Abstract
Background
Meniscal injury is one of the most common indications for knee surgery. The advent of meniscal repair techniques has facilitated meniscal preservation in suitable cases. Meniscal substitution with scaffolds may be advantageous following partial meniscal resection. There are three main scaffolds in current clinical use; Collagen Meniscal Implant (CMI Stryker Corporation, Kalamazoo, MI, USA), Actifit (Actifit, Orteq Ltd, London, UK) and NUsurface (Active Implants, LLC). The purpose of this systematic review was to compare clinical outcomes and failure rates of patients who have had implantation with these meniscal scaffolds.
Methods
MEDLINE and EMBASE databases were searched for studies that included patients who had surgical implantation with Actifit or CMI. Eligibility criteria included papers that described both clinical outcomes and failure rates of these implants, a mean follow up of 5 years and studies published in English. A Google search was also performed to identify any grey literature.
Results
Five Level IV studies were found for Actifit. One Level II, one Level III and four Level IV studies were found for the CMI implant. One Level II study was identified for the NUsurface scaffold with a follow-up 12 months and was included for completeness. Overall, 262 patients were treated with Actifit, 109 with CMI and 65 with NUsurface. Failure rates for Actifit were 18% (range 6.3–31.8%) with a mean follow up of 66.8 months, and for CMI 6.5% (range 0–11.8%) with a mean follow up of 97.1 months. The NUsurface failure rate was 16.9% at 12 months. Clinical outcomes such as VAS, Tegner and Lysholm scores improved significantly post-operatively. However, there was a high volume of concurrent procedures, such as anterior cruciate ligament reconstructions and high tibial osteotomies in each study group; 118 (45%) for Actifit and 53 (45%) for CMI.
Conclusion
The evidence for meniscal scaffold use is insufficient to suggest that they could potentially improve clinical outcomes in patients post-meniscal resection. This is largely due to the high proportion of concurrent procedures performed at index procedure for both CMI and Actifit. On the basis of current evidence, the use of meniscal scaffolds as a sole treatment for partial meniscal defects cannot be recommended, owing to the relatively high failure rate and paucity of clinical data.
Similar content being viewed by others
Introduction
Meniscal injury is one of the most common indications for knee surgery [1]. The advent of meniscal repair techniques has facilitated meniscal preservation in suitable cases [2]. The extent of meniscal resection, however, is proportional to the risk of developing secondary osteoarthritis [3]. Meniscal preservation techniques have been developed to reduce this risk. The success of meniscal repair depends on location and blood supply, with peripheral or ‘red on red’ or ‘red on white’ tears being amenable to surgery [4]. The majority of meniscal tears are, unfortunately, central ‘white on white’ tears with poor blood supply. In these cases, partial meniscectomy is necessary. Meniscal allograft transplantation (MAT) can be implanted following complete meniscal loss, and recently meniscal scaffolds have been developed to replace partial defects [5, 6].
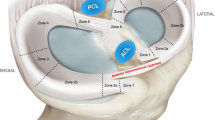
Meniscal scaffolds provide a template for cells and may allow the formation of meniscal-like tissues [7]. There are three main implants in current clinical use. Collagen Meniscal Implant (CMI Stryker Corporation, Kalamazoo, MI, USA) is a collagen scaffold harvested from bovine Achilles tendons, which allows the ingrowth of cells into the menisci [8, 9]. It requires an outer rim of meniscus with an attachment to the anterior/posterior horn [10]. The Actifit (Actifit, Orteq Ltd, London, UK) implant is a synthetic scaffold composed of polyurethrane (20%) and prolactone (80%), [7] which is biodegradable, with a predicted degradation time of 4–6 years [5]. More recently, a polymeric medial implant, the NUsurface (Active Implants, LLC) has been developed [11]. NUsurface is a polyurethrane product made from ultra-high-molecular-weight polyethylene [11] (see Fig. 1).
The goals of meniscal surgery are to improve pain and stability, restore activity levels and reduce the risk of secondary osteoarthritis [12]. The role of meniscal scaffolds in attaining these goals is still uncertain. The purpose of this systematic review is to compare the current evidence of clinical outcome and failure rates of patients who have had surgical implantation of the Actifit, CMI, or NUsurface scaffold.
