Abstract
Background
Conventional right ventricular apex (RVa) pacing increases the risk of pacing-induced cardiomyopathy (PICM), especially in elderly patients with a higher ventricular pacing burden. Left bundle branch area pacing (LBBAP) has been suggested as an alternative to conventional RVa pacing. However, there is a lack of evidence that LBBAP may reverse PICM. We report a case of a reversal of PICM after LBBAP.
Case presentation
An 81-year-old woman with a history of complete atrioventricular block and baseline QRS duration of 142 ms received permanent pacemaker implantation with dual pacing. The ventricular lead was placed at the apical direction and paced QRS duration was 146 ms. After 8 months, the patient visited with acute heart failure. The patient’s ventricular pacing burden was > 99%, and echocardiography found severe depression of left ventricular ejection fraction (LVEF, 30%), left ventricular dyssynchrony, and global hypokinesia. Despite 3 months of optimal medical management of heart failure, there was minimal improvement in LVEF (35%) and ventricular dyssynchrony persisted. The patient's presentation was consistent with PICM. LBBAP was performed with a stylet-driven lead and a delivery sheath (Biotronik Selectra 3D, Biotronik, Berlin, Germany). The lead was placed at the area of the left bundle branch trunk and non-selective LBBAP was achieved with a left ventricular activation time of 71 ms, paced QRS duration of 110 ms, and bipolar stimulation to QRS end of 136 ms. After a month, echocardiography found improved LVEF (53%) and N-terminal Pro-B-Type natriuretic peptide was decreased from 1011 to 645 pg/mL. The patient was relieved from dyspnea.
Conclusions
We report a case that PICM was resolved after LBBAP. LBBAP could be a rescue therapy for PICM induced by conventional RVa pacing.
Similar content being viewed by others
Background
Although leadless pacemakers are now available, a pacemaker with right ventricular pacing by a transvenous lead remains the major therapeutic option for patients with symptomatic bradyarrhythmia. In the case of right ventricular pacing, the ventricular conduction becomes discordant from the physiologic condition, and the left bundle branch block is induced. Such non-physiologic conduction may induce pacing-induced cardiomyopathy (PICM) in high-risk patients [1]. Risk factors for PICM include a high burden of ventricular pacing, wider paced QRS duration, and baseline left bundle branch block [2]. In this case report, the patients had a complete atrioventricular block, which necessitates virtually continuous ventricular pacing. In such cases, conduction system pacing such as left bundle branch area pacing (LBBAP) and his-bundle pacing may reduce the risk of PICM by preserving physiologic ventricular conduction.
Recently, LBBAP has gained attention as an alternative to his-bundle pacing [3]. LBBAP fixes ventricular leads inside the interventricular septum near the left bundle or its branches. Depending on the lead placement, LBBAP can recruit septal myocardium and left bundle branches. As a result, LBBAP can achieve more physiologic pacing and result in narrower-paced QRS durations than right ventricular pacing. LBBAP has been suggested as a primary option when a pacing burden is expected to be high [4]. However, there is not enough evidence whether LBBAP can resolve PICM which was induced by traditional right ventricular pacing.
In this article, we report a case of a reversal of PICM after LBBAP.
Case presentation
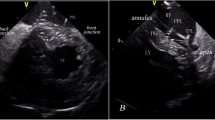
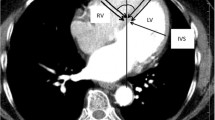
An 81-year-old woman with a history of hypertension, dyslipidemia, and diabetes visited an emergency department due to dyspnea and fatigue. Her blood pressure was 161/44 mmHg, and her heart rate was 44 beats per min. Pulmonary edema and cardiomegaly were observed in a chest X-ray, and an electrocardiogram showed diffuse conduction system disorder including complete atrioventricular block, incomplete right bundle branch block, and left anterior fascicular block with a QRS duration of 142 ms (Fig. 1). Congestive heart failure with a complete atrioventricular block was diagnosed, and cardiac evaluations including echocardicular end-diastolic dimension [LVEDD] of 48 mm), and there was no evidence of ischemic insults or cardiomyopathy. However, diastolic dysfunction and moderate degenerative aortic stenosis were observed; E/E′ = 45, septal E′ = 3.8 cm/s, and left atrial volume index = 42.7 ml/m2, aortic valve peak velocity = 3.5 m/s, mean of aortic valve pressure gradient = 27 mmHg, and aortic valve area = 1.5 cm2. Coronary angiography only revealed focal 50% stenosis at the distal left circumflex artery. A permanent pacemaker with dual pacing was implanted via the left subclavian vein. The ventricular lead was placed in the right ventricular apex (RVa), and the resultant paced QRS duration was slightly increased by 4 ms from the baseline measurement before pacemaker implantation (Fig. 2). The sensing, capture threshold, and impedance of the leads were normal, and the lower rate of ventricular pacing was set to 60 pacing per min.
