Abstract
Introduction
In chronic arthropathies, there are several mechanisms of joint destruction. In recent years, studies have reported the implication of receptor activator of nuclear factor kappa-B ligand (RANKL) and osteoprotegerin (OPG) in the process of activation and differentiation of osteoclasts, a key cell in the development of bone erosion. The RANKL/OPG ratio is increased in the serum of patients with malignant diseases and lytic bone disease, as well as rheumatoid arthritis (RA). The objective of this study was to measure and compare the concentrations of OPG and RANKL in the synovial fluid (SF) of patients with rheumatoid arthritis, spondyloarthritis (SpA) and osteoarthritis (OA).
Methods
This was an observational and cross-sectional study with 83 patients, 33 with RA, 32 with SpA and 18 with OA, followed up regularly in the outpatient clinics of the Rheumatology Department of the Clinics Hospital of the Ribeirão Preto Medical School-USP. All patients were assessed for indications for arthrocentesis by the attending physicians at the time of SF collection and were evaluated for demographic variables and medication use. Disease activity was assessed in individuals with RA and SpA. The quantification of SF OPG and RANKL levels was performed by ELISA, and the correlations of the results with clinical, laboratory and radiological parameters were assessed.
Results
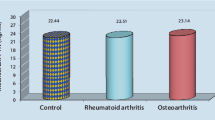
We found no statistically significant difference in the RANKL and OPG levels among the groups. Patients with RA showed a positive correlation between the SF cell count and RANKL level (r = 0.59; p < 0.05) and the RANKL/OPG ratio (r = 0.55; p < 0.05). Patients with OA showed a strong correlation between C-reactive protein (CRP) and the RANKL/OPG ratio (r = 0.82; p < 0.05). There was no correlation between the OPG and RANKL levels and markers of inflammatory activity or the disease activity index in patients with RA or SpA.
Conclusion
Within this patient cohort, the RANKL/OPG ratio was correlated with the SF cell count in patients with RA and with serum CRP in patients with OA, which may suggest a relationship with active inflammation and more destructive joint disease.
Similar content being viewed by others
Introduction
Chronic arthropathies are a heterogeneous set of diseases characterized by a broad range of synovial inflammation. Among the main chronic arthropathies are immune-mediated arthropathies, such as rheumatoid arthritis (RA) and the spondyloarthritis (SpA) disease spectrum, and primary osteoarthritis (OA), the best example of a primarily degenerative arthropathy [1]. All of these can lead to joint dysfunction, with different degrees of morbidity [2]. Although they have different clinical manifestations, these diseases share events in the pathogenesis of joint injury, such as cartilage destruction, subchondral cysts formation and bone erosion [3,4,5]. In RA and SpA, bone erosion is directly related to the progression of joint dysfunction [6, 7]. OA is associated with loss of articular cartilage and joint tissue destruction along with bone remodeling (mostly sclerosis and osteophytes) resulting in altered joint function and pain. Subchondral cysts are common in long-term disease, but joint erosions are not a characteristic and seen in only a small subset of patients [8].
Among the important cellular events related to osteochondral erosion is osteoclast activation. Synovial and cell surface markers of osteoclasts, both of which have a pro-erosive effect, are found in the synovium and bone erosion sites in chronic arthropathies [9, 10]. Receptor activator of nuclear factor kappa-B activator receptor ligand (RANKL) and osteoprotegerin (OPG) are essential in the recruitment and action of these cells [11]. RANKL is a surface molecule, a potent stimulator of bone resorption involved in the differentiation and activation of osteoclasts. OPG is produced and released by activated osteoblasts and inhibits osteoclast maturation and activation. The relationship between RANKL and OPG levels is an indirect measure of osteoclastogenesis at the site evaluated [11]. The RANKL/OPG ratio is high in the serum of patients with malignant diseases and tumor bone lysis [12, 13]. A high RANKL/OPG ratio has also been observed in the synovial fluid (SF) and serum of patients with RA and SpA, but the clinical relevance of this observation is still unclear [14,15,16]. It is also known that the expression of RANKL in the synovial tissue of patients with RA is higher in active disease than in less-active disease and in SpA and OA [17, 18]. In patients with OA, the serum RANKL/OPG ratio may be related to more severe forms of the disease [19].
