliTaq Gold®, DNA Polymerase, UP (Ultra Pure), Uracil-DNA Glycosylase (UDG), deoxyribonucleotide triphosphates (dNTPs) with deoxyuridine triphosphate (dUTP), ROX™ Passive Reference and Buffer components optimized for sensitivity, precision, specificity, and duplexing. The PCR reaction exploits the 5′ nuclease activity of AmpliTaq® Gold DNA Polymerase, UP (Ultra Pure) to cleave a TaqMan® probe during PCR. The TaqMan probe contains a reporter dye at the 5′ end of the probe and a quencher dye at the 3′ end of the probe. Cleavage of the probe separates the reporter dye and the quencher dye, resulting in increased fluorescence of the reporter. Accumulation of PCR products is detected directly by monitoring the increase in fluorescence of the reporter dye. The nuclease activity is fork-like and structure dependent. When the probe is intact, the proximity of the reporter dye to the quencher dye results in suppression of the reporter fluorescence primarily by Förster-type energy transfer, the probe specifically anneals to the target. The 5′ to 3′ nucleolytic activity of the AmpliTaq Gold, UP enzyme cleaves the probe between the reporter and the quencher only if the probe hybridizes to the target. The probe fragments are then displaced from the target, and polymerization of the strand continues. The 3′ end of the probe is blocked to prevent extension of the probe during PCR. This process occurs in every cycle, and it does not interfere with the exponential accumulation of product. Increased fluorescence signal is detected only if the target sequence is complementary to the probe and if it is amplified during PCR.
Housekeeping gene GABDH was used as an endogenous control to normalize the amount of total mRNA in each sample of HOTAIR between different samples. HOTAIR expression was measured as fold of control using the equation of ΔRn = (Rn+)−(Rn−), (where Rn+ = emission Intensity of Reporter PCR with template Emission Intensity of Passive Reference and Rn− = Emission Intensity of Reporter PCR without template or early cycles of a real-time Emission Intensity of Passive Reference reaction). Then the results is calculated as 2−∆∆CT (where CT= cycle threshold).
Statistical analysis
Descriptive and analytical statistical procedures were conducted. Data entry and statistical analysis of collected data was performed using Statistical Package for Social Science (SPSS version 23.0). Comparison between two groups regarding qualitative data was performed using chi-square test (X2). The comparison between two independent groups regarding quantitative data with parametric distribution was done by using independent t test (t) while comparison between two independent groups regarding quantitative data with non-parametric distribution was done by using Mann-Whitney test. Parameters correlations (correlation coefficient “r”) were performed by using Spearman’s correlation. Kruskal-Wallis test and post HOC analysis were performed for comparison among groups regarding quantitative non-parametric data. Receiver operating characteristic (ROC) as a graphical plot was done to determine the best cut-off value for HOTAIR as a diagnostic marker for AML. Kaplan-Meier analysis with log-rank test was used to assess the relation of HOTAIR with relapse free survival (event free survival (EFS)). Regarding power of significance, the probability level (p value) was considered significant (S) if p value was < 0.05, non-significant (NS) if p value was ≥ 0.05 and highly significant (HS) if p value was <0.01.
Results
Demographic, clinical, hematological, and cytogenetic data are shown in Tables 1 and 2.
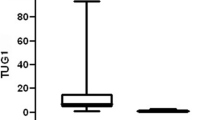
HOTAIR was expressed in all AML patients ranging from 1.11-16.11 (median: 4.23; interquartile range (IQR): 3.6-5.5) (Table 3). HOTAIR expression was higher in AML patients group than control group and this difference was statistically highly significant with p value= 0.00 (Table 3 and Fig. 2).
As regards FAB subtypes, highest levels of HOTAIR expression were seen in M0-1 (median: 16.11, IQR: 16.11-16.11) followed by M4 (median: 4.34, IQR: 3.84-4.64), M5 AML (median: 4.53, IQR: 4.03-5.5), and mixed phenotype acute leukemia (AL) (median: 4.96, IQR: 4.1-9.57) than other FAB classification subtypes. The least expression was seen in M3 (median: 3.32, IQR: 2.38-4.23). However, statistical analysis revealed no significant relation between HOTAIR expression and FAB subtypes (p value = 0.408) (Table 4).