Results of permanent pacemaker implantation with dual pacing. A The ventricular lead was placed at the RVa. B After pacemaker implantation and heart failure management, pulmonary edema was resolved. C An electrocardiogram during RVa pacing showed paced QRS duration of 146 ms. LAO left anterior oblique view, RAO right anterior oblique view, RVa right ventricular apex
Eight months later, the patient visited the emergency department again due to dyspnea. Device interrogation did not find any abnormality or device malfunction, but the ventricular pacing burden of 100%. Echocardiography found severe depression of left ventricular systolic function (LVEF = 30%), increased left ventricular volume (LVEDD = 55 mm), ventricular dyssynchrony, and global hypokinesia while the progression of underlying aortic stenosis was not revealed by dobutamine stress echocardiography. Chest X-ray suggested pulmonary congestion, but an electrocardiogram showed a slightly increased paced QRS duration since the implantation (from 146 to 152 ms). Optimal medical management of heart failure was initiated with carvedilol, candesartan, spironolactone, furosemide, and dapagliflozin. However, due to low blood pressure, increasing the dose of carvedilol and candesartan was limited.
Despite 3 months of optimal medical management of heart failure, the patient complained of persisting fatigue and dyspnea. The follow-up echocardiography found that severe left ventricular dysfunction (LVEF = 35%, LVEDD = 46 mm) and ventricular dyssynchrony persisted (Additional file 1: Video 1). Because the clinical presentation was consistent with PICM and there were no further correctable factors, LBBAP was planned to improve cardiac function by correcting ventricular dyssynchrony. Before LBBAP, RVa-paced QRS duration and stimulation to the QRS end were 172 and 191 ms, respectively (Fig. 3). LBBAP was performed with a stylet-driven lead with a delivery sheath of 39 cm (Biotronik Selectra 3D, Biotronik, Berlin, Germany). The lead was placed at the area of the left bundle branch trunk, and a septogram was performed to confirm the penetration depth of the lead (Additional file 2: Video 2). Non-selective LBBAP was achieved with a left ventricular activation time of 71 ms, paced QRS duration of 110 ms, and bipolar stimulation to QRS end of 136 ms (Figs. 3, 4). The echocardiography immediately after LBBAP found a resolution of ventricular dyssynchrony during ventricular pacing. There was no complication due to LBBAP.
Measurements during LBBAP. A Baseline and RVa-paced QRS durations were 172 ms and 191 ms, respectively. B After bipolar LBBAP, LVAT was 71 ms, Bipolar stim to QRS duration was 136 ms, and LBB-paced QRS duration was 110 ms. Non-selective LBBAP was achieved. LBB left bundle branch, LBBAP left bundle branch area pacing, LVAT left ventricular activation time, RVa right ventricular apex
Results of LBBAP. A, B Old ventricular lead was abandoned, and a new ventricular lead was used for LBBAP (yellow arrow: the pacing lead for LBBAP). C LBB-paced QRS duration was 110 ms. LAO left anterior oblique view, LBB left bundle branch, LBBAP left bundle branch area pacing, LVAT left ventricular activation time, RAO right anterior oblique view
After 2 weeks, the capture threshold (0.5 V with a pulse width of 0.4 ms) and lead impedance (630 ohms) remained stable. Compared to the baseline workup before LBBAP, the 1-month follow-up echocardiography found improved left ventricular ejection fraction (from 35 to 53%, Additional file 3: Video 3) and N-terminal Pro-B-Type natriuretic peptide decreased from 1011 to 645 pg/mL. The patient was relieved from dyspnea and fatigue.