Methods
A literature search was performed using the OVID web interface. MEDLINE and EMBASE databases were searched for studies related to outcomes following implantation with the Actifit/polyurethrane implant up to 15 November 2020. The review was performed in accordance with PRISMA guidelines [13]. Terms searched for included ‘meniscus’, ‘synthetic’ and ‘implant. A Google search was also performed to identify any grey literature. The initial search yielded 142 studies. Subsequently, titles and abstracts were reviewed according to the inclusion and exclusion criteria.
A separate literature search was also performed to identify papers related to the CMI implant. Search terms such as ‘meniscal scaffold’, ‘collagen meniscal implant’ and ‘CMI’ were used. This search yielded 532 studies. Titles and abstracts were reviewed for both categories and included/excluded on the basis of the following criteria outlined below.
One reviewer (S.K.) conducted the original literature research as well as the inclusion and exclusion of articles on the basis of the criteria. Subsequently, the included articles were assessed by two reviewers (S.K. and J.S.) and the results were collated. Studies were excluded on the basis of the following criteria: (1) studies not written in English, (2) only abstract or conference data, (3) animal studies.
Individual patient inclusion criteria varied within the papers selected, and indication for meniscal repair included both traumatic and degenerative causes.
All papers included for this review addressed the use of partial meniscal implants; however, depending on the implant used, these could be lateral or medial.
Outcomes
Studies were included if they reported pre- and post-operative clinical outcomes of patients post-implantation and if they reported failure/reoperation rates. Each study was then assessed and reviewed by two reviewers. Twelve studies were included: five Actifit and six CMI studies (both with a mean follow-up of 5 years), and one NUsurface with a follow-up of 1 year.
Results
Five Level IV studies were found for Actifit. One Level II, one Level III and four Level IV studies were found for the CMI implant. One Level II study was identified for the NUsurface scaffold. The studies with patient demographics are summarised in Table 1.
All of the studies listed had a greater propensity of male to female subjects. All of the CMI and NUsurface implant lesions were medial, whereas the Actifit implant was used for medial (169) and lateral lesions (93). The follow-up of patients ranged from 60 to 72 months for Actifit, 60–120 months for CMI, and up to 12 months for the NUsurface scaffold. Overall, there were more patients treated by Actifit (262) compared with the CMI (109) and NUsurface (65) implant. Mean follow-up times were 66.8 months for Actifit, 97.1 months for CMI, and 12 months for NUsurface. Mean age was 36 years for Actifit, 35 years for CMI and higher for NUsurface at 48.7 years.
Patient inclusion and exclusion criteria
The inclusion and exclusion criteria for these studies are summarised in Table 2. Two of the included CMI studies did not state exclusion criteria [21, 22]. Although there is a significant variability in criteria, the majority of studies included patients with irreparable meniscal tears (acute or chronic were both included), and excluded patients who had evidence of moderate-to-severe OA, and patients with unstable knees.
Clinical outcomes
Clinical outcomes improved in the Actifit, CMI and NUsurface scaffolds (Table 3). The visual analogue score (VAS) and Lysholm, Tegner and KOOS scores improved post-operatively with the Actifit implant. One study did not show a significant improvement in Tegner scores [17], and another did not show a significant improvement in KOOS scores [14]. The NUsurface preliminary data recorded the KOOS score only, which was found to improve across all five domains and were statistically significant, although mean differences were reported instead of absolute values [11]. The CMI scaffold data showed an improvement in the VAS score, Lysholm score and Tegner Score post-operatively. However, one case series of eight patients did not report mean clinical improvement values [21], and another study did not report absolute values [23].
Concurrent procedures performed
All of the Actifit [14,15,16,17,18] and five out of six CMI studies [19, 21,22,23,24] had some form of concurrent procedure performed on the operated knees (Table 4). The Actifit studies had 118 (45%) patients undergo a concurrent procedure, and the CMI patients had 53 (45%). The commonest procedure performed for the Actifit patients was a high tibial osteotomy (HTO) (54), followed by an ACL repair (47). Out of 53 patients who had concurrent procedures in the CMI studies, the most common procedure was ACL reconstruction (45), followed by HTO (2). The concurrent procedures for the NUsurface implant have not been recorded, however, the inclusion criteria for the study included patients who have normal leg alignment and had not undergone ACL reconstructions in the prior 9 months [11].
