Abstract
Background
The critically endangered kākāpō is a flightless, nocturnal parrot endemic to Aotearoa New Zealand. Recent efforts to describe the gastrointestinal microbial community of this threatened herbivore revealed a low-diversity microbiota that is often dominated by Escherichia-Shigella bacteria. Given the importance of associated microbial communities to animal health, and increasing appreciation of their potential relevance to threatened species conservation, we sought to better understand the development of this unusual gut microbiota profile. To this end, we conducted a longitudinal analysis of faecal material collected from kākāpō chicks during the 2019 breeding season, in addition to associated nest litter material.
Results
Using an experimental approach rarely seen in studies of threatened species microbiota, we evaluated the impact of a regular conservation practice on the developing kākāpō microbiota, namely the removal of faecal material from nests. Artificially removing chick faeces from nests had negligible impact on bacterial community diversity for either chicks or nests (p > 0.05). However, the gut microbiota did change significantly over time as chick age increased (p < 0.01), with an increasing relative abundance of Escherichia-Shigella coli over the study period and similar observations for the associated nest litter microbiota (p < 0.01). Supplementary feeding substantially altered gut bacterial diversity of kākāpō chicks (p < 0.01), characterised by a significant increase in Lactobacillus bacteria.
Conclusions
Overall, chick age and hand rearing conditions had the most marked impact on faecal bacterial communities. Similarly, the surrounding nest litter microbiota changed significantly over time since a kākāpō chick was first placed in the nest, though we found no evidence that removal of faecal material influenced the bacterial communities of either litter or faecal samples. Taken together, these observations will inform ongoing conservation and management of this most enigmatic of bird species.
Similar content being viewed by others
Introduction
The kākāpō (Strigops habroptilus) is a critically endangered parrot endemic to Aotearoa New Zealand. Kākāpō are unusual among parrots as they are flightless, nocturnal, exhibit body size sexual dimorphism and undergo lek mating [1]. From only 51 individuals in 1995, today approximately 250 kākāpō are protected on five predator-free islands. These populations are intensively managed by New Zealand’s Department of Conservation (NZDOC, Te Papa Atawhai) to ensure the survival of this unique species.
Kākāpō only mate when plentiful fruit arise following a heavy podocarp mast every 2–4 years, creating highly irregular breeding cycles and slow population growth rates in addition to frequent infertile eggs and embryo deaths [2,3,4]. In current management practice, fertile eggs are usually removed from the nest shortly before hatching to enhance juvenile survival. Newly hatched chicks are hand reared for one or two days before their release into an appropriate nest, after which chick weight and health are closely monitored. Nest monitoring is a vital component of kākāpō conservation as chick health can decline rapidly due to insufficient feeding, especially if rimu fruit fail to ripen. Large amounts of chick faecal material also accumulate in nests which is regularly removed by NZDOC staff to theoretically prevent disease and infection as only a small number of chicks often survived in previous breeding seasons. However, this practice is not based on scientific testing and it remains unknown whether removing this faecal material (and corresponding microorganisms) may affect development of the kākāpō chick microbiota. Conceivably, removing faecal material may deprive kākāpō chicks of an important environmental source of gut microbiota.
Kākāpō harbour a relatively low-diversity gut microbiota that is frequently dominated (up to ~ 99% of 16S rRNA gene amplicons) by Escherichia-Shigella [5,6,7,8]. Members of the bacterial phyla Firmicutes and Proteobacteria often dominate avian intestinal microbiotas, irrespective of host phylogeny or ecology [9,10,11,12]. However, an increased abundance of Enterobacteriaceae (the family to which Escherichia-Shigella belongs) in the gut has been associated with sterility in crested ibis (Nipponia nippon) [13] and mortality in juvenile ostrich (Struthio camelus) [14]. Nonetheless, Enterobacteriaceae frequently inhabit the avian digestive tract [10,11,12, 15], suggesting a non-pathogenic role for these bacteria in many birds. Marked variation in the relative abundance of Escherichia-Shigella among kākāpō individuals has, thus far, not been significantly associated with supplemental feeding, geographic location, age, sex or antibiotic use [6, 7] (West et al. in prep.).
We sought to determine the impact of the current management practice of removing faecal material from nests on the developing kākāpō gut microbiota. In a longitudinal study during the 2019 breeding season, we analysed > 400 samples of chick faeces and corresponding nest litter collected from Whenua Hou/Codfish Island and Pukenui/Anchor Island (Additional file 1: Fig. S1). By experimentally manipulating (removing) faecal matter from some kākāpō nests but not others, we were able to directly evaluate the impact of this practice and inform ongoing conservation efforts.
Materials and methods
Sample collection
Faecal and litter material were collected from 67 kākāpō chicks and 34 nests, respectively (Table 1), from Whenua Hou (46° 47′ S, 167° 38′ E) and Pukenui (45° 45′ S, 166° 31′ E) islands (Additional file 1: Fig. S1) between late February–mid May 2019. Samples were placed directly into 5 mL sterile polypropylene tubes containing RNAlater, then stored overnight at 4 °C and subsequently at − 20 °C until shipping on ice to Waipapa Taumata Rau University of Auckland. We aimed to obtain faecal and litter samples for each chick and nest every fortnight for 10 weeks, with additional samples collected following introduction of a new chick to a nest and follow-up faecal samples collected at least six months later (defined as sub-adult samples). However, the nature of endangered species research is such that sample collection for some chicks and nests occurred less or more frequently than every two weeks, reflecting the workload of island staff and volunteers. An aspergillosis outbreak among kākāpō on Whenua Hou further impacted the study, with all chicks rapidly removed from nests on this island and screened for infection (samples were collected from these Whenua Hou chicks during their stay in the hand rearing facility). Aspergillosis is a respiratory infection caused by Aspergillus fungi to which birds are particularly susceptible [16, 17]. Despite these events, we collected and analysed longitudinal samples for more than half of the newly hatched chicks and their corresponding nests.
Collected metadata included hatch date, sample collection date, location at time of sampling, disease status, nest type (e.g. base of tree, hole within log, behind large rock, under vegetation (‘Open’) or reconstructed by NZDOC staff into an A-Frame) and whether faeces were artificially removed from nests (Additional file 2). Of the 34 nests for which litter samples were successfully analysed, 11 nests were not manipulated (i.e. faeces left in the nest), 22 nests had faecal material removed by NZDOC staff and for one nest this information was not recorded (‘Unknown’; Table 1). Faecal removal varied with chick age and genetic priority but was carried out daily for the first 3 days following hatching or chick introduction to a nest, then every 2–4 days until chicks were 14 days old, every 2–7 days from 15 to 28 days, and every 4–7 days from 29 days until chicks fledged at ~ 10 weeks old. The number of chicks per nest varied, with 10 nests hosting only one chick, 13 nests hosting two and 11 nests hosting three chicks. Metadata were tested as covariates against multiple measures of bacterial diversity and overall microbiota composition.