Discussion
In this article, we reported a case of resolved PICM after LBBAP. Although it is difficult to diagnose PICM, this case is considered that PICM occurred for the following reasons. First, considering the temporal relationship, the patient’s heart failure developed 8 months after RVa pacing. A high burden of RVa pacing (100%) might have accelerated the progression of PICM. Second, no other causes for heart failure were identified other than a high burden of RVa pacing. Although the patient had moderate aortic stenosis, the aortic stenosis did not progress significantly to aggravate heart failure during the follow-up. Therefore, it was difficult to consider underlying aortic stenosis as a cause of heart failure. Third, the patient’s clinical presentation was at high risk for PICM. According to the previous literature, older age [5], high right ventricular pacing burden [2, 6, 7], longer paced QRS duration ≥ 150 ms [7], longer native QRS duration > 115 ms [8], native left bundle branch block [2], and ventricular dyssynchrony during pacing [6] have been known to predictors of PICM. Among the known risk factors of PICM, the patient had an old age (81 years), high burden of ventricular pacing (100%), long native QRS duration (142 ms), and ventricular dyssynchrony during RVa pacing.
Diastolic dysfunction could be another risk factor in developing PICM in our case. According to Jeong et al., RVa pacing under diastolic dysfunction may be associated with an increased risk of PICM among patients with preserved EF [9]. Before the RVa pacing, the patient had diastolic dysfunction (LVEF = 68%, E′ = 3.8 cm/s, E/ E′ = 45, left atrial volume index = 42.7 cc/cm2), and it becomes aggravated after the RVa pacing for 8 months (LVEF = 30%, E′ = 2.4 cm/s, E/ E′ = 58, left atrial volume index = 45.6 cc/cm2). If diastolic dysfunction is present, the left bundle branch block induced by RVa pacing may further aggravate the cardiac workload.
In such a patient with a high risk of PICM, right ventricular pacing may be targeted to non-apical sites such as the right ventricular septum. However, Bansal et al. [6] reported that there was no significant difference in the incidence of PICM between right ventricular pacing sites. For patients expected to have a high pacing burden, his bundle pacing could be an alternative to right ventricular pacing. However, in such a case, the current recommendation remained at Class IIb [4].
Cardiac resynchronization therapy (CRT) could be another alternative strategy to conventional pacing. Khurshid et al. [10] investigated 69 PICM patients and reported that 72.2% of the patients could be recovered from PICM within a median of 7 months. The therapeutic effect of CRT among patients with PICM has also been shown by a recent meta-analysis [11]. There is a report that his bundle pacing might be more effective in improving LVEF than CRT among PICM patients [12]. However, it should be cautiously interpreted because the study has a limited cohort size and there was no guarantee that CRT was fully optimized.
Although the population where LBBAP should be preferred to CRT remains unclear, there have been reports that LBBAP might be superior to biventricular pacing such that LBBAP results in better resynchronization and hemodynamic improvement in nonischemic heart failure patients with left bundle branch block [13, 14]. Therefore, LBBAP might be preferred to CRT if a patient has nonischemic heart failure with the left bundle branch block. Another situation that LBBAP might be preferred to CRT is PICM as in our case. As we described before, a previous study observed that his bundle pacing was associated with a better improvement in LVEF than CRT in patients with PICM [12]. Although LBBAP is different from his bundle pacing, both target conduction system pacing, which is believed to be more physiologic than biventricular pacing. However, to define a population where LBBAP should be preferred to CRT, we need more studies at present.
In our case, LBBAP may also be preferred to conventional right ventricular pacing. Because LBBAP uses a cardiac conduction system and is believed to be physiologic, LBBAP may prevent PICM in patients with a higher risk of PICM. However, there has been no head-to-head comparison between LBBAP and right ventricular pacing yet in patients with bradyarrhythmia requiring a high burden of ventricular pacing. Also, the predictors of PICM improvement with LBBAP have not been well known. Therefore, LBBAP as a first-line in patients with a high risk of PICM needs more evidence. If several studies (NCT05129098, NCT05015660, and NCT04624763) are reported in the future, it would be helpful in determining LBBAP or right ventricular pacing as a first-line in patients with a high risk of PICM.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- LBBAP:
-
Left bundle branch area pacing
- PICM:
-
Pacing-induced cardiomyopathy
- CRT:
-
Cardiac resynchronization therapy
- RVa:
-
Right ventricular apex
References
Merchant FM, Mittal S. Pacing-induced cardiomyopathy. Card Electrophysiol Clin. 2018;10:437–45.
Cho SW, Gwag HB, Hwang JK, et al. Clinical features, predictors, and long-term prognosis of pacing-induced cardiomyopathy. Eur J Heart Fail. 2019;21:643–51.
Vijayaraman P, Ponnusamy S, Cano O, et al. Left bundle branch area pacing for cardiac resynchronization therapy: results from the International LBBAP Collaborative Study Group. JACC Clin Electrophysiol. 2021;7:135–47.