There was no statistically significant relation between HOTAIR expression and each of age and sex (p value= 0.943 and 0.476 respectively) in AML patients. In addition there was no statistically significant relation between HOTAIR expression and hepatosplenomegaly (HSM) among AML patients (p value= 0.956) (Table 5).
As regards the correlation between HOTAIR expression and hematological data among AML group, there was a positive significant correlation between HOTAIR expression and total leucocytic count (TLC) (p = 0.036). However. there was no statistical correlation between AML patients and hemoglobin (Hb) or platelets (PLT) (p value = 0.686, p value = 0.208 respectively). As regards BM blast cell count, there was no statistically significant correlation between HOTAIR expression and blasts count among AML cases (p value = 0.968) (Table 6 and Fig. 3).
Receiver operating characteristic (ROC) curve was used to assess the best cut-off point with best sensitivity and specificity for the diagnosis of AML. This study revealed that HOTAIR expression value of 3.25 turned to be the best cut-off value that could discriminate between AML patients and control group. The diagnostic performance was evident by area under the curve (AUC) of 0.978 with a diagnostic accuracy of 97.8%, diagnostic sensitivity of 88.57%, specificity of 100%, positive predictive value (PV) of 100%, and negative predictive value of 88.2% (Table 7 and Fig. 4).
According to patient response to chemotherapy at day 28, most patients with higher HOTAIR expression are non-responsive (BM blast% > 5% at day 28 of starting chemotherapy) (median: 5.03 and 4.6; IQR: 3.78-8 and 3.47-5.03 respectively); nevertheless, the association between HOTAIR expression and response to chemotherapy was found to be statistically insignificant (p = 0.195) (Table 5).
Follow-up of the patients was done after day 28 till either the end of the study or last contact with the patient to find patients who were relapsed. In this study, 11 AML cases out of 35 AML cases were relapsed. Ten of AML relapsed cases had HOTAIR expression > 3.25-folds of control, while one of the 11 relapsed cases had HOTAIR expression < 3.25-folds of control; however, this was statistically insignificant. Kaplan-Meier analysis by log-rank test shows that cases with HOTAIR > 3.25-folds of control have lower mean EFS (11.0 ± 0.828) than cases with HOTAIR < 3.25-folds of control who have higher EFS (8.87 ± 0.816). This was statistically insignificant (p value = 0.694) (Table 8 and Fig. 5). There was no significant association between HOTAIR expression and standard prognostic factors in AML (Tables 9 and 10).
Interestingly, there was an M0-1 FAB classified case who showed resistance to therapy with no remission whose HOTAIR expression level was the highest (16.11-folds of control). In contrary, M3 AML cases showed the lowest HOTAIR expression (Table 4).
Discussion
Although HOTAIR has been implicated in the onset of a variety of tumors, its role in hematological tumor formation remains unclear. HOTAIR acts as a scaffold for histone modification complexes and is involved in epigenetic gene regulation [15]. The present study aimed at elucidation of the value of HOTAIR expression as a diagnostic and prognostic marker in AML by investigating its level of expression and assessing its relation to various clinical, biological, and standard prognostic factors.
In this study, the expression of LncRNA HOTAIR is observed in all de novo AML patients and this expression was significantly higher in AML patients than controls. This expression showed insignificant association with different FAB subtypes which may be due to small sample size or due to different subtypes involved in the study. This is in accordance with Fouad and Salah [15] who held a study at Cairo University aimed at studying HOTAIR LncRNA expression in Egyptian AML patients and revealed that HOTAIR is upregulated in AML. Also, this goes with Gao et al. [16] who found that HOTAIR was elevated in AML cells. This is also supported by Hao and Shao [8] who could not thoroughly assess whether there is any difference in HOTAIR levels between AML subtypes also due to small sample size.
