Abstract
Background
Machine learning is a promising and powerful technology with increasing use in orthopedics. Periprosthetic joint infection following total knee arthroplasty results in increased morbidity and mortality. This systematic review investigated the use of machine learning in preventing periprosthetic joint infection.
Methods
A systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. PubMed was searched in November 2022. All studies that investigated the clinical applications of machine learning in the prevention of periprosthetic joint infection following total knee arthroplasty were included. Non-English studies, studies with no full text available, studies focusing on non-clinical applications of machine learning, reviews and meta-analyses were excluded. For each included study, its characteristics, machine learning applications, algorithms, statistical performances, strengths and limitations were summarized. Limitations of the current machine learning applications and the studies, including their ‘black box’ nature, overfitting, the requirement of a large dataset, the lack of external validation, and their retrospective nature were identified.
Results
Eleven studies were included in the final analysis. Machine learning applications in the prevention of periprosthetic joint infection were divided into four categories: prediction, diagnosis, antibiotic application and prognosis.
Conclusion
Machine learning may be a favorable alternative to manual methods in the prevention of periprosthetic joint infection following total knee arthroplasty. It aids in preoperative health optimization, preoperative surgical planning, the early diagnosis of infection, the early application of suitable antibiotics, and the prediction of clinical outcomes. Future research is warranted to resolve the current limitations and bring machine learning into clinical settings.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Total knee arthroplasty (TKA) is a common procedure for severe knee osteoarthritis and other end-stage joint conditions. One complication of TKA is periprosthetic joint infection (PJI). The incidence of PJI ranges from 1% to 2% following primary TKA [1]. This complication results in an increased cost of treatments, a prolonged hospital stay, an increase in pain, and an increase in morbidity and mortality [2, 3]. Currently, machine learning (ML) is becoming a promising and powerful technology in the prevention of PJI as it may benefit the prediction, diagnosis, treatment and prognosis of PJI.
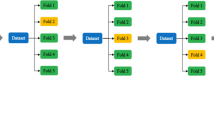
ML is a form of artificial intelligence. It may imitate human thinking and may even exceed human capability [4]. To build a ML model, massive datasets and outcomes could be split into a training set and a test set, and input into a computer (Fig. 1) [5]. Then, the computer may find an association between the data and generate an algorithm accordingly. Various hyperparameters could be adjusted to improve the algorithm’s performance [6]. The final algorithm could be used to generate decisions in future unseen datasets [5].
ML is a promising field with surging applications. Some ML models have been developed for the prevention of PJI. A complete prevention strategy for PJI usually includes four key principles, i.e., early prediction, diagnosis, antibiotic application, and prognosis. The ML models built by Yeo et al. [7] and Kuo et al. [8] enabled early prediction and diagnosis of PJI respectively, allowing for patient-specific surgical planning and detection of infection. Luftinger et al. [9] reviewed multiple ML models for determining the antibiotic susceptibility status of common PJI pathogens, allowing for an early prescription of antibiotics for PJI management. Wouthuyzen et al. [10] reviewed an ML model that could predict outcomes more accurately than two statistical risk scores for debridement, antibiotics and implant retention (DAIR). One of the common drawbacks of the models was the difficulty in interpreting the results. Although a few studies investigated the use of ML in the prevention of PJI, to our best knowledge, so far, no systematic reviews on the prevention of PJI covered all four aforementioned key principles.
This systematic review investigated the use of ML designed for prophylaxis of PJI. We also elaborated on and summarized the efficiency of the prevention strategy based on the four key principles, i.e., early prediction, diagnosis, antibiotic application, and prognosis.
Materials and methods
Search and selection
A systematic review of the literature published from 2006 to 2022 was conducted based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [11]. PubMed was searched in November 2022 using the following keywords: ‘periprosthetic joint infection’, ‘prosthetic joint infection’, ‘PJI’, ‘infection’, ‘artificial intelligence’, ‘AI’, ‘machine learning’, ‘ML’, ‘deep learning’, ‘joint replacement’ and ‘arthroplasty’. References of eligible studies were included in the search for additional results.
Two independent reviewers reviewed the studies. Discrepancies between the reviewers were resolved by comparing notes. All studies that examined the clinical applications of ML in the prevention of PJI following TKA were included. Non-English studies, studies with no full text available, studies focusing on non-clinical applications of ML, reviews and meta-analyses were excluded. The retrieved studies were first screened for possible relevance to the review topic by reading the titles and abstracts. Then, full texts were perused to further confirm eligibility.