Glikson M, Nielsen JC, Kronborg MB, et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J. 2021;42:3427–520.
Li DL, Yoneda ZT, Issa TZ, Shoemaker MB, Montgomery JA. Prevalence and predictors of pacing-induced cardiomyopathy in young adult patients (<60 years) with pacemakers. J Cardiovasc Electrophysiol. 2021;32:1961–8.
Bansal R, Parakh N, Gupta A, et al. Incidence and predictors of pacemaker-induced cardiomyopathy with comparison between apical and non-apical right ventricular pacing sites. J Interv Card Electrophysiol. 2019;56:63–70.
Khurshid S, Liang JJ, Owens A, et al. Longer paced QRS duration is associated with increased prevalence of right ventricular pacing-induced cardiomyopathy. J Cardiovasc Electrophysiol. 2016;27:1174–9.
Khurshid S, Epstein AE, Verdino RJ, et al. Incidence and predictors of right ventricular pacing-induced cardiomyopathy. Heart Rhythm. 2014;11:1619–25.
Jeong HK, Kim HW, Kim SS, et al. Impact of diastolic dysfunction in patients with preserved ejection fraction undergoing permanent cardiac pacemaker placement. Int J Arrhythm. 2022. https://doi.org/10.1186/s42444-022-00078-8.
Khurshid S, Obeng-Gyimah E, Supple GE, et al. Reversal of pacing-induced cardiomyopathy following cardiac resynchronization therapy. JACC Clin Electrophysiol. 2018;4:168–77.
Lu W, Lin J, Dai Y, Chen K, Zhang S. The therapeutic effects of upgrade to cardiac resynchronization therapy in pacing-induced cardiomyopathy or chronic right ventricular pacing patients: a meta-analysis. Heart Fail Rev. 2022;27:507–16.
Gardas R, Golba KS, Soral T, et al. The effects of his bundle pacing compared to classic resynchronization therapy in patients with pacing-induced cardiomyopathy. J Clin Med. 2022;11:5723.
Liang Y, Wang J, Gong X, et al. Left bundle branch pacing versus biventricular pacing for acute cardiac resynchronization in patients with heart failure. Circ Arrhythm Electrophysiol. 2022;15:e011181.
Wang Y, Zhu H, Hou X, et al. Randomized trial of left bundle branch vs biventricular pacing for cardiac resynchronization therapy. J Am Coll Cardiol. 2022;80:1205–16.
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
SK contributed to conceptualization, data curation, formal analysis, investigation, methodology, writing of original draft, review, and editing. SRL contributed to conceptualization, data curation, formal analysis, investigation, methodology, project administration, supervision, validation, review, and editing. EKC contributed to conceptualization, investigation, methodology, supervision, and validation. SO contributed to conceptualization, investigation, methodology, supervision, and validation. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study conforms to the ethical guidelines of the Declaration of Helsinki revised in 2013 and was approved by the Institutional Review Board of Seoul National University Hospital (No. H-2206-204-1335). Informed consent was waived because the study was retrospective and only used anonymized data.
Consent for publication
Not applicable.
Competing interests
Kwon S, Lee SR, Oh S: None to disclose. Choi EK: Research grants or speaking fees from Abbott, Bayer, BMS/Pfizer, Biosense Webster, Chong Kun Dang, Daewoong Pharmaceutical Co., Daiichi-Sankyo, DeepQure, Dreamtech Co., Ltd., Jeil Pharmaceutical Co. Ltd, Medtronic, Samjinpharm, Seers Technology, and Skylabs. Stock options from Seers Technology, and Skylabs.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Video 1. The apical 4-chamber view after right ventricular pacing. The left ventricular ejection fraction was 35% and there was ventricular dyssynchrony during pacing. The patient’s presentation was consistent with pacing-induced cardiomyopathy.
Additional file 2: Video 2. The septogram for LBBAP confirmed the lead depth and its position inside the interventricular septum.
Additional file 3: Video 3. The apical 4-chamber view a month after LBBAP. Left ventricular ejection fraction was improved to 53% and pacing-induced ventricular dyssynchrony was resolved.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kwon, S., Lee, SR., Choi, EK. et al. Reversal of pacing-induced cardiomyopathy after left bundle branch area pacing: a case report. Int J Arrhythm 24, 5 (2023). https://doi.org/10.1186/s42444-023-00087-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42444-023-00087-1