Only one Actifit study stratified patients into different sub-groups for patients who had undergone scaffold implantation between those that had undergone ACL reconstructions or HTO [16]. However, it is unclear whether or not there was a cohort of patients who had scaffolds only [16]. They found that at 5 years, patients who had undergone an ACL reconstruction had statistically significant improvements in VAS, Lysholm and KOOS symptoms scores [16]. Comparison of the HTO versus patients without HTO showed no statistically significant differences in clinical outcome scores at 5 years [16].
Failures
The failure rates for Actifit, CMI and NUsurface scaffolds are summarised in the figure above. Failure rates for the Actifit ranged from 6.3% to 31.8% with a total failure rate of 18.0% at 66.8 months; for the CMI, the range was 0–11.8% with a total failure rate of 6.5% at 97.1 months. The NUsurface failure rate was 16.9% at 12 months from one study (Table 5).
Discussion
The current information from literature on meniscal scaffolds is insufficient to suggest that they could potentially improve clinical outcomes in patients post-meniscal resection. This is largely due to the high proportion of concurrent procedures performed at surgery in both the Actifit and CMI studies. Only one Actifit [16] and one CMI study [20] stratified concurrent procedures into sub-groups for separate statistical analysis. This makes meaningful direct comparison of clinical outcomes following isolated meniscal defects treated by meniscal scaffolds not possible.
The failure rates for Actifit and NUsurface were higher than that for the CMI implant. The mean failure rate for Actifit was 18.0% (range of 6.3–31.8%), subsequent procedures included removal of the implant, conversion to MAT, scaffold breakage, and conversion to UKR/TKR. The Actifit implant was used for both medial and lateral meniscal substitution, whereas the CMI and NUsurface scaffolds were used only for the medial side. The failure rate for CMI was 6.5% (range 0–11.8%), with subsequent recorded procedures not consistently recorded. Lastly, the failure rate for NUsurface was 16.9% after 12 months, with patients undergoing device repositioning, replacement, removals and conversion to UKR [11]. Survivorship of the Actifit and CMI scaffolds is comparable to medial MAT, which have been shown to be 86.2% at 5 years [25], and 73.5% at 10 [26]. Given the variability in recording of failures and volume of concurrent procedures, failures were not sub-analysed for individual concurrent procedures. This issue has been recognised previously in the literature [17], and further investigation into outcomes and complications associated with concurrent procedures would be beneficial.
Secondary prevention of osteoarthritis
The evidence for the chondroprotective effects, and thus secondary prevention of OA of meniscal scaffolds, is not sufficient. One study showed that at 5 years, the Actifit scaffold showed a small increase in the volume of meniscal tissue [22]. An alternate study evaluated International Cartilage Repair Society (ICRS) scores and demonstrated a worsening of the cartilage status of the Actifit implant in 7/15 patients who underwent post-operative MRI scans [15].
For CMI, one study which performed MRI scans to assess the Yulish scores (an MRI scoring system for cartilage defects) showed a normal cartilage signal in over 60% of patients at 5 years post-operation [23]. Second-look arthroscopies showed that the implant was present but reduced in size [23]. Another study assessed progression of osteoarthritis in CMI patients using Rosenberg X-ray views [24]. They found that only one of their patients progressed from an Ahlbäck grade 0 to Ahlbäck grade 2 [24]. However, there are studies that have shown no statistically significant improvement in Yulish scores post CMI scaffold implantation [19]. Overall, there is limited evidence of the chondroprotective effects of the Actifit and CMI meniscal scaffolds.
There are several limitations of this review. Firstly, there was a high volume of concurrent procedures performed at the time of meniscal implantation. In addition, there was also a significant variability in the clinical outcomes reported. These factors make direct meaningful comparison of clinical outcomes impossible. In addition to this, the inclusion criteria for the individual studies included in this review also varied. Given the high level of heterogeneity, meta-analyses and statistical comparison were felt not to be appropriate at this stage. To evaluate clinical outcomes and failure rates, further randomised control trials with comparable clinical outcomes and without concurrent procedures are required. This review is also susceptible to publication bias, as there is significant variability in the criteria for failure in each of the studies.