Quality assessment
Two independent reviewers assessed the methodological quality by employing the National Institutes of Health quality assessment tool for case-control studies [12]. Discrepancies in the quality rating were resolved through discussion. The total quality score was calculated as the number of ‘yes’ over the number of questions, with questions answered with ‘not applicable’ excluded. The quality was rated as good (>75%), fair (50%–75%) or poor (<50%). The total score and quality rating are listed in Table 1.
Data extraction
Data extracted from each study consisted of three parts: (1) the characteristics of the studies, including the first author, the title, the journal of publication, the year of publication and the cohort size, (2) the details of the ML models, including their applications, algorithms and statistical performances and (3) the strengths and limitations of the studies.
Results
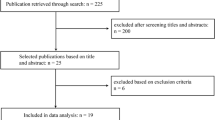
The initial search identified 87 studies (Fig. 2). Seventy-one studies were excluded after reading the titles and abstracts against the inclusion and exclusion criteria. Full texts were obtained and reviewed for the remaining 16 studies. Five studies were removed, with 11 studies included for the final analysis [7, 8, 13,14,15,16,17,18,19,20,21]. The included studies targeted four areas of ML application: PJI prediction, diagnosis, antibiotic application, and prognosis (Fig. 3). The features of the studies are summarized in Table 2, in terms of PJI prediction, diagnosis and prognosis, and in Table 3 in terms of pathogens. The details of the ML models, strengths and limitations of the studies are summarized in Table 4 for PJI prediction, Table 5 for PJI diagnosis, Tables 6 and 7 for PJI pathogens, and Table 8 for PJI prognosis. The two most common metrics used by the included studies to evaluate the performance of the ML models in the classification of individuals was the area under the receiver operating characteristic curve (AUC) and accuracy. The receiver operating characteristics curve is a probability curve plotted with true positive rates against false positive rates. The AUC represents the degree of classification ability. The value of AUC ranges from 0 (poor model performance) to 1 (perfect model performance). The value of accuracy ranges from 0% (poor performance) to 100% (perfect performance). Other common metrics used were the Brier score, F1 score, sensitivity, specificity, calibration intercept and predictive value.
Prediction
Yeo et al. [7] used an artificial neural network to develop an ML model for the preoperative risk prediction of both superficial surgical site infection and PJI following TKA. About 10,000 primary TKA patients were included. The average follow-up time lasted for about 3 years. The patients’ demographic and operational variables were collected. The model performance was good, with an AUC of 0.84 and a Brier score of 0.054 (a Brier score close to zero indicates good accuracy of probabilistic prediction). Several important variables for prediction were identified, including Charlson comorbidity score, obesity, smoking and diabetes.
Diagnosis
Kuo et al. [8] developed a diagnostic model using a two-level stacked generalization architecture with a support vector machine as a meta-classifier. A small cohort of 323 patients was included. The model performance in diagnosing chronic PJI was compared with that of the 2018 European Bone and Joint Infection Society criteria. They applied an if-then rule and a decision diagram to visualize the decision pathway of the ML model. With an AUC of 0.988, the model performed better than the criteria of the International Consensus Meeting (ICM) with an AUC of 0.958. The accuracy of the model was 96.4%. The model not only identified most of the common important features listed in the 2018 ICM criteria for PJI diagnosis but also considered additional important features, such as hemoglobin and prothrombin time, and set up different baseline values for these features that were individualized to each patient.
An ML model based solely on pathological information is also available for diagnosing PJI. Using pathological data, Tao et al. [13] trained a resNet34 deep-learning convolutional network model to diagnose PJI. The cohort comprised 20 revision total knee and hip arthroplasty patients from the Chinese People’s Liberation Army General Hospital, who were classified into infected and non-infected based on the 2018 ICM guidelines. Frozen pathological sections collected were converted into electronic images with 461 positive and 461 negative images for model training.
Comparing the performance of the different ML models for PJI diagnosis, Kuo’s [8] model, which utilized a wide variety of demographic, biomedical, comorbidity, surgical and ICM-related data, demonstrated a greater performance in PJI diagnosis, yielding an AUC of 0.988 and an accuracy of 96.4%. This was followed by Tao’s [13] model with an AUC of 0.814 and an average accuracy of 93.3%. This might be due to a narrower and more specialized spectrum of data used by Tao’s model as compared to Kuo’s. Nevertheless, both of them provide a new research direction for PJI diagnosis.