Of the 67 chicks sampled in this study, 63 were initially hatched and hand reared in on-island captive facilities for 1–2 days during which time they were provided with the commercial feeding formula ‘Kaytee exact Hand Feeding’ (Kaytee, Wisconsin). The remaining four chicks were left to hatch naturally in their respective nests. Seven of the 63 chicks initially hatched in hand rearing facilities were ultimately wholly hand reared until fledging and not placed out in nests on the islands. The remaining 56 chicks initially hatched in hand rearing were then assigned and transferred out to a nest (not necessarily that of their biological mother) with 1–3 chicks occupying a given nest. In response to sub-optimal weight gain or failing health, chicks were often moved among nests or briefly hand reared until a suitable adoptive mother was identified. We refer to the movement of chicks among different nests as ‘chick movement’ (Table 1). Movement among nests was not restricted to the faecal experiment group to which chicks were originally assigned, and hence some chicks were moved among nests that had faeces removed and those that did not (see the ‘Mixed’ category for number of chicks and samples collected under these conditions in Table 1). Movement among nests also meant that the number of chicks for a given nest fluctuated over time and was thus excluded as a testable metadata covariate. Samples collected from chicks being hand reared were not confined to the brief window post-hatching, but occurred throughout the 10-week sampling period as chicks were moved in and out of the facility, particularly during the aspergillosis outbreak.
Between one and six samples were collected per chick or nest, comprising a total of 287 faecal samples and 124 litter samples, respectively (Table 1). Where a faecal sample could not be attributed to a single chick in a nest of multiple individuals, all chicks were listed for that sample, hereafter referred to as a ‘pooled sample’. Faecal samples were collected from one additional nest for which litter samples were not successfully sequenced (Table 1). Overall, 13 nests were located on Pukenui, of which seven nests experienced the movement of chicks and six did not (Table 1). Of the 21 nests located on Whenua Hou, 15 nests experienced chick movement, whilst six nests did not. As many chicks spent some time in and out of the hand-rearing facility, we have faecal samples collected under captive conditions for 22 chicks. Due to the coincident aspergillosis outbreak among kākāpō on Whenua Hou, our data comprise samples from 17 infected chicks and 12 nests where Aspergillus was detected in the mother and/or chicks (Table 1). Also included are data from seven chicks and two nests that came into contact with infected individuals but were not diagnosed with aspergillosis or considered a site of infection (‘Linked’, Table 1).
DNA extraction, PCR and 16S rRNA gene sequencing
DNA was extracted using a modified version of a bead-beating method described by Perry et al. [7], details of which are provided in Additional file 1. This modified protocol reduces the presence of abundant PCR inhibitors and co-precipitation of excess salts.
PCR amplification of the V3-V4 region of the bacterial 16S rRNA gene was performed using primers 341F (5′-TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG-3′) and 785R (5′-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGGACTACHVGGGTATCTAATCC-3′) [18] with Illumina-compatible Nextera adaptors (underlined). A KAPA 3G Plant PCR kit (Sigma-Aldrich, New Zealand) was used for amplification with thermocycling conditions as described by Perry et al. [7]. Amplicon size and absence of a band for negative controls (DNA extraction controls and no-template PCR controls) were verified on a 1% agarose gel with SYBR Safe DNA Gel Stain (Invitrogen, New Zealand). PCR products were purified using AMPure XP beads (Beckman Coulter, New Zealand) or the Zymo ZR-96 DNA Clean-Up Kit (Ngaio Diagnostics Ltd, New Zealand). DNA concentration was quantified on an EnSpire Multimode Plate Reader (Perkin Elmer) using a Qubit High Sensitivity dsDNA kit (Invitrogen, New Zealand). Samples were normalised to 5 ng/µL for library preparation and sequencing by Auckland Genomics Ltd on an Illumina MiSeq with 2 × 300 bp chemistry.
Sequence data analysis
16S rRNA gene amplicon sequences were processed in R (version 4.0.1 [19]) using DADA2 (version 1.16 [20]) following the authors’ recommended workflow for paired-end big data [20]. Following initial primer removal, forward and reverse reads were trimmed to 280 bp and 240 bp, respectively. Sequence reads shorter than the truncated value were discarded, as were reads where truncQ < 2 or where the number of expected errors exceeded 3 for forward and reverse reads (-maxEE parameter). Unique amplicon sequence variants (ASVs) were generated, then taxonomy assigned (assignTaxonomy) using the SILVA 138 ribosomal RNA database (silva_nr99_v138_wSpecies_train_set) [21]. Following removal of sequence chimeras and non-target sequences including chloroplasts and mitochondria, the ASV table and taxonomic assignments were merged with corresponding metadata to create separate phyloseq objects for faecal and litter samples using the R (version 4.1.3 [22]) package phyloseq (version 1.38.0 [23]). Low-abundance ASVs (total relative abundance < 0.001%) and ASVs not assigned to phylum level were also discarded. Samples were normalised using scaling with ranked subsampling (SRS), which better preserves the original community structure with fewer subsampling errors than rarefaction [24]. Faecal samples were normalised to 1,200 reads/sample and litter samples to 1,850 reads/sample. ASVs are numbered separately for faecal and litter data in decreasing order of their relative sequence abundance in the respective data set and thus will differ between the two.
To explore variation in bacterial communities among samples grouped by significant covariates (i.e. chick age, faecal removal, location, nest type, disease status), normalised data were transformed to Bray–Curtis and generalised UniFrac (gUniFrac) dissimilarity matrices and ordinated with principal coordinate analysis (PCoA) using the vegan (version 2.5–7 [25]) and GUniFrac (version 1.6 [26]) packages in R. Generalised UniFrac ordinations are included as Additional file 1: Figs. S2 and S3. We tested for significant associations with PERMANOVA (vegan::adonis) where we controlled for variation with repeated sampling (see Additional file 3) as well as among nests (where hand rearing wasn’t a confounding factor). Significant PERMANOVA models were further subjected to pairwise comparison testing using a modified version of the pairwise.adonis2 function in the pairwiseAdonis package (version 0.4 [27]) where we similarly controlled for variation with repeated sampling. We used vegan functions betadisper and permutest to test for homogeneous group dispersion.
Observed ASV richness and Inverse Simpson alpha-diversity indices were calculated on normalised data using phyloseq and associations with metadata covariates tested using Kruskal–Wallis tests (vegan), followed by generalised and standard linear mixed modelling (lme4 package version 1.1–28 [28]), for observed and Inverse Simpson indices respectively, with likelihood ratio testing to assess the significance of mixed models. Post-hoc pairwise comparisons were performed for significant models using Dunn’s test (dunn.test package version 1.3.5 [29]) with Benjamini–Hochberg p-value correction [30]. We further tested the effect of chick age and location on the log ratio-transformed relative abundance of abundant bacterial genera (> 3% abundance across dataset) in faecal samples using linear mixed models, controlling for variation among chicks, chick age and location with the lme4 package. Again, we tested for significant relationships using likelihood ratio tests comparing null models with target models for each covariate and genus. A differential abundance analysis was employed using the DESeq2 package (version 1.34.0 [31]) to further investigate ASVs that were differentially abundant in samples collected from chicks under hand rearing versus those out in nests.