In contrary, Sayed et al. [17] examined the expression of HOTAIR messenger ribonucleic acid (mRNA) in the blood samples of 25 Iranian AML patients in comparison with 50 healthy controls and investigated the correlation between this lncRNA expression levels and the disease using quantitative real-time RT-PCR. They also categorized their samples regarding the gender into two separate groups of males and females. They demonstrated no significant differences in HOTAIR lncRNA expression level between Iranian AML patients and healthy individuals or between males and females.
According to the current study, upregulated HOTAIR expression can be used as a diagnostic marker in AML patients with the best cut-off point of expression 3.25. This cut-off point has a diagnostic accuracy of 97.8% with diagnostic sensitivity of 88.57%, specificity of 100%, positive predictive value of 100%, and negative predictive value of 88.2%.
Studying the correlation between HOTAIR expression and different clinicopathological factors that assigned for risk stratification as indicated by 2017 European Leukemia Net (ELN) recommendations from an international expert panel [18] showed a statistically significant correlation between HOTAIR expression level and TLC, but there was no significant correlation with other laboratory, demographic, or clinical data.
The current study showed no association between HOTAIR expression and prognosis and EFS, although higher HOTAIR expression was observed in patients not responding to therapy and those with shorter EFS. In addition, HOTAIR expression could not be used as an independent prognostic marker. Interestingly, there was an M0-1 FAB classified case who showed the highest HOTAIR expression level (HOTAIR: 16.11-folds of control) and resistance to therapy with no remission (Table 4). This supports that HOTAIR is involved in the development and progression of AML and that HOTAIR knockdown may be a new prospective for AML treatment.
In accordance to this study, meta-analysis which studied five researches covering a number of 531 AL and lymphoma patients demonstrated that HOTAIR expression is related to a poor prognosis, but did not significantly affect the EFS in patients with AL [19].
Another study by Zhang et al. [20] suggested that upregulated HOTAIR expression is associated with poor prognosis and reduced EFS. Multivariate analysis showed that age, peripheral blood leucocyte count, and high expression of HOTAIR were independent prognostic indicators for EFS.
Gao et al. [16] provided evidence that HOTAIR may act as an oncogenic gene in AML and that its overexpression was associated with aggressive tumor progression. This indicated that it has a possible prognostic value in AML and that it may represent a potential biomarker of poor prognosis and a potential therapeutic target for AML intervention.
This study showed increased HOTAIR expression in AML patients, but this upregulated expression was not correlated with poor prognosis which may need to be assessed as the biological importance of HOTAIR in AML could not be ignored. Further studies are needed to find the role of HOTAIR in development, prognosis, and targeted treatment of AML through its silencing.
Conclusion
This study showed that the expression of HOTAIR is upregulated in newly diagnosed adult AML patients and that it can be used as a diagnostic biomarker in these patients. Accordingly, HOTAIR might have a role in pathogenesis of AML and can be used as a target for treatment by knocking it down. However, it is not associated with outcome or prognosis in AML patients. Hence, larger studies on AML patients with its different subgroups and longer duration of follow-up (3-5 years) are recommended to explore the impact of HOTAIR expression on the duration of survival and EFS of patients after treatment.
Limitations
The main limitation of our study was relatively small sample size. For this reason, impact of HOTAIR expression as a prognostic marker in AML patients could not be thoroughly assessed.