Antibiotic application
Staphylococcus aureus
Davis et al. [14] developed an adaptive boosting ML classifier for the prediction of methicillin resistance status in Staphylococcus aureus. A total of 606 bacterial genomes were collected from the Pathosystems Resource Integration Center database. The DNA k-mer counts were used to represent the antimicrobial resistance regions within a bacterial genome, and were then used to train the algorithm, which resulted in an outstanding performance with an AUC of 0.991 and an accuracy of 99.5%, similar to the model developed by Drouin et al. [15], which demonstrated a high accuracy of 98.7% for Staphylococcus aureus.
Enterococcus faecium
Drouin et al. [15] developed a set covering machine model to predict the antibiotic susceptibility status of 12 pathogens, including Staphylococcus aureus, Enterococcus faecium, Escherichia coli, Klebsiella pneumoniae and Pseudomonas aeruginosa, using 56 different antibiotics. The DNA k-mer counts were also extracted from the Pathosystems Resource Integration Center database. The model had a very high accuracy of 100% for Enterococcus faecium. The model was also highly interpretable and had a short computing time as a result of the implementation of the sample compression theory and the addition of some comprehensive tutorials that could guide users with no prior knowledge of ML to interpret the model.
Escherichia coli
Moradigaravand et al. [16] trained a gradient-boosting decision tree as an ML model to predict the resistance of Escherichia coli to 11 common antibiotics. Data including gene contents, isolation year, population structure and information on polymorphism were collected from 1936 whole genome sequencing samples of Escherichia coli strains for model training. The performance of the ML model was compared with that of a rule-based method developed in the same study, and the result demonstrated that the ML model, with an average accuracy of 91.0%, outperformed the rule-based method as well as the model developed by Drouin et al. [15], which achieved an accuracy of 81.8% for Escherichia coli.
Klebsiella pneumoniae
Drouin et al. [15] developed a model based on a binary phenotype classification, i.e., susceptible or resistant, and achieved an accuracy of 95.0% for Klebsiella pneumoniae. In another study by Nguyen et al. [17], the minimal inhibitory concentration (MIC) of Klebsiella pneumoniae was predicted by using a gradient-boosting tree model to determine the level of antibiotic resistance. The model was trained on 1668 isolates and achieved an overall accuracy of 92.0%.
Pseudomonas aeruginosa
Using genomic sequences and transcriptional data from 414 Pseudomonas aeruginosa samples, Khaledi et al. [18] developed a support vector machine classifier for predicting the susceptibility of Pseudomonas aeruginosa to four commonly used anti-pseudomonas antibiotics, including ceftazidime, meropenem, ciprofloxacin and tobramycin. The model achieved a high sensitivity and predictive value of 0.8–0.9 or > 0.9, which was comparable to the model of Drouin et al. [15], with an accuracy of 93.9% for Pseudomonas aeruginosa. Although more and more studies have developed predictive models for different species, there is still little effort in developing software that provides easy accessibility to the public who may not have high-standard computing hardware. Considering this, Aun et al. [19] developed a simple software called ‘PhenotypeSeeker’ that used MIC values as well as binary phenotypes to determine Pseudomonas aeruginosa resistance to ciprofloxacin using two regression models. This method achieved an accuracy of 88.0%. K-mers of 200 genomes were collected to develop the model. With assembled genomes, the model could be built in less than 5 h per phenotype and could generate a phenotype prediction in just a second.
Prognosis
Shohat et al. [20] developed a random forest analysis model to predict DAIR failure using the patients’ demographics, medical comorbidities, microbiology, operative findings and laboratory findings. The cohort of total knee and hip arthroplasty patients contained 609 TKA cases. Significant predictors for the knee cohort and early acute PJI patients were positive blood cultures and high C-reactive protein, whereas days of symptoms and immunosuppression were more significant in late acute PJI patients. The model performance was acceptable with an AUC of 0.74.
Another model targeting revision TKA (rTKA) failure rate was developed by Klemt et al. [21], who used an artificial neural network to predict recurrent PJI following rTKA. The model was established by using 618 PJI cases with rTKA as the treatment. The model achieved an AUC of 0.84, a Brier score of 0.053 (close to zero indicating good accuracy of the probabilistic prediction), and a calibration intercept of 0.06 (indicating a slight underestimation of the risk prediction). Irrigation and debridement with or without modular component exchange during rTKA, more than four prior open surgeries, metastatic disease and drug abuse were identified as statistically significant variables for the prediction.