Kākāpō chicks were weighed regularly throughout the breeding season, enabling linear comparisons between weight gain (as a proportion of first weight) and gut microbial diversity. We utilised linear modelling for alpha-diversity comparisons (lme4) and PERMANOVA (vegan::adonis2) for analysing associations with beta-diversity.
Finally, we created a separate taxonomic level which concatenated genus- and species-level assignments together and agglomerated the phyloseq object to this taxonomy group. The plyr package (version 1.8.7 [32]) was then employed to group less abundant ASVs into the category ‘Others’ based on a per-species mean relative abundance of < 0.3% for faecal samples and < 0.5% for litter samples. The data were plotted against location for faecal samples and against chick age or ‘days since first chick’ for faecal and litter samples, respectively.
All data were visualised using R packages ggplot2 (version 3.3.5 [33]), ggpubr (version 0.4.0 [34]), cowplot (version 1.1.1 [35]), and Manu (‘kākāpō’ colour palette specifically designed from kākāpō plumage; version 0.0.1 [36, 37]).
Results
Bacterial diversity of faecal and litter samples
In total, 33,067,910 raw 16S rRNA gene amplicon paired sequence reads were obtained, with 19,117,949 merged reads remaining after quality and chimera filtering. Of these filtered reads, 99.99% were taxonomically assigned to at least phylum level, spanning 26,602 unique ASVs. Average sequencing depth across all samples was 45,518.93 ± 27,751.97 reads. After removing non-target and low-abundance ASVs, faecal and litter samples were subsampled separately to a minimum read count of 1,200 reads and 1,850 reads, respectively. Four faecal and five litter samples were discarded due to read counts not meeting these thresholds. We ultimately identified 1,065 unique ASVs across 287 faecal samples and 4,403 unique ASVs across 124 litter samples. The number of ASVs per sample ranged from 1 to 180 for faeces and 3–531 for litter, with an average of 37 and 187 ASVs, respectively.
Overall, 96.2% of faecal sample reads could be assigned to genus, but only 78.1% for litter samples. Similarly, 78.7% of faecal sample reads, but only 33.5% for litter, could be assigned to species (however, species-level assignments based solely on 16S rRNA gene data should be interpreted with caution as the inherent conservation of rRNA genes means that fine-scale taxonomic information is not always obtainable from these analyses). Proteobacteria was by far the most abundant phylum for both sample types (Table 2). The most prevalent ASVs in faecal samples were F_ASV1_Escherichia-Shigella coli (detected in 100% of faecal samples), F_ASV2_Streptococcus gallolyticus (58%) and F_ASV4_Tyzzerella unclassified (41%). Despite being the third most abundant ASV of the faecal microbiota, F_ASV3_Lactobacillus gasseri was only present in 18% of samples (n = 51). L_ASV1_Escherichia-Shigella coli, L_ASV2_Rahnella1 unclassified and L_ASV7_Rahnella1 unclassified were the three most prevalent ASVs across all litter samples (present in 100%, 79% and 77% of samples, respectively).
Neither bacterial alpha- nor beta-diversity of faecal samples differed significantly with movement of chicks among nests or aspergillosis infection (Table 3). These observations were maintained under generalised (observed richness) and standard (Inverse Simpson diversity) linear mixed modelling (lmm) while additionally accounting for variation with chick age and identity, as well as location and nest residency (1 | location:nest) (Additional file 1: Table S1). Metadata covariates significantly associated with variation in faecal bacterial diversity (alpha and/or beta) included removal of faecal material from nests (‘faecal experiment’), location, chick age, nest type and nest of residence (Table 3; Additional file 1: Table S1). However, the significance of these associations appeared to be driven by samples collected from chicks under hand rearing conditions. Following the exclusion of samples collected in hand rearing, only chick age and nest of residence (Additional file 1: Fig. S4) covariates retained significant associations with bacterial diversity (Table 3), though age had a small effect size (R2 = 0.06) compared to nest of residence (R2 = 0.36). Both these covariates also exhibited significant heterogeneous group dispersion (Additional file 1: Table S2). Under generalised lmm, chick age was significantly associated with observed species richness while accounting for chick identity and nest residency (Additional file 1: Table S1). However, no significant associations were found between Inverse Simpson diversity and chick age under lmm (Additional file 1: Table S1). We further modelled the overall effect of hand rearing on bacterial alpha-diversity, accounting for sample variation with chick age and identity. Again, hand rearing was significantly associated with observed species richness but not Inverse Simpson diversity (Additional file 1: Table S1). We found no interaction between chick age and hand rearing.
Overall, faecal samples collected from chicks being hand reared were highly dissimilar to all other faecal samples regardless of location (Fig. 1A), nest type (Additional file 1: Fig. S5) or age (Fig. 1C). By contrast, bacterial diversity of litter samples was only significantly associated with temporal collection across all three alpha- and beta-diversity tests (i.e. number of days since a chick was first placed in the nest and subsequent litter samples were collected; Table 3; Additional file 1: Table S1; Fig. 2). Bacterial community separation along PCoA ordination axes was largely due to Escherichia-Shigella coli dominance in many kākāpō chick faecal samples (Fig. 1B) and nest litter samples (Fig. 2B).
Bray–Curtis dissimilarity distances based on 16S rRNA gene sequences for faecal samples visualised via principal coordinate analysis (PCoA) ordination. Each dot of the PCoA represents the microbiota of a single kākāpō chick faecal sample. Samples are shaped by location and coloured by A whether samples were collected while the chick was in the hand rearing facility versus in a nest, B relative abundance of Escherichia-Shigella coli, and C chick age at sample collection. Panel D depicts the most influential ASV vectors plotted using the vegan::envfit function
Bray–Curtis dissimilarity distances of 16S rRNA gene sequences for litter samples visualised via principal coordinate analysis (PCoA) ordination. Each dot of the PCoA represents the microbiota of a single nest litter sample. Samples are shaped by island location and coloured by A island location, B relative abundance of Escherichia-Shigella coli, and C number of days since the nest sampled first housed a chick. Panel D depicts the most influential ASV vectors plotted using the vegan::envfit function
Chick weight gain over the 10-week sampling period was inversely correlated with both observed species richness (p < 0.001, F = 13.97, R2 = 0.06, t = − 3.74) and Inverse Simpson diversity (p < 0.001, F = 13.22, R2 = 0.06, t = − 3.64). However, these observations were not maintained for beta-diversity under PERMANOVA (p = 0.14, F = 1.58, R2 = 0.008).