Availability of data and materials
The data within this paper and other findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- AL:
-
Acute leukemia
- AML:
-
Acute myeloid leukemia
- AUC:
-
Area under the curve
- BM:
-
Bone marrow
- Cat.:
-
Catalog
- CBC:
-
Complete blood picture
- CD:
-
Cluster of differentiation
- cDNA:
-
Complementary deoxyribonucleic acid
- CI:
-
Confidence interval
- CML:
-
Chronic myelogenous leukemia
- CT:
-
Cycle threshold
- DNA:
-
Deoxyribonucleic acid
- EFS:
-
Event free survival
- ELN:
-
European Leukemia Net
- FAB:
-
French-American-British
- HB:
-
Hemoglobin
- HOTAIR:
-
Homeobox transcript antisense intergenic ribonucleic acid
- HOX:
-
Homeobox
- HOXC:
-
Homeobox C
- HOXC11:
-
Homeobox C11
- HOXC12:
-
Homeobox C12
- HS:
-
Highly significant
- HSM:
-
Hepatosplenomegaly
- Inv:
-
Inversion
- IPT:
-
Immunophenotyping
- IQR:
-
Interquartile range
- K2-EDTA:
-
K2-ethylene diamine tetraacetic acid
- LncRNA:
-
Long non-coding ribonucleic acid
- MDS:
-
Myelodysplastic syndrome
- ncRNA:
-
Non-coding ribonucleic acid
- No.:
-
Number
- NS:
-
Non-significant
- PB:
-
Peripheral blood
- PLT:
-
Platelets
- PV:
-
Predictive value
- p value:
-
Probability value
- qRT-PCR:
-
Quantitative reverse transcription polymerase chain reaction
- r:
-
Correlation coefficient
- Rn−:
-
Emission Intensity of Reporter PCR without template
- Rn+:
-
Emission Intensity of Reporter PCR with template Emission Intensity of Passive Reference
- RNA:
-
Ribonucleic acid
- RNAase:
-
Ribonuclease
- ROC:
-
Receiver operating characteristic
- RT:
-
Reverse transcription
- S:
-
Significant
- SD:
-
Standard deviation
- SE:
-
Standard error
- SPSS:
-
Statistical Package for Social Science
- t:
-
Independent t test
- t:
-
Translocation
- TLC:
-
Total leucocytic count
- UP:
-
Ultra pure
- WHO:
-
World Health Organization
- X 2 :
-
Chi-square test
References
Kreitz J, Schönfeld C, Seibert M, Stolp V, Alshamleh I, Oellerich T, Steffen B, Schwalbe H, Schnütgen F, Kurrle N, Serve H (2019) Metabolic plasticity of acute myeloid leukemia. Cells 8(8):805. https://doi.org/10.3390/cells8080805
Jones L, McCarthy P, Bond J (2020) Epigenetics of paediatric acute myeloid leukaemia. Br J Hematol 188(1):63–76. https://doi.org/10.1111/bjh.16361
Chmelarova M, Palicka V (2019) Epigenetics in cancer: a promising path to follow? Clin Chem Lab Med 57(7):927–931. https://doi.org/10.1515/cclm-2019-0010
Roberti A, Valdes AF, Torrecillas R, Fraga MF, Fernandez AF (2019) Epigenetics in cancer therapy and nanomedicine. Clin Epigenetics 11(1):81. https://doi.org/10.1186/s13148-019-0675-4
ZHU HP (2020) Silence of HOTAIR inhibits insulin secretion and proliferation in pancreatic β cells. Eur Rev Med Pharmacol Sci 24:784–792. https://doi.org/10.1186/s13148-019-0675-4
Tang Q, Hann SS (2018) HOTAIR: an oncogenic long non-coding RNA in human cancer. Cell Physiol Biochem 47:893–913. https://doi.org/10.1159/000490131
Toy HI, Okmen D, Kontou PI, Georgakilas AG, Pavlopoulou A (2019) HOTAIR as a prognostic predictor for diverse human cancers: a meta- and bioinformatics analysis. Cancers 11(6):778. https://doi.org/10.3390/cancers11060778
Hao S, Shao Z (2015) HOTAIR is upregulated in acute myeloid leukemia and that indicates a poor prognosis. Int J Clin Exp Pathol 8(6):7223–7228
Zhao X, Tian X (2019) Knockdown of long noncoding RNA HOTAIR inhibits cell growth of human lymphoma cells by upregulation of miR-148b. J Cell Biochem 120(8):12348–12359. https://doi.org/10.1002/jcb.28500
Wang H, Li Q, Tang S, Li M, Feng A, Qin L, Liu Z, Wang X (2017) The role of long noncoding RNA HOTAIR in the acquired multidrug resistance to imatinib in chronic myeloid leukemia cells. Hematol J 22(4):208–216. https://doi.org/10.1080/10245332.2016.1258152
Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Arber DA, Hasserjian RP, Le Beau MM, Orazi A, Siebert R (2017) WHO classification of tumours of haematopoietic and lymphoid tissues,2016 (Revised 4th edition). In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J (eds) International Agency for Research on Cancer, pp 15–28
Bennet JM, Catovsky D, Daniel MT et al (1976) Proposed revised criteria for the classification of acute myeloid leukemias (FAB cooperative group). Br J Haematol 33:451–458
Qiagen, 2013-20 , accessed 27 January 2021, <https://www.qiagen.com/us/products/discovery-and-translational-research/pcr-qpcr-dpcr/real-time-pcr-enzymes-and-kits/reverse-transcription-cdna-synthesis-qpcr/quantitect-whole-transcriptome-kit/?clear=true#productdetails.>. Accessed 27 Jan 2021.