Discussion
Prediction
In clinical practice, TKA patients at high risk of developing PJI are identified based on the presence or absence of risk factors. However, there is currently no universal guideline on this matter, and clinicians can only predict the risk of PJI based on their experiences. Whenever a patient’s condition is complex or the dataset is incomplete, the difficulty of prediction may increase, requiring more time for an accurate prediction. The ML model presented by Yeo et al. [7] may be a promising alternative to the manual risk prediction method.
Applying ML models in PJI prediction following TKA has several benefits: First, the early preoperative risk prediction of PJI may assist in preoperative treatment decisions by allowing patients to weigh potential risks against benefits [7]. Second, the ML model may assist in the preoperative optimization of the patient’s condition [22] by identifying and correcting modifiable high-risk variables prior to surgery. Third, the ML model may be able to identify relationships between variables even in a complex and incomplete dataset. It may generate a prediction faster than a manual prediction, facilitating preoperative decision-making.
Diagnosis
To date, there is no universal definition of PJI. Nonetheless, a widely accepted definition has been introduced by the Musculoskeletal Infection Society, which was endorsed at the 2013 ICM. The strength of the definition was further enhanced in a new version formulated by the Musculoskeletal Infection Society in 2018 [23]. Yet, having a fixed list of criteria and being non-specific to individual cases, the definition may not be able to provide personalized diagnostic approaches. The ML model suggested by Kuo et al. [8] may be an alternative for PJI diagnosis as it could provide a patient-specific explanation and aid in individualized decisions for PJI diagnosis. The decision diagram and the if-then rule used may also provide a more comprehensive explanation of the decision compared to the importance level presented by most other studies.
Currently, there is also no gold standard for pathological PJI diagnosis. The 2018 ICM pathological criteria [23] suggested that more than five neutrophils per high-power field observed in five high-power fields are needed for pathological diagnosis of PJI, whereas the European Bone and Joint Infection Society definition in 2021 [24] suggested that at least five neutrophils per high-power field observed in at least one high-power field are enough to suggest a possible PJI. The model suggested by Tao et al. [13] may be an alternative approach with the following advantages: First, the ML model may avoid the controversy of neutrophil number and positive high-power field number, as the diagnosis does not rely on the neutrophil count alone. It is also accompanied by several infection indicators such as tissue edema, capillary hyperplasia, neutrophil infiltration and proliferation. This could be a more comprehensive approach than the current pathological diagnostic criteria. Second, the ML method may be more accurate as it covers the entire pathological section and does not rely on the neutrophil count alone. In contrast, manual diagnostic methods only select suspected sections for recognition, which often omit pathological sections that may be infected, and only rely on the neutrophil count, which may be confused by the diverse morphology of neutrophils that may be similar to other inflammatory cells. Third, the ML method may shorten the time spent on pathological diagnosis. This is because ML can process multiple images at the same time. It may also be more powerful at recognizing pathological features than the manual method, enabling early diagnosis and thus early surgical intervention for PJI. Lastly, pathological diagnosis by ML could be more objective than the manual method which heavily depends on pathologists’ experience in recognizing pathological features.
Antibiotic application
If a diagnosis of PJI is established, an antibiotic prescription will be urgent. However, it was anticipated that antibiotic resistance would result in a decrease in the effectiveness of antibiotics [25]. In view of this, it is important to know the antimicrobial susceptibility status before prescribing antibiotics for PJI treatment. Currently, microbial resistance to antibiotics can be identified by several methods, each with its own downsides. First, culture-dependent antimicrobial susceptibility testing, although commonly used, usually takes 12–48 h and is time-consuming [26]. For slow-growing microorganisms, days to weeks may be needed. For non-culturable PJI pathogens, there may even be no results [9]. Second, another method is the polymerase chain reaction-based method, which is limited by the completeness of the database of known antimicrobial resistance marker genes [9]. An alternative approach may be the next-generation sequencing-based predictive antimicrobial susceptibility testing with an ML model [14,15,16,17,18,19]. Some of the ML models included in the analysis used the binary phenotype classification, i.e., susceptible or resistant [14,15,16, 18]. Another predicted outcome used was the MIC, which further assessed the degree of susceptibility status [17, 19, 27].