Influence of human intervention on the bacterial biota
The gut microbiotas of chicks being hand reared in captivity (even for brief periods of time) exhibited significantly lower ASV richness (Fig. 3A), though similar community evenness (Fig. 3B), compared with chicks living in nests, and were dominated by Clostridium, Lactobacillus and Streptococcus bacteria (Figs. 1D, 3C; Additional file 1: Fig. S6). Lactobacillus gasseri and Streptococcus gallolyticus had significantly greater relative abundance (up to 20-fold based on DESeq differential abundance Wald tests) in faecal samples collected from chicks being hand reared (Fig. 3C; Additional file 1: Fig. S6). One faecal sample dominated by L. gasseri but collected from a nest on Whenua Hou represents a 19 d old chick that had been recently moved into the nest from captivity (Fig. 3C). Samples from chicks in the older (43–56, 57–70 and 71–120 d) age categories dominated by L. gasseri represent chicks reclaimed from nests and taken back to the hand rearing facility for aspergillosis screening or sub-optimal weight gain (Fig. 4). Comparatively, faecal samples collected from wild chicks had a greater relative abundance of Escherichia-Shigella, Pseudomonas, Rhodanobacter, Acidocella, Acinetobacter and Tyzzerella species, among other bacteria (Figs. 1D, 3C; Additional file 1: Fig. S6). The bacterial communities of faecal samples collected from chicks being hand reared had significantly greater dispersion (p < 0.001; Additional file 1: Table S2) than the communities of all other faecal samples even when grouped by multiple covariates, including location, faecal experiment and nest type (the only exception where the microbiotas of chicks being hand reared did not have the greatest dispersion was for those grouped by nest of residence). The gut bacterial communities of Pukenui chicks varied slightly more (i.e. had greater dispersion) than chicks on Whenua Hou.
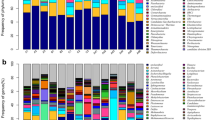
A Observed richness and B Inverse Simpson alpha-diversity indices for kākāpō chick faecal samples grouped by location. Significant Dunn’s test pairwise comparisons with Benjamini–Hochberg adjustment between location groups in the box-plots are denoted by asterisks (*p < 0.05, **p < 0.01, ***p < 0.001). Boxes represent the median (within-box horizontal line), 25th (lower hinge) and 75th (upper hinge) percentiles. Whiskers extend to the smallest and largest values within 1.5 times interquartile range above the 25th and 75th percentiles, respectively. Data beyond the end of the whiskers are outliers and plotted individually. C Species-level taxonomic distribution of bacteria by the relative abundance of 16S rRNA gene sequences within location groups (at time of sampling) for individual kākāpō chick faecal samples (samples for a given chick may include those collected both in captivity and in the nest). Individual samples are ordered within location groups chronologically and alphabetically. Taxa with mean relative 16S rRNA gene sequence abundance < 0.3% are grouped as ‘Other species’
A 16S rRNA gene sequence-based taxonomic distribution of bacteria within age groups and individual kākāpō chick faecal samples at the species level. Bars on the aggregated graph (left) are in the same order as the sample-level profile age groups from left to right. Individual samples are ordered within age groups chronologically and alphabetically. Taxa with mean relative 16S rRNA gene sequence abundance < 0.3% are grouped as ‘Other species’. Underlined samples are those collected from chicks being hand reared at the time of collection. B Observed richness and C Inverse Simpson (plotted on a log10 scale) alpha-diversity indices for kākāpō chick faecal samples grouped by chick age. The y-axes for corresponding bar- and box-plots are identical. Significant Dunn’s test pairwise comparisons with Benjamini–Hochberg adjustment between age groups in the box plot are denoted by asterisks (*p < 0.05, **p < 0.01, ***p < 0.001). Both box- and bar- plots show a decrease in bacterial community diversity with increasing chick age. Box-plot details are as described for Fig. 3
Removing faecal material from nests did not significantly alter the bacterial diversity of either faecal or litter samples (Table 3; Additional file 1: Table S1; Additional file 1: Fig. S7). For this analysis we excluded faecal and litter samples collected from the ‘Unknown’ nest (for which faecal manipulation was not recorded), while faecal samples collected from kākāpō chicks moved among nests under different faecal manipulation regimens were grouped into a ‘Mixed’ category. Samples from chicks under captive, hand rearing conditions had significantly lower bacterial richness than those from chicks located in nests (Additional file 1: Fig. S7A). By contrast, the bacterial richness of ‘Faeces in’, ‘Faeces removed’ and ‘Mixed’ nest groups did not differ significantly from each other (Additional file 1: Tables S1 and S3; Additional file 1: Fig. S7A). Within each faecal experiment group, overall bacterial diversity (measured by Bray–Curtis distances) of faecal and litter samples exhibited a broad range of dissimilarity to other samples within the group (Additional file 1: Fig. S7B). In contrast, gut microbiotas of chicks in captivity were all highly dissimilar to one another, and as a group differed significantly from all other faecal experiment groups (Additional file 1: Table S3; Additional file 1: Fig. S7B).
Variation over time in the bacterial biota of chicks and nests
There was strong evidence for an age-related shift in faecal bacterial diversity of kākāpō chicks (Table 3; Fig. 4), with a general decline in microbiota richness and diversity with increasing chick age (Fig. 4B, C). In particular, the relative abundance of rarer species (species with mean relative abundance < 0.3% across all faecal samples; Fig. 4A) decreased markedly with a concomitant increase in relative abundances of Tyzzerella and Escherichia-Shigella species (though this conceivably reflects data compositionality [38]). However, Escherichia-Shigella is also relatively abundant in younger age groups and often dominates the kākāpō chick gut microbiota regardless of age (Fig. 4A).
Using linear mixed models (Additional file 1: Table S4), we found a positive relationship between Escherichia-Shigella relative abundance and kākāpō chick age (χ2 = 30.09, p < 0.001). The relative abundance of Tyzzerella was also significantly greater in sub-adults than in chicks (χ2 = 13.73, p = 0.03). Both Escherichia-Shigella and Tyzzerella were much more abundant in chicks residing in nests than those being hand reared at time of sample collection (χ2 = 48.63, p < 0.001 and χ2 = 42.90, p < 0.001, respectively). Clostridium sensu stricto 1 exhibited significantly greater relative abundance in hand rearing samples (χ2 = 104.99, p < 0.001) and in young kākāpō chicks < 14 d old compared to all subsequent age groups (χ2 = 22.06, p = 0.001). The former association may be due to most chicks < 14 d old residing in the hand rearing facility. The relative abundance of Lactobacillus was also significantly associated with chick age (χ2 = 46.65, p < 0.001), though Streptococcus was not (p = 0.19). Both genera were significantly more abundant in chicks being hand reared than those in nests (χ2 = 72.64, p < 0.001 and χ2 = 15.65, p < 0.001, respectively). The observation that Clostridium sensu stricto 1, Lactobacillus and Streptococcus had significantly greater abundance in samples collected from the captive facility is further supported by differential abundance analyses between hand rearing and wild samples within each age group (Additional file 1: Fig. S6). Samples collected from chicks in nests were significantly enriched with a more diverse assemblage of bacteria than that found in hand rearing samples (Additional file 1: Fig. S6).