Thermofisher scientific, TaqMan® Assays and Arrays, accessed 27 January 2021, <https://www.thermofisher.com/order/genome-database/?pearUXVerSuffix=pearUX2&elcanoForm=true#!/ge/assays/ge_all/?keyword=HOTAIR%20taqman%20gene%20expression%20master%20mix%20.>. Accessed 27 Jan 2021.
Fouad NB, Salah M (2019) Study of HOTAIR LncRNA expression in Egyptian AML patients in context to FLT3-ITD and NPM1 mutations status. OncNet Newsletter Lymphoma Leukaemia Myeloma Congress 4773. http://oncnet.com/meeting-materials/lymphoma-and-myeloma/4773.
Gao J, Wang F, Wu P, Chen Y, Jia Y (2020) Aberrant LncRNA Expression in Leukemia. J Cancer 11(14):4284–4296. https://doi.org/10.7150/jca.42093
Sayad A, Hajifathali A, Hamidieh AA, Roshandel E, Taheri M (2017) HOTAIR long noncoding RNA is not a biomarker for acute myeloid leukemia (AML) in Iranian patients. Asian Pacific J Cancer Prev 18(6):1581–1584. https://doi.org/10.22034/apjcp.2017.18.6.1581
Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, Dombret H, Ebert BL, Fenaux P, Larson RA, Levine RL, Lo-Coco F, Naoe T, Niederwieser D, Ossenkoppele GJ, Sanz M, Sierra J, Tallman MS, Tien H, Wei AH, Löwenberg B, Bloomfield CD (2017) Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 129(4):424–447. https://doi.org/10.1182/blood-2016-08-733196
Lin Y, Fang Z, Lin Z, Li Z, Zhao J, Luo Y and Xu B (2018): The prognostic impact of long noncoding RNA HOTAIR in leukemia and lymphoma: a meta-analysis, Hematology, ISSN: 1024-5332 (Print) 1607-8454 (Online). (doi: https://doi.org/10.1080/10245332.2018.1446572)
Zhang YY, Huang SH, Zhou HR, Chen CJ, Tian LH, Shen JZ (2016) Role of HOTAIR in the diagnosis and prognosis of acute leukemia. Oncol Rep 36(6):3113–3122. https://doi.org/10.3892/or.2016.5147
Acknowledgements
The authors gratefully thank professor Eman Omar (Professor of Clinical Pathology, Faculty of Medicine—Ain Shams University) for her many useful tips which helped us in writing our methodology.
Funding
Self-funded.
Author information
Authors and Affiliations
Contributions
RA performed bone marrow aspiration, collected the samples of the patients in addition to their demographic, clinical, and laboratory data, and wrote the manuscript. RA, AA, MF, SA, and YN analyzed and interpreted the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The approval of the study was taken from the Institutional Ethics Committee of the Faculty of Medicine, Ain Shams University (Ethical Committee’s reference number: 138/2018; 27 May 2018). Written informed consent was taken from all patients who were invited to participate in the research.
Consent for publication
Not applicable
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Saad, R.A.A.A.M., Osman, A.A., Hassan, M.F.A.F. et al. HOTAIR expression and prognostic impact in acute myeloid leukemia patients. Egypt J Med Hum Genet 22, 36 (2021). https://doi.org/10.1186/s43042-021-00147-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43042-021-00147-y