Applying ML in the antibiotic prescription for PJI has several advantages: First, no prior knowledge of the resistance mechanism of the microbial strains is required to use the models [15,16,17,18], allowing for a more extensive application of the models. Second, the prediction could be generated in a short time, allowing for an early prescription of antibiotics. Third, ML models may identify the antibiotic susceptibility status of non-culturable PJI pathogens, which are the causative pathogens in 5%–42% of PJI [28], hence allowing for a more effective treatment prescription.
Prognosis
Treatment options for PJI include DAIR, one- or two-stage rTKA, arthrodesis and amputation [29]. Among them, DAIR and rTKA are the two most common choices, but the success rates of these treatments vary greatly. For acute postoperative PJI, DAIR has a failure rate of 0–69%, whereas for late chronic PJI, the failure rate is 38%–72% [30,31,32,33,34,35,36,37,38,39,40,41]. One-stage rTKA has a failure rate of 27% and the rate of two-stage is less than 10% [42,43,44,45,46,47,48,49,50]. To improve the prognosis after treatment, an early and correct decision on the treatment option, and preoperative optimization of the patient’s condition are important.
Currently, there is a guideline for DAIR recommendation. However, the Infectious Diseases Society of America guideline published in 2013 may have multiple weaknesses, such as no separate guidance between early and late acute PJI, and little consideration of patient- and implant-related variables [51]. Alternatively, Shohat et al. [20] and Klemt et al. [21] demonstrated that ML models could provide more comprehensive treatment guidance than traditional guidelines. One special feature of Shohat’s [20] model is the separate analysis of the DAIR failure rate in early and late acute PJI patients given the reported difference in their failure rates [30,31,32,33,34,35,36,37,38,39,40,41]. The ML model developed by Klemt et al. [21] also out-performed a prior model with a conventional statistical approach [52].
Adapting ML models in prognostic prediction has several advantages: First, the prediction of treatment failure risk may assist clinicians and patients in making early preoperative treatment decisions, to better allocate resources, with revision surgery reserved for patients at high risk and DAIR for patients at low risk. Second, an individualized prediction may lead to more patient-specific guidance than conventional guidelines due to the involvement of more patient-specific variables. Third, ML models allow for preoperative optimization of patients’ conditions, thereby reducing the failure rate by correcting modifiable risk factors prior to surgery. Lastly, the risk prediction may allow for early preparation for prescriptions, lengthened hospital stays, and subsequent treatment planning for possible treatment failure in high-risk patients.
Limitations
While ML could be an effective tool for preventing PJI, it does have several practical limitations that restrict its use. First, ML algorithms have ‘black box problems’, meaning that their decision-making processes are not transparent, their results may not be interpretable, and their flaws may not be readily detectable [53]. It is therefore essential to evaluate and validate the algorithm extensively before putting it into clinical practice [54]. Second, ML algorithms are likely to overfit in imbalanced datasets. In the case of overfitting, an algorithm with high accuracy may not perform well when tested on an unseen dataset [5, 55]. Third, a large database with millions to trillions of data points may be required for training and testing [5], and a separate set of local training data has to be available to adapt the algorithm to a new population [56]. Hence, hospitals with small data sizes may need data sharing, which presents a problem of data protection and privacy infringement [57, 58]. With the emergence of massive databases, such as the National Inpatient Sample datasets and the American College of Surgeons-National Surgical Quality Improvement Program database, more abundant datasets will be available to facilitate future research on ML applications.
Although ML application is gaining more attention, there are research gaps to be bridged. The studies reviewed above were not of prospective nature and were not externally validated. There is also a paucity of research in PJI-related areas. Future studies on the AI-based prediction of the risk of PJI with a longer follow-up time are needed and should cover more commonly used antibiotics and pathogens in investigating the susceptibility of the microbes. In addition, most of the current models did not assess the impact of the severity of disease factors on the outcome [7, 8, 13,14,15,16,17,18,19,20,21]. For example, only the presence or absence of hypertension was examined for its association with PJI risk following primary TKA without taking into account the severity of hypertension. This provides a new direction for future research work.
Conclusion
Machine learning may be a favorable alternative to manual methods in the prevention of PJI after TKA. It aids in preoperative patient optimization, preoperative surgical planning, early diagnosis of infection, early application of suitable antibiotics, and the prediction of clinical outcomes. Although ML applications are potentially beneficial to the prevention of PJI, some current limitations need to be overcome in order to ensure that ML is a non-inferior or even superior option to manual approaches and, therefore, worth application in clinical settings.