Though we observed no significant effect of faecal removal, chick movement, disease (aspergillosis), nest architecture or geographic location on the microbiota of litter samples, we found evidence for a shift in the litter bacterial community over time (since a kākāpō chick was first placed in the nest; Table 3; Additional file 1: Table S1; Figs. 2, 5). The bacterial diversity of litter samples collected in the month following first chick introduction differed significantly from those collected several weeks later, but not to samples collected between 71 and 90 d (Fig. 2C, 5; Additional file 1: Table S3). Bacterial communities of litter samples collected < 14 d following chick introduction had significantly greater dispersion than those of other subsequent time brackets (p = 0.0006; Additional file 1: Table S2). Samples collected between 15 and 56 d post chick introduction had significantly lower bacterial diversity than those collected in the first 14 d post-introduction (Fig. 5). Overall, litter samples collected several weeks following chick introduction typically hosted a greater relative abundance of Yersiniaceae (bacterial family to which Rahnella1, L_ASV3_Unclassified and L_ASV10_Unclassified belong) and Enterobacteriaceae compared to those collected < 14 d following introduction of the first chick to the nest (Fig. 2C).
A 16S rRNA gene sequence-based taxonomic distribution of bacteria by days since the nest sampled first housed a chick and individual nest litter faecal samples at the species level. Bars on the aggregated graph (left) are in the same order as the sample-level profile groups from left to right. Individual samples are ordered within groups chronologically and alphabetically. Taxa with mean relative 16S rRNA gene sequence abundance < 0.5% are grouped as ‘Other species’. B Observed richness and C Inverse Simpson (plotted on a log10 scale) alpha-diversity indices for litter samples grouped by days since the first chick was introduced to the nest. The y-axes for corresponding bar- and box-plots are identical. Significant Dunn’s test pairwise comparisons with Benjamini–Hochberg adjustment between groups in the box-plots are denoted by asterisks (*p < 0.05, **p < 0.01, ***p < 0.001). Box-plot details are as described for Fig. 3
Discussion
There is mounting evidence that the microbiota of threatened species can be altered by factors including captivity [39,40,41,42], habitat fragmentation [43,44,45], climate change [46, 47] and medical treatment [48, 49], though how such changes may impact animal health remains uncertain [50]. While kākāpō reside on secluded offshore islands with limited human contact, they are, nonetheless, an intensively managed species, particularly during breeding seasons. Kākāpō chicks are monitored closely throughout their first year, with most being hatched in breeding facilities and briefly provided with supplemental feed before being distributed, and later moved, among nests to be cared for by female kākāpō. Nests are also regularly monitored and excess faecal material removed in an attempt to prevent infection in vulnerable chicks. How these combined anthropogenic interventions affect the development of the kākāpō gut microbiota was, until now, largely unknown.
Hand rearing, but not faecal removal from nests, influences the developing kākāpō gut microbiota
Here we show that the gastrointestinal bacterial community of kākāpō chicks is affected by hand rearing practices but not by removal of faecal material from nests. Removing faecal material from nests also did not affect the composition or diversity of bacterial communities associated with litter samples collected concurrently with chick faecal samples. However, faecal samples collected from chicks under captive conditions at the time differed significantly in bacterial community richness and composition compared to those collected in nests.
Samples associated with hand rearing had lower bacterial richness and were dominated by Clostridium sp., Lactobacillus gasseri and Streptococcus gallolyticus, all members of the Firmicutes phylum. We previously showed [6, 7] that several bacterial taxa, identified as Clostridium, Lactobacillus and Pseudomonas, were significantly enriched in the gut microbiota of kākāpō chicks in captivity. However, in neither of the earlier studies did the overall composition of the chick gut microbiota differ significantly from that of adults, nor could it be attributed to supplemental feed. Interestingly, domestic broiler chickens (Gallus gallus domesticus) supplied with Bacillus licheniformis-fermented products experience a similar increase in Firmicutes bacteria, particularly Lactobacillus species, and a reduction of Proteobacteria members [51, 52]. This fermented product is a listed ingredient of the Kaytee exact Hand Feeding formula fed to kākāpō chicks, as are dried Bacillus subtilis and dried Aspergillus oryzae fermentation extracts. L. gasseri is barely represented in the faecal samples of chicks in nests, suggesting that captive conditions, including supplemental feeding, facilitate its dominance in guts of chicks being hand reared, which is quickly lost upon chicks (re-)entering the nest. Furthermore, birds fed starch-heavy diets, including corn, soy and grain, typically host a significantly greater abundance of Lactobacillus than those consuming foliage [12]. The Kaytee feed consumed by young kākāpō contains corn, soy, wheat and oat, thereby facilitating the growth and metabolism of Lactobacillus species. We thus find compelling evidence that the Kaytee supplemental feed does, in fact, significantly alter the gut bacterial composition of kākāpō chicks, particularly the abundance of L. gasseri (Figs. 1C, 4A). As this study was designed to test the effect of removal faecal material from nests and not rearing method per se, unfortunately only three chicks sampled never spent time in the captive facility during the course of this experiment. Future research specifically regarding the effect of rearing method on the developing kākāpō gut microbiota would benefit from a more appropriately designed experiment for this purpose.
Clostridium species were typically identified at greater relative abundance in captive chicks. However, this observation may be confounded by almost all young kākāpō being hatched and reared in captivity before being transferred to wild nests, and may reflect age more than captivity per se. By contrast, Streptococcus gallolyticus was reasonably prevalent and abundant across all chick faecal samples, though especially plentiful in samples from hand reared chicks (Figs. 1B, 4A). The distinct presence of Clostridium and Streptococcus species in young chicks supports the general theory that avian gut Firmicutes facilitate chick growth during early development by providing abundant short-chain fatty acids which are easily absorbed across the intestinal epithelium [15, 53,54,55]. Hird et al. [10] previously identified Streptococcus as a core member of the gut microbiota across 59 avian species.
We found an inverse relationship between gut bacterial richness and chick weight gain over the 10-week sampling period. Whether this reflects a biological relationship between weight gain and microbial diversity remains unknown; conceivably, the significance of this association may reflect a decrease in bacterial richness with age rather than a direct relationship with body condition.
Some of the observed variation in gut microbiota diversity among kākāpō chicks is also likely attributable to the mother’s consumption of adult supplementary feed. Female kākāpō are passively provided Harrison’s High Potency Coarse pellets throughout the breeding season at electronic feed stations, and while most mothers preferentially feed their chicks rimu berries, some consumed HPC pellets intermittently. This behaviour varied substantially among females, and even within a single individual throughout the course of this study, and was thus not explicitly tested.
The kākāpō gut microbiota varies with chick age
Bacterial ASV richness significantly decreased with increasing kākāpō chick age. The bacterial profile of faecal samples collected from sub-adult individuals (at least 200 d post-hatching) resembled those of adult kākāpō, with low bacterial diversity and dominance by Escherichia-Shigella coli and/or Tyzzerella sp. (West et al., in prep.). Although bacterial richness was significantly decreased in sub-adult faecal samples, community evenness was relatively similar. This is consistent with our observation that only a few taxa often dominated the faecal samples of chicks despite hosting a greater variety of species than their subsequent sub-adult samples. Similar to our observations, the gut microbiotas of recently hatched great tits (Parus major), black-legged kittiwakes (Rissa tridactyla), dunlin (Calidris alpina), red phalaropes (Phalaropus fulicarius) and crested ibis (Nipponia nippon) host a diverse assemblage of bacteria with considerable inter-individual variation [13, 54, 56]. Moreover, the nest itself can influence the gut microbiota of juvenile great tits, in which microbiotas of foster chicks diverged from that of their true siblings and were more similar within the same nest [54]. In our study, nest of residence significantly influenced bacterial alpha- and beta-diversity in the kākāpō gut. However, we hesitate to interpret these results further given sample sizes among nests varied substantially and exhibited significant heterogeneous dispersion. Given the apparent significance of nest of residence, source tracking analysis may be worth exploring in the future in order to determine the extent of microbial input from the nest environment to the chicks.