Conclusion
On the basis of current evidence, the use of meniscal scaffolds as a sole treatment for partial meniscal defects cannot be recommended, owing to the relatively high failure rate and paucity of clinical data. The evidence for their chondroprotective effects, and thus prevention of secondary OA, remains inconclusive. Further high-quality comparative randomised control trials are required before meniscal scaffolds can be recommended for routine clinical use.
Availability of data and materials
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
References
Monk P, Garfjeld Roberts P, Palmer AJ, Bayliss L, Mafi R, Beard D, Hopewell S, Price A (2017) The urgent need for evidence in arthroscopic meniscal surgery. Am J Sports Med 45(4):965–973. https://doi.org/10.1177/0363546516650180
Crook TB, Ardolino A, Williams LA, Barlow IW (2009) Meniscal allograft transplantation: a review of the current literature. Ann R Coll Surg Engl 91(5):361–365. https://doi.org/10.1308/003588409X428559
Papalia R, Del Buono A, Osti L, Denaro V, Maffulli N (2011) Meniscectomy as a risk factor for knee osteoarthritis: a systematic review. Br Med Bull 99:89–106. https://doi.org/10.1093/bmb/ldq043
Mordecai SC, Al-Hadithy N, Ware HE, Gupte CM (2014) Treatment of meniscal tears: an evidence based approach. World J Orthop 5(3):233–241. https://doi.org/10.5312/wjo.v5.i3.233
de Caro F, Perdisa F, Dhollander A, Verdonk R, Verdonk P (2020) Meniscus scaffolds for partial meniscus defects. Clin Sports Med 39(1):83–92. https://doi.org/10.1016/j.csm.2019.08.011
Winkler PW, Rothrauff BB, Buerba RA et al. (2020) Meniscal substitution, a developing and long-awaited demand. J Exp Orthop. 7(1):55. https://doi.org/10.1186/s40634-020-00270-6
Winkler PW, Rothrauff BB, Buerba RA, Shah N, Zaffagnini S, Alexander P, Musahl V (2020) Meniscal substitution, a developing and long-awaited demand. J Exp Orthop 7(1):55. https://doi.org/10.1186/s40634-020-00270-6
Houck DA, Kraeutler MJ, Belk JW, McCarty EC, Bravman JT (2018) Similar clinical outcomes following collagen or polyurethane meniscal scaffold implantation: a systematic review. Knee Surg Sports Traumatol Arthrosc 26(8):2259–2269. https://doi.org/10.1007/s00167-018-4838-1
Duarte-Silva M, Guerra-Pinto F, Camelo-Barbosa N, Beja-da-Costa P (2019) Integration and vascular ingrowth of a collagen meniscal implant: a case report. Malays Orthop J 13(2):38–41. https://doi.org/10.5704/MOJ.1907.007
Rodkey WG, Steadman JR, Li ST (1999) A clinical study of collagen meniscus implants to restore the injured meniscus. Clin Orthop Relat Res. https://doi.org/10.1097/00003086-199910001-00027
McKeon BP, Zaslav KR, Alfred RH, Alley RM, Edelson RH, Gersoff WK, Greenleaf JE, Kaeding CC (2020) Preliminary results from a US clinical trial of a novel synthetic polymer meniscal implant. Orthop J Sports Med 8(9):2325967120952414. https://doi.org/10.1177/2325967120952414
Butt U, Vuletić F, Stenhouse G, Hudetz D, Bradbury N (2021) Meniscal scaffold for the treatment of partial meniscal defect-clinical and radiological outcomes in a two-year follow-up. Int Orthop 45(4):977–983. https://doi.org/10.1007/s00264-020-04811-7
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535. https://doi.org/10.1136/bmj.b2535
Leroy A, Beaufils P, Faivre B, Steltzlen C, Boisrenoult P, Pujol N (2017) Actifit® polyurethane meniscal scaffold: MRI and functional outcomes after a minimum follow-up of 5 years. Orthop Traumatol Surg Res 103(4):609–614. https://doi.org/10.1016/j.otsr.2017.02.012
Dhollander A, Verdonk P, Verdonk R (2016) Treatment of painful, irreparable partial meniscal defects with a polyurethane scaffold: midterm clinical outcomes and survival analysis. Am J Sports Med 44(10):2615–2621. https://doi.org/10.1177/0363546516652601