Availability of data and materials
All data analyzed during this study are included in this published article.
Abbreviations
- AUC:
-
Area under the receiver operating characteristic curve
- DAIR:
-
Debridement, antibiotics and implant retention
- ICM:
-
International Consensus Meeting
- MIC:
-
Minimum inhibitory concentration
- ML:
-
Machine learning
- PJI:
-
Periprosthetic joint infection
- rTKA:
-
Revision total knee arthroplasty
- TKA:
-
Total knee arthroplasty
References
Ahmed SS, Haddad FS. Prosthetic joint infection. Bone Joint Res. 2019;8(11):570–2.
Zmistowski B, Karam JA, Durinka JB, Casper DS, Parvizi J. Periprosthetic joint infection increases the risk of one-year mortality. J Bone Joint Surg Am. 2013;95(24):2177–84.
Premkumar A, Kolin DA, Farley KX, Wilson JM, McLawhorn AS, Cross MB, et al. Projected economic burden of periprosthetic joint infection of the hip and knee in the United States. J Arthroplasty. 2021;36(5):1484-9.e3.
Deo RC. Machine learning in medicine. Circulation. 2015;132(20):1920–30.
Choi RY, Coyner AS, Kalpathy-Cramer J, Chiang MF, Campbell JP. Introduction to machine learning, neural networks, and deep learning. Transl Vis Sci Technol. 2020;9(2):14.
Kurmis AP, Ianunzio JR. Artificial intelligence in orthopedic surgery: evolution, current state and future directions. Arthroplasty. 2022;4(1):9.
Yeo I, Klemt C, Robinson MG, Esposito JG, Uzosike AC, Kwon YM. The Use of artificial neural networks for the prediction of surgical site infection following TKA. J Knee Surg. 2023;36(6):637–43.
Kuo FC, Hu WH, Hu YJ. Periprosthetic joint infection prediction via machine learning: comprehensible personalized decision support for diagnosis. J Arthroplasty. 2022;37(1):132–41.
Luftinger L, Ferreira I, Frank BJH, Beisken S, Weinberger J, von Haeseler A, et al. Predictive antibiotic susceptibility testing by next-generation sequencing for periprosthetic joint infections: potential and limitations. Biomedicines. 2021;9(8):910.
Wouthuyzen-Bakker M, Shohat N, Parvizi J, Soriano A. Risk scores and machine learning to identify patients with acute periprosthetic joints infections that will likely fail classical irrigation and debridement. Front Med (Lausanne). 2021;8:550095.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Study quality assessment tools: NIH National Heart, Lung, and Blood Institute; 2021. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. Accessed 28 Nov 2022.
Tao Y, Hu H, Li J, Li M, Zheng Q, Zhang G, et al. A preliminary study on the application of deep learning methods based on convolutional network to the pathological diagnosis of PJI. Arthroplasty. 2022;4(1):49.
Davis JJ, Boisvert S, Brettin T, Kenyon RW, Mao C, Olson R, et al. Antimicrobial resistance prediction in PATRIC and RAST. Sci Rep. 2016;6:27930.
Drouin A, Letarte G, Raymond F, Marchand M, Corbeil J, Laviolette F. Interpretable genotype-to-phenotype classifiers with performance guarantees. Sci Rep. 2019;9(1):4071.
Moradigaravand D, Palm M, Farewell A, Mustonen V, Warringer J, Parts L. Prediction of antibiotic resistance in Escherichia coli from large-scale pan-genome data. PLoS Comput Biol. 2018;14(12):e1006258.
Nguyen M, Brettin T, Long SW, Musser JM, Olsen RJ, Olson R, et al. Developing an in silico minimum inhibitory concentration panel test for Klebsiella pneumoniae. Sci Rep. 2018;8(1):421.
Khaledi A, Weimann A, Schniederjans M, Asgari E, Kuo TH, Oliver A, et al. Predicting antimicrobial resistance in Pseudomonas aeruginosa with machine learning-enabled molecular diagnostics. EMBO Mol Med. 2020;12(3):e10264.
Aun E, Brauer A, Kisand V, Tenson T, Remm M. A k-mer-based method for the identification of phenotype-associated genomic biomarkers and predicting phenotypes of sequenced bacteria. PLoS Comput Biol. 2018;14(10):e1006434.