The observation that samples from chicks being hand reared exhibited greater within-group dispersion than those collected from chicks in nests is likely confounded by many hand rearing samples representing chicks < 14 d old, a period during which inter-individual variation is substantially higher than that of older chicks in other avians [13, 15, 54, 56]. That a wide variety of bacteria in chicks are not found in adult conspecifics has also been attributed to rapid colonisation of the gut, followed by a subsequent taxonomic shift towards a stable adult microbiota [54, 56, 57]. These studies mostly observed increases in facultative and obligate anaerobes of Firmicutes bacteria as the intestinal environment becomes less aerobic from the respiration of initial colonisers, including aerobic members of the Enterobacteriaceae family such as Escherichia-Shigella [13, 15, 54, 56]. Unlike other avian hosts, the relative abundance of Escherichia-Shigella increased with kākāpō chick age and remained the dominant bacterial genus in sub-adult samples collected more than > 200 d post-hatching (Fig. 4A). The dominance of Escherichia-Shigella species in the kākāpō chick gut microbiota, and the presence of F_ASV1_ES. coli in 100% of faecal samples, is consistent with previous findings for both adults and chicks [5,6,7] and suggests this pattern represents a typical “wild” bacterial profile for the kākāpō species. It’s possible that, given the long-standing geographic isolation of Aotearoa New Zealand, we find unique ecological systems in the kākāpō gut that are not currently found in other avian species.
Nest microbiota varies with kākāpō chick occupancy
Similarly to faecal samples, the bacterial richness of nest litter samples decreased over time. The most abundant ASV detected in litter samples, Escherichia-Shigella coli, was the same as that identified for faecal samples and was similarly present in 100% of litter samples. We assume that the presence of kākāpō mothers (though not their faeces) and chick faecal material in the nest facilitates the prevalence of this ASV in the nest environment. Litter samples collected after the first month of chick residence generally exhibited an increased relative abundance of Enterobacteriaceae and Yersiniaceae bacteria (Figs. 2, 5), likely due to accumulation of faecal material over time. The presence of chicks in the nest may have also encouraged proliferation of specific bacteria and thus reduced overall diversity of litter samples with each subsequent collection. Interestingly, the microbiota composition of nest litter did not differ significantly between Pukenui and Whenua Hou islands, perhaps reflecting similar overall vegetation types (dominated by endemic podocarp-hardwood forest [58]). The similarity of litter microbiota from Pukenui and Whenua Hou may also reflect female kākāpō often choosing to nest at the base of hollow rātā trees in the 2019 breeding season. Overall, we find substantial evidence that the presence of kākāpō chicks in nests significantly alters the bacterial community of their surrounding environment.
Conservation implications
The practice of removing faecal material from nests was discontinued for the 2022 breeding season since it had no discernible impact on overall chick health (as observed by NZDOC staff) nor on the developing kākāpō gut microbiota or that of the surrounding nest environment. However, the marked impact of supplemental feeding on the chick gut microbiota has important implications for management of this critically endangered species and suggests that further consideration of the hand rearing diet for young kākāpō may be warranted. Experimental studies in threatened species research, where feasible, are an important tool to advance our understanding of animal health and aid conservation management programmes [59].
Concluding remarks
Our primary aim was to explore the influence of current conservation management practices on development of the kākāpō gut microbiota, particularly the regular removal of faecal material from nests during the breeding season. Ultimately, we found no evidence that removing faecal material altered the bacterial composition of faecal or litter samples. However, while previous research regarding the gut microbiota of young kākāpō suggested that neither age nor supplemental feeding significantly influenced bacterial composition [6, 7], the current study provides substantial evidence to revise these earlier findings. Chicks provided supplemental feed in hand rearing facilities hosted significant abundances of Lactobacillus gasseri that were absent from wild chicks. This bacterium was likely linked to the Kaytee exact supplemental feed, which contains Bacillus-based fermented products. Furthermore, by increasing sample size and longitudinal collection of faecal samples, we revealed that bacterial diversity of the chick gastrointestinal tract decreases significantly with age. This trend is often associated with the gut microbiota of avian nestlings. However, we find a unique shift in taxonomy towards Proteobacteria dominance, particularly Escherichia-Shigella bacteria, that epitomises the adult kākāpō gut microbiota. Our findings have already provided pertinent information for current conservation efforts of the critically endangered kākāpō and will hopefully aid future research in this field.
References
Powlesland RG, Merton DV, Cockrem JF. A parrot apart: the natural history of the kakapo (Strigops habroptilus), and the context of its conservation management. Notornis. 2006;53:3–26.
Merton DV, Morris RB, Atkinson IAE. Lek behaviour in a parrot: the kakapo Strigops habroptilus of New Zealand. Ibis. 1984;126:277–83. https://doi.org/10.1111/j.1474-919x.1984.tb00250.x.
White KL, Eason DK, Jamieson IG, Robertson BC. Evidence of inbreeding depression in the critically endangered parrot, the kakapo. Anim Conserv. 2015;18:341–7. https://doi.org/10.1111/acv.12177.
Savage JL, Crane JMS, Hemmings N. Low hatching success in the critically endangered kākāpō (Strigops habroptilus) is driven by early embryo mortality not infertility. Anim Conserv. 2021. https://doi.org/10.1111/acv.12746.
Waite DW, Deines P, Taylor MW. Gut microbiome of the critically endangered New Zealand parrot, the kakapo (Strigops habroptilus). PLoS ONE. 2012;7: e35803. https://doi.org/10.1371/journal.pone.0035803.
Waite DW, Eason DK, Taylor MW. Influence of hand rearing and bird age on the fecal microbiota of the critically endangered kakapo. Appl Environ Microbiol. 2014;80(4650–4658):4650–8. https://doi.org/10.1128/AEM.00975-14.
Perry EK, Digby A, Taylor MW. The low-diversity fecal microbiota of the critically endangered kākāpō is robust to anthropogenic dietary and geographic influences. Front Microbiol. 2017;8:2033. https://doi.org/10.3389/fmicb.2017.02033.
Waite DW, Dsouza M, Sekiguchi Y, Hugenholtz P, Taylor MW. Network-guided genomic and metagenomic analysis of the faecal microbiota of the critically endangered kakapo. Sci Rep. 2018;8:8128. https://doi.org/10.1038/s41598-018-26484-4.
Waite DW, Taylor MW. Characterizing the avian gut microbiota: Membership, driving influences, and potential function. Front Microbiol. 2014;5:223. https://doi.org/10.3389/fmicb.2014.00223.