There are few comparative data regarding the levels of these proteins in the SF of individuals with chronic arthropathies. We compared the synovial concentrations of RANKL and OPG, in addition to the RANKL/OPG ratio, in patients with RA, SpA and OA. In addition, we evaluated whether the synovial levels of these proteins are correlated with markers of inflammatory activity, disease activity indices (in RA and SpA) and the presence of subchondral cysts (OA) and bone erosions.
Methods
Study design and patients
This was a cross-sectional study involving a convenience sampling of patients with rheumatic disease undergoing arthrocentesis (diagnostic or therapeutic), from whom synovial fluid was obtained from May 2020 to April 2022, at the Rheumatology Outpatient Clinic of the Hospital of the Clinics of the School of Medicine of Ribeirão Preto of the University of São Paulo (HCRP-USP).
The inclusion criteria for the sample were a medical indication for arthrocentesis and joint aspiration; age over 18 years; and understanding and signing of the informed consent form. Patients with RA were classified according to the American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) criteria [20]. Patients with SpA met the criteria of the Assessment of SpondyloArthritis international Society (ASAS) group [21], and those with OA met the criteria of the OsteoArthritis Research Society International (OARSI) [22].
The exclusion criteria were as follows: other chronic inflammatory diseases that could interfere with the inflammatory response, such as other rheumatic diseases, acute or chronic infectious diseases (including septic arthritis), and neoplasia under treatment or for which treatment had been completed less than 5 years prior.
One hundred ninety-one patients were evaluated for eligibility. For each of them, the SF was obtained from one knee. Nineteen patients had a presumptive diagnosis of chronic inflammatory arthropathy without meeting any of the classification criteria, which led to their noninclusion. Eighty-nine patients were excluded for the following reasons: septic arthritis (43 cases); microcrystalline arthritis (27 cases), other rheumatic diseases (12 cases), trauma-related arthritis (6 cases) and neoplasia (1 case). Thus, 83 patients were analyzed: 33 with RA, 32 with SpA and 18 with OA (Fig. 1). The SpA group consisted of patients with psoriatic arthritis (PsA, n = 13), ankylosing spondylitis (AS, n = 7), enteropathic arthritis (EpA, n = 7) and reactive arthritis (RA, n = 5). All patients signed informed consent forms, and the study was approved by the Research Ethics Committee of the HCRP-USP (opinion number: 59784122.8.0000.5440).
Clinical and laboratory evaluations
Patient information including sex, age, time since diagnosis, associated diseases and medication use was collected. For patients with RA, disease activity was evaluated as the Disease Activity Score 28 (DAS28) [23]. All patients had their levels of rheumatoid factor (RF) and anti-cyclic citrullinated peptide (anti-CCP) antibody previously measured. For the SpA group, DAS28 was used to evaluate disease activity whether the disease was restricted to peripheral joints. If the patients had axial involvement, ASDAS-CRP was used to assess disease activity [24]. In order to evaluate if SF levels OPG and RANKL were influenced by disease activity, RA and SpA groups were further divided taking into account values of DAS28 ≥ 3.2 or ASDAS-CRP ≥ 2.1 (for axial SpA only). Regarding the OA group, activity evaluation was not performed because there is no specific score. Each patient was classified according to the Kellgren-Lawrence system to assess the stage of OA at the punctured knee [25].
The erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) level were determined for all patients on the day of arthrocentesis. Radiographs of the hands and/or knees were analyzed by a single physician member of the team (SCLA) to establish the presence of erosion or subchondral cysts. The physician was blinded to the information of the recruited patients.
Experimental procedures
The SF was collected in an EDTA tube and analyzed within two hours after collection to determine the total and differential leukocyte counts. A 1 mL aliquot of SF was stored at − 80 °C until the day of the RANKL and OPG measurements. Levels of RANKL and OPG in the samples were determined by ELISA according to the manufacturer’s protocols (Duo Set Human Osteoprotegerin, Duo Set Human TRANCE/RANKL, R&D Systems, Minnesota, USA).
Statistical analysis
Absolute numbers and percentages were compared with Fisher’s exact test. Continuous variables are presented as the mean (or median) and standard deviation (or interquartile range). ANOVA (or the Kruskal‒Wallis test) was used to compare the serum levels of RANKL and OPG and the RANKL/OPG ratio. The Pearson or Spearman correlation coefficients (if the sample distribution was different from a normal distribution) were used to evaluate the degree of correlation between RANKL and OPG values and clinical and laboratory parameters. For all tests, p < 0.05 was considered statistically significant.