Shohat N, Goswami K, Tan TL, Yayac M, Soriano A, Sousa R, et al. 2020 Frank Stinchfield Award: identifying who will fail following irrigation and debridement for prosthetic joint infection. Bone Joint J. 2020;102-B(7_Supple_B):11–9.
Klemt C, Laurencin S, Uzosike AC, Burns JC, Costales TG, Yeo I, et al. Machine learning models accurately predict recurrent infection following revision total knee arthroplasty for periprosthetic joint infection. Knee Surg Sports Traumatol Arthrosc. 2022;30(8):2582–90.
Batailler C, Shatrov J, Sappey-Marinier E, Servien E, Parratte S, Lustig S. Artificial intelligence in knee arthroplasty: current concept of the available clinical applications. Arthroplasty. 2022;4(1):17.
Parvizi J, Tan TL, Goswami K, Higuera C, Della Valle C, Chen AF, et al. The 2018 definition of periprosthetic hip and knee infection: an evidence-based and validated criteria. J Arthroplasty. 2018;33(5):1309-14.e2.
McNally M, Sousa R, Wouthuyzen-Bakker M, Chen AF, Soriano A, Vogely HC, et al. The EBJIS definition of periprosthetic joint infection. Bone Joint J. 2021;103-B(1):18–25.
Holmes AH, Moore LSP, Sundsfjord A, Steinbakk M, Regmi S, Karkey A, et al. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet. 2016;387(10014):176–87.
van Belkum A, Bachmann TT, Ludke G, Lisby JG, Kahlmeter G, Mohess A, et al. Developmental roadmap for antimicrobial susceptibility testing systems. Nat Rev Microbiol. 2019;17(1):51–62.
Kowalska-Krochmal B, Dudek-Wicher R. The minimum inhibitory concentration of antibiotics: methods, interpretation, clinical relevance. Pathogens. 2021;10(2):165.
Kalbian I, Park JW, Goswami K, Lee YK, Parvizi J, Koo KH. Culture-negative periprosthetic joint infection: prevalence, aetiology, evaluation, recommendations, and treatment. Int Orthop. 2020;44(7):1255–61.
Springer BD, Parvizi J. Periprosthetic joint infection of the hip and knee. New York: Springer; 2013.
Kuiper JW, Willink RT, Moojen DJ, van den Bekerom MP, Colen S. Treatment of acute periprosthetic infections with prosthesis retention: review of current concepts. World J Orthop. 2014;5(5):667–76.
Laffer RR, Graber P, Ochsner PE, Zimmerli W. Outcome of prosthetic knee-associated infection: evaluation of 40 consecutive episodes at a single centre. Clin Microbiol Infect. 2006;12(5):433–9.
Rasul AT Jr, Tsukayama D, Gustilo RB. Effect of time of onset and depth of infection on the outcome of total knee arthroplasty infections. Clin Orthop Relat Res. 1991;273:98–104.
Rodríguez D, Pigrau C, Euba G, Cobo J, García-Lechuz J, Palomino J, et al. Acute haematogenous prosthetic joint infection: prospective evaluation of medical and surgical management. Clin Microbiol Infect. 2010;16(12):1789–95.
Theis JC, Gambhir S, White J. Factors affecting implant retention in infected joint replacements. ANZ J Surg. 2007;77(10):877–9.
Tintle SM, Forsberg JA, Potter BK, Islinger RB, Andersen RC. Prosthesis retention, serial debridement, and antibiotic bead use for the treatment of infection following total joint arthroplasty. Orthopedics. 2009;32(2):87.
Vilchez F, Martínez-Pastor JC, García-Ramiro S, Bori G, Tornero E, García E, et al. Efficacy of debridement in hematogenous and early post-surgical prosthetic joint infections. Int J Artif Organs. 2011;34(9):863–9.
Qasim SN, Swann A, Ashford R. The DAIR (debridement, antibiotics and implant retention) procedure for infected total knee replacement - a literature review. SICOT J. 2017;3:2.
Geurts JA, Janssen DM, Kessels AG, Walenkamp GH. Good results in postoperative and hematogenous deep infections of 89 stable total hip and knee replacements with retention of prosthesis and local antibiotics. Acta Orthop. 2013;84(6):509–16.
Gardner J, Gioe TJ, Tatman P. Can this prosthesis be saved?: implant salvage attempts in infected primary TKA. Clin Orthop Relat Res. 2011;469(4):970–6.