Hird SM, Sánchez C, Carstens BC, Brumfield RT. Comparative gut microbiota of 59 neotropical bird species. Front Microbiol. 2015;6:1403. https://doi.org/10.3389/fmicb.2015.01403.
Song S, Sanders JG, Delsuc F, Metcalf J, Amato K, Taylor MW, et al. Comparative analyses of vertebrate gut microbiomes reveal convergence between birds and bats. mBio. 2020;11:e02901–19.
Xiao K, Fan Y, Zhang Z, Shen X, Li X, Liang X, et al. Covariation of the fecal microbiome with diet in nonpasserine birds. mSphere. 2021;6:e00308–21. https://doi.org/10.1128/msphere.00308-21.
Ran J, Wan Q-H, Fang S-G. Gut microbiota of endangered crested ibis: Establishment, diversity, and association with reproductive output. PLoS ONE. 2021;16:e0250075. https://doi.org/10.1371/journal.pone.0250075.
Videvall E, Song SJ, Bensch HM, Strandh M, Engelbrecht A, Serfontein N, et al. Early-life gut dysbiosis linked to juvenile mortality in ostriches. Microbiome. 2020;8:147. https://doi.org/10.1186/s40168-020-00925-7.
Zhu Y, Li Y, Yang H, He K, Tang K. Establishment of gut microbiome during early life and its relationship with growth in endangered crested ibis (Nipponia nippon). Front Microbiol. 2021;12:2276. https://doi.org/10.3389/fmicb.2021.723682.
Beernaert LA, Pasmans F, Waeyenberghe LV, Haesebrouck F, Martel A. Aspergillus infections in birds: a review. Avian Pathol. 2010;39:325–31. https://doi.org/10.1080/03079457.2010.506210.
Leishangthem G, Singh N, Brar R, Banga H. Aspergillosis in avian species: a review. J Poult Sci Technol. 2015;3:1–14.
Klindworth A, Pruesse E, Schweer T, Peplies J, Quast C, Horn M, Glöckner FO. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucl Acids Res. 2013. https://doi.org/10.1093/nar/gks808.
R Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2019.
Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13:581–3. https://doi.org/10.1038/nmeth.3869.
Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, et al. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013;41:D590-596.
R Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2022.
McMurdie PJ, Holmes S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE. 2013;8:e61217. https://doi.org/10.1371/journal.pone.0061217.
Beule L, Karlovsky P. Improved normalization of species count data in ecology by scaling with ranked subsampling (SRS): application to microbial communities. PeerJ. 2020;8:e9593. https://doi.org/10.7717/peerj.9593.
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, et al. Vegan: community ecology package. https://CRAN.R-project.org/package=vegan. 2020.
Chen J. GUniFrac: generalized UniFrac distances, distance-based multivariate methods and feature-based univariate methods for microbiome data analysis. R package version 1.6. 2018.
Martinez Arbizu P. pairwiseAdonis: Pairwise multilevel comparison using adonis. 2020.
Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:1–48. https://doi.org/10.18637/jss.v067.i01.
Dinno A. dunn.test: Dunn’s test of multiple comparisons using rank sums. https://CRAN.R-project.org/package=dunn.test. 2017.
Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B (Methodol). 1995;57:289–300. https://doi.org/10.1111/j.2517-6161.1995.tb02031.x.
Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. https://doi.org/10.1186/s13059-014-0550-8.
Wickham H. The split-apply-combine strategy for data analysis. J Stat Softw. 2011;40:1–29.
Wickham H. ggplot2: elegant graphics for data analysis. New York: Springer; 2016.
Kassambara A. ggpubr: ‘ggplot2’ based publication ready plots. https://CRAN.R-project.org/package=ggpubr. 2020.
Wilke CO. cowplot: streamlined plot theme and plot annotations for ‘ggplot2’. https://CRAN.R-project.org/package=cowplot. 2020.
Ram K, Wickham H, Richards C, Baggett A. A Wes Anderson palette generator. https://github.com/karthik/wesanderson. 2018.
Thomson G. NZ bird colour palettes. https://github.com/G-Thomson/Manu. 2020.
Gloor GB, Macklaim JM, Pawlowsky-Glahn V, Egozcue JJ. Microbiome datasets are compositional: and this is not optional. Front Microbiol. 2018;8:2224. https://doi.org/10.3389/fmicb.2017.02224.
McKenzie VJ, Song SJ, Delsuc F, Prest TL, Oliverio AM, Korpita TM, et al. The effects of captivity on the mammalian gut microbiome. Integr Comp Biol. 2017;57:690–704. https://doi.org/10.1093/icb/icx090.
Eliades SJ, Brown JC, Colston TJ, Fisher RN, Niukula JB, Gray K, et al. Gut microbial ecology of the critically endangered Fijian crested iguana (Brachylophus vitiensis): effects of captivity status and host reintroduction on endogenous microbiomes. Ecol Evol. 2021;11:4731–43. https://doi.org/10.1002/ece3.7373.
San Juan PA, Castro I, Dhami MK. Captivity reduces diversity and shifts composition of the brown kiwi microbiome. Anim Microbiome. 2021;3:48. https://doi.org/10.1186/s42523-021-00109-0.
West AG, DeLaunay A, Marsh P, Perry EK, Jolly M, Gartrell BD, et al. Gut microbiota of the threatened takahē: biogeographic patterns and conservation implications. Anim Microbiome. 2022;4:11. https://doi.org/10.1186/s42523-021-00158-5.
Amato KR, Yeoman CJ, Kent A, Righini N, Carbonero F, Estrada A, et al. Habitat degradation impacts black howler monkey (Alouatta pigra) gastrointestinal microbiomes. ISME J. 2013;7:1344–53. https://doi.org/10.1038/ismej.2013.16.
Barelli C, Albanese D, Donati C, Pindo M, Dallago C, Rovero F, et al. Habitat fragmentation is associated to gut microbiota diversity of an endangered primate: implications for conservation. Sci Rep. 2015;5:14862. https://doi.org/10.1038/srep14862.
Ingala MR, Becker DJ, Holm JB, Kristiansen K, Simmons NB. Habitat fragmentation is associated with dietary shifts and microbiota variability in common vampire bats. Ecol Evol. 2019;9:6508–23. https://doi.org/10.1002/ece3.5228.
Bestion E, Jacob S, Zinger L, Di Gesu L, Richard M, White J, Cote J. Climate warming reduces gut microbiota diversity in a vertebrate ectotherm. Nat Ecol Evol. 2017;1:1–3. https://doi.org/10.1038/s41559-017-0161.
Hughes TP, Kerry JT, Álvarez-Noriega M, Álvarez-Romero JG, Anderson KD, Baird AH, et al. Global warming and recurrent mass bleaching of corals. Nature. 2017;543:373–7. https://doi.org/10.1038/nature21707.