Results
Of the 83 patients recruited, 54 (65%) were women, and the median age was 56 (18–82) years. The clinical and laboratory characteristics of the patients are shown in Table 1.
Of the 65 patients with immune-mediated arthropathy, 20 (31%) had been diagnosed less than 2 years prior, 6 were treatment-naive, 16 were on monotherapy with disease-modifying antirheumatic drugs (DMARDs) and 33 (51%) were treated with biological DMARDs (bDMARDs), both with and without concomitant synthetic DMARD use. Among the DMARDs, methotrexate was the most used, followed by leflunomide and sulfasalazine. Among the bDMARDs, anti-tumor necrosis factor (TNF) drugs were the most frequent. No patient was using prednisone at a dosage greater than 5 mg/day.
The RA group had a median disease duration of 4 (2–11) years. Active smoking, positive RF, positive anti-CCP antibody and erosion on hand or knee radiography were observed in 40%, 70%, 78% and 61% of patients, respectively. For 73% of the patients, we found an increase in the ESR and/or CRP level. The median ESR was 23 (7–37) mm/h, and the median CRP level was 0.95 (0.4–3.1) mg/dL. Fifteen (45%) of RA patients had a DAS28 ≥ 3.2.
In the SpA group, the mean disease duration was 4 (0.87–7.7) years. The ESR and/or CRP level were increased in 75% of these patients, and 44% had bone erosions on hand or knee radiography. DAS ≥ 3.2 or ASDAS-CRP ≥ 2.1 was found in 14 (44%) of the SpA patients. In the OA group, the mean disease duration was 5.6 (0.5–16) years, and 78% had been diagnosed more than two years prior and were in an advanced stage of joint involvement (Kellgren-Lawrence classification III or IV). Subchondral cysts or bone erosions in hand or knee x-rays were observed in 8 (44%) patients.
The SF leukocyte count was higher in patients with immune-mediated chronic arthropathy than in OA patients. There was no difference between the RA and SpA groups (Table 1).
The levels of RANKL and OPG and the RANKL/OPG ratio in the SF of the 3 groups are shown in Table 2. There was no significant difference between the groups. The correlations with the laboratory and radiographic parameters are shown in Table 3. In patients with RA, there was a positive correlation between the SF cell count and RANKL level (r = 0.59, p < 0.05) and the RANKL/OPG ratio (r = 0.55, p < 0.05). There was no correlation between RANKL and OPG levels and CRP, erosion or DAS28. In patients with OA, there was a strong positive correlation between CRP level and the RANKL/OPG ratio (r = 0.82, p < 0.05), but there was no correlation with the other parameters evaluated. In the SpA group, no statistically significant correlations were observed.
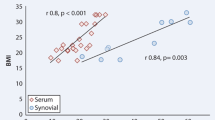
When analyzing the samples together, considering all patients in the study, we observed that the CRP level was weakly and negatively correlated with the OPG level (r = − 0.26, p = 0.02) and moderately positively correlated with the RANKL level (r = 0.40, p = 0.008) (Fig. 2).
Discussion
This study aimed to quantify the RANKL and OPG levels in the SF of patients with RA, SpA and OA and evaluate their correlations with clinical and laboratory parameters of each disease. There was no statistical difference in the synovial levels of RANKL or OPG or the RANKL/OPG ratio between the groups.
In patients with RA, the synovial RANKL level and the RANKL/OPG ratio were significantly correlated with the cell count in the SF. There was no statistical significance with other parameters of disease activity or severity, such as markers of inflammatory activity, DAS28 and erosions on hand radiography. Unlike what was observed in our study, Ellabban et al. detected higher serum and synovial RANKL levels in patients with RA than in patients with OA and a significant correlation between synovial levels and parameters of disease severity and activity [26]. Skoumal et al. found that the OPG and RANKL levels in the SF were negatively correlated in RA, showing significantly low OPG and high RANKL levels [16]. In addition, they observed that the OPG/RANKL ratio was significantly related to radiographic changes. Haynes et al., in a semiquantitative and quantitative analysis via immunostaining, showed that the expression level of OPG in synovial tissue was markedly decreased in patients with active RA compared with patients with inactive RA or OA or with healthy individuals [18]. Similarly, Kong et al. demonstrated that the SF OPG levels were lower in RA than in OA [27]. The lower synovial OPG