Chiu FY, Chen CM. Surgical débridement and parenteral antibiotics in infected revision total knee arthroplasty. Clin Orthop Relat Res. 2007;461:130–5.
Silva M, Tharani R, Schmalzried TP. Results of direct exchange or debridement of the infected total knee arthroplasty. Clin Orthop Relat Res. 2002;404:125–31.
Zywiel MG, Johnson AJ, Stroh DA, Martin J, Marker DR, Mont MA. Prophylactic oral antibiotics reduce reinfection rates following two-stage revision total knee arthroplasty. Int Orthop. 2011;35(1):37–42.
Bengtson S, Knutson K. The infected knee arthroplasty. A 6-year follow-up of 357 cases. Acta Orthop Scand. 1991;62(4):301–11.
Booth RE Jr, Lotke PA. The results of spacer block technique in revision of infected total knee arthroplasty. Clin Orthop Relat Res. 1989;248:57–60.
Goldman RT, Scuderi GR, Insall JN. 2-stage reimplantation for infected total knee replacement. Clin Orthop Relat Res. 1996;331:118–24.
Haleem AA, Berry DJ, Hanssen AD. Mid-term to long-term followup of two-stage reimplantation for infected total knee arthroplasty. Clin Orthop Relat Res. 2004;428:35–9.
Insall JN, Thompson FM, Brause BD. Two-stage reimplantation for the salvage of infected total knee arthroplasty. J Bone Joint Surg Am. 1983;65(8):1087–98.
Segawa H, Tsukayama DT, Kyle RF, Becker DA, Gustilo RB. Infection after total knee arthroplasty. A retrospective study of the treatment of eighty-one infections. J Bone Joint Surg Am. 1999;81(10):1434–45.
Park SJ, Song EK, Seon JK, Yoon TR, Park GH. Comparison of static and mobile antibiotic-impregnated cement spacers for the treatment of infected total knee arthroplasty. Int Orthop. 2010;34(8):1181–6.
Mortazavi SM, Molligan J, Austin MS, Purtill JJ, Hozack WJ, Parvizi J. Failure following revision total knee arthroplasty: infection is the major cause. Int Orthop. 2011;35(8):1157–64.
Osmon DR, Berbari EF, Berendt AR, Lew D, Zimmerli W, Steckelberg JM, et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56(1):e1–25.
Klemt C, Tirumala V, Smith EJ, Padmanabha A, Kwon YM. Development of a preoperative risk calculator for reinfection following revision surgery for periprosthetic joint infection. J Arthroplasty. 2021;36(2):693–9.
Price WN. Big data and black-box medical algorithms. Sci Transl Med. 2018;10(471):eaao5333.
Topol EJ. High-performance medicine: the convergence of human and artificial intelligence. Nat Med. 2019;25(1):44–56.
Hinterwimmer F, Lazic I, Langer S, Suren C, Charitou F, Hirschmann MT, et al. Prediction of complications and surgery duration in primary TKA with high accuracy using machine learning with arthroplasty-specific data. Knee Surg Sports Traumatol Arthrosc. 2023;31:1323–33.
Kelly CJ, Karthikesalingam A, Suleyman M, Corrado G, King D. Key challenges for delivering clinical impact with artificial intelligence. BMC Med. 2019;17(1):195.
Gerke S, Minssen T, Cohen G. Ethical and legal challenges of artificial intelligence-driven healthcare. In: Bohr A, Memarzadeh K, editors. Artificial intelligence in healthcare. Massachusetts: Academic; 2020. p. 295–336.
Purnomo G, Yeo S-J, Liow MHL. Artificial intelligence in arthroplasty. Arthroplasty. 2021;3(1):37.
Acknowledgements
The authors thank Mr. Leung Jeffrey Ho Yu for selecting eligible articles and conducting the quality assessment.
Funding
Nil.
Author information
Authors and Affiliations
Contributions
Y.Y.C. (Conception, articles review and quality assessment, analysis, manuscript writing); P.K.C. (Conception, revision comments provision); V.W.K.C., A.C., M.H.L., M.H.C., H.F., K.Y.C. (Revision comments provision). All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
All authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chong, Y.Y., Chan, P.K., Chan, V.W.K. et al. Application of machine learning in the prevention of periprosthetic joint infection following total knee arthroplasty: a systematic review. Arthroplasty 5, 38 (2023). https://doi.org/10.1186/s42836-023-00195-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42836-023-00195-2