Vlcková K, Gomez A, Petrželková KJ, Whittier CA, Todd AF, Yeoman CJ, et al. Effect of antibiotic treatment on the gastrointestinal microbiome of free-ranging Western lowland gorillas (Gorilla g. gorilla). Microbial Ecol. 2016;72:943–54. https://doi.org/10.1007/s00248-016-0745-5.
Moustafa MAM, Chel HM, Thu MJ, Bawm S, Htun LL, Win MM, et al. Anthropogenic interferences lead to gut microbiome dysbiosis in Asian elephants and may alter adaptation processes to surrounding environments. Sci Rep. 2021;11:741. https://doi.org/10.1038/s41598-020-80537-1.
West AG, Waite DW, Deines P, Bourne DG, Digby A, McKenzie VJ, Taylor MW. The microbiome in threatened species conservation. Biol Conserv. 2019;229:85–98. https://doi.org/10.1016/j.biocon.2018.11.016.
Chen Y-C, Yu Y-H. Bacillus licheniformis–fermented products improve growth performance and the fecal microbiota community in broilers. Poult Sci. 2019;99:1432–43. https://doi.org/10.1038/nmeth.3869.
Cheng Y-H, Horng Y-B, Chen W-J, Hua K-F, Dybus A, Yu Y-H. Effect of fermented products produced by Bacillus licheniformis on the growth performance and cecal microbial community of broilers under coccidial challenge. Animals. 2021;11:1245. https://doi.org/10.3390/ani11051245.
den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud D-J, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54:2325–40. https://doi.org/10.1194/jlr.R036012.
Teyssier A, Lens L, Matthysen E, White J. Dynamics of gut microbiota diversity during the early development of an avian host: evidence from a cross-foster experiment. Front Microbiol. 2018;9:1524. https://doi.org/10.3389/fmicb.2018.01524.
Bodawatta KH, Hird SM, Grond K, Poulsen M, Jønsson KA. Avian gut microbiomes taking flight. Trends Microbiol. 2022;30:268–80. https://doi.org/10.1016/j.tim.2021.07.003.
Grond K, Lanctot RB, Jumpponen A, Sandercock BK. Recruitment and establishment of the gut microbiome in arctic shorebirds. FEMS Microbiol Ecol. 2017;93:fix142. https://doi.org/10.1093/femsec/fix142.
Kohl KD, Brun A, Caviedes-Vidal E, Karasov WH. Age-related changes in the gut microbiota of wild House Sparrow nestlings. Ibis. 2018;161:184–91. https://doi.org/10.1111/ibi.12618.
Whitehead J, Case B, Wilson K-J, Molles L. Breeding variation in female kakapo (Strigops habroptilus) on Codfish Island in a year of low food supply. N Z J Ecol. 2012;36:64–74.
Diaz J, Reese AT. Possibilities and limits for using the gut microbiome to improve captive animal health. Anim Microbiome. 2021;3:89. https://doi.org/10.1186/s42523-021-00155-8.
Acknowledgements
We thank all Department of Conservation (Te Papa Atawhai) staff and volunteers involved with collection of samples for this study. AGW thanks Graduate Women New Zealand, the Todd Foundation and Kate Edger Educational Charitable Trust for generous scholarships which helped fund this work. The authors also acknowledge the use of New Zealand eScience Infrastructure (NeSI) high performance computing facilities, consulting support and training services as part of this research. New Zealand's national facilities are provided by NeSI and funded jointly by NeSI's collaborator institutions and through the Ministry of Business, Innovation & Employment's Research Infrastructure programme. URL https://www.nesi.org.nz.
Kākāpō Aspergillosis Research Consortium members
Andrew Digby1, Doug Armstrong2, Darius Armstrong-James3, Mike Bromley4, Elizabeth Buckley5, James Chatterton6, Murray P. Cox2, Robert A. Cramer7, Jodie Crane1, Peter K. Dearden8, Daryl Eason1, Matthew C. Fisher3, Sara Gago4, Brett Gartrell9, Neil J. Gemmell10, Travis R. Glare11, Joseph Guhlin10, Jason Howard12, Donnabella Lacap-Bugler5, Marissa Le Lec10, Xiao Xiao Lin2, Lotus Lofgren13, John Mackay14, Jacques Meis15, Kaesi A. Morelli7, John Perrott5, Megan Petterson16, Miguel Quinones-Mateu17, Johanna Rhodes3, Joanna Roberts18, Jason Stajich13, Michael W. Taylor19, Scott J. Tebbutt20, Amber Truter-Meyer5, Lydia Uddstrom1, Lara Urban10, Norman van Rhijn4, Deidre Vercoe1, Elisa Vesely7, Bevan S. Weir16, Annie G. West19, David J. Winter21, Juliana Yeung22.
1Kākāpō Recovery Team, Department of Conservation, New Zealand; 2School of Natural Sciences, Massey University, New Zealand; 3Faculty of Medicine, Imperial College London, United Kingdom; 4School of Biological Sciences, The University of Manchester, United Kingdom; 5School of Science, Auckland University of Technology, New Zealand; 6Auckland Zoo, New Zealand; 7Geisel School of Medicine, Dartmouth College, New Hampshire, United States of America; 8Department of Biochemistry, The University of Otago, New Zealand; 9Wildbase Research Centre, Massey University, New Zealand; 10Department of Anatomy, The University of Otago, New Zealand; 11Bio-Protection Research Centre, Lincoln University, New Zealand; 12Novogene, North Carolina, United States of America; 13Microbiology and Plant Pathology, University of California, Riverside, United States of America; 14dnature diagnostics and research, New Zealand; 15Canisius Wilhelmina Hospital, Department of Medical Microbiology and Infectious Diseases Nijmegen, the Netherlands; 16Manaaki Whenua Landcare Research, New Zealand; 17Department of Microbiology and Immunology, The University of Otago, New Zealand; 18Flowjoanna, Palmerston North, New Zealand; 19School of Biological Sciences, The University of Auckland, New Zealand; 20Department of Medicine, The University of British Columbia, Canada; 21School of Fundamental Sciences, Massey University, New Zealand; 22AgResearch, New Zealand.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Research was supported by Waipapa Taumata Rau University of Auckland, New Zealand Department of Conservation (Te Papa Atawhai), Graduate Women New Zealand, the Todd Foundation, and the Kate Edger Educational Charitable Trust.
Author information
Authors and Affiliations
Consortia
Contributions
MWT and AD conceived the experiment and designed the study with AGW. AD and KRT coordinated and collected samples. GL contributed to statistical analyses and AGW conducted all laboratory work and sequence data analyses and wrote the manuscript. All authors reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Samples collected for this study were approved by NZDOC and did not require ethics approval from NZDOC Animal Ethics Committee, which upholds NZDOC’s obligations under the New Zealand Animal Welfare Act.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. Additional information, tables and figures.
Additional file 2
. Sample metadata table.
Additional file 3
. RStudio data analysis.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
West, A.G., Digby, A., Lear, G. et al. Influence of management practice on the microbiota of a critically endangered species: a longitudinal study of kākāpō chick faeces and associated nest litter. anim microbiome 4, 55 (2022). https://doi.org/10.1186/s42523-022-00204-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42523-022-00204-w