Toanen C, Dhollander A, Bulgheroni P, Filardo G, Zaffagnini S, Spalding T, Monllau JC, Gelber P, Verdonk R, Beaufils P, Pujol N, Bulgheroni E, Asplin L, Verdonk P (2020) Polyurethane meniscal scaffold for the treatment of partial meniscal deficiency: 5-year follow-up outcomes: a European Multicentric Study. Am J Sports Med 48(6):1347–1355. https://doi.org/10.1177/0363546520913528
Monllau JC, Poggioli F, Erquicia J, Ramírez E, Pelfort X, Gelber P, Torres-Claramunt R (2018) Magnetic resonance imaging and functional outcomes after a polyurethane meniscal scaffold implantation: minimum 5-year follow-up. Arthroscopy 34(5):1621–1627. https://doi.org/10.1016/j.arthro.2017.12.019
Filardo G, Kon E, Perdisa F, Sessa A, Di Martino A, Busacca M, Zaffagnini S, Marcacci M (2017) Polyurethane-based cell-free scaffold for the treatment of painful partial meniscus loss. Knee Surg Sports Traumatol Arthrosc 25(2):459–467. https://doi.org/10.1007/s00167-016-4219-6
Zaffagnini S, Marcheggiani Muccioli GM, Lopomo N, Bruni D, Giordano G, Ravazzolo G, Molinari M, Marcacci M (2011) Prospective long-term outcomes of the medial collagen meniscus implant versus partial medial meniscectomy: a minimum 10-year follow-up study. Am J Sports Med 39(5):977–985. https://doi.org/10.1177/0363546510391179
Steadman JR, Rodkey WG (2005) Tissue-engineered collagen meniscus implants: 5- to 6-year feasibility study results. Arthroscopy 21(5):515–525. https://doi.org/10.1016/j.arthro.2005.01.006
Zaffagnini S, Giordano G, Vascellari A, Bruni D, Neri MP, Iacono F, Kon E, Presti ML, Marcacci M (2007) Arthroscopic collagen meniscus implant results at 6 to 8 years follow up. Knee Surg Sports Traumatol Arthrosc 15(2):175–183. https://doi.org/10.1007/s00167-006-0144-4
Bulgheroni E, Grassi A, Bulgheroni P, Marcheggiani Muccioli GM, Zaffagnini S, Marcacci M (2015) Long-term outcomes of medial CMI implant versus partial medial meniscectomy in patients with concomitant ACL reconstruction. Knee Surg Sports Traumatol Arthrosc 23(11):3221–3227. https://doi.org/10.1007/s00167-014-3136-9
Bulgheroni P, Murena L, Ratti C, Bulgheroni E, Ronga M, Cherubino P (2010) Follow-up of collagen meniscus implant patients: clinical, radiological, and magnetic resonance imaging results at 5 years. Knee 17(3):224–229. https://doi.org/10.1016/j.knee.2009.08.011
Monllau JC, Gelber PE, Abat F, Pelfort X, Abad R, Hinarejos P, Tey M (2011) Outcome after partial medial meniscus substitution with the collagen meniscal implant at a minimum of 10 years’ follow-up. Arthroscopy 27(7):933–943. https://doi.org/10.1016/j.arthro.2011.02.018
Verdonk PC, Demurie A, Almqvist KF, Veys EM, Verbruggen G, Verdonk R (2005) Transplantation of viable meniscal allograft: survivorship analysis and clinical outcome of one hundred cases. J Bone Joint Surg Am 87(4):715–724
Novaretti JV, Patel NK, Lian J et al (2019) Long-Term Survival Analysis and Outcomes of Meniscal Allograft Transplantation With Minimum 10-Year Follow-Up: A Systematic Review. Arthroscopy. 35(2):659–667. https://doi.org/10.1016/j.arthro.2018.08.031
Funding
No funding was received by any of the authors.
Author information
Authors and Affiliations
Contributions
S.K. collected papers for review, performed statistical analysis and was a significant contributor to writing the manuscript. J.S. performed part of statistical analysis and contributed to writing the manuscript. I.B. provided expert guidance and contributed to writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kohli, S., Schwenck, J. & Barlow, I. Failure rates and clinical outcomes of synthetic meniscal implants following partial meniscectomy: a systematic review. Knee Surg & Relat Res 34, 27 (2022). https://doi.org/10.1186/s43019-022-00155-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43019-022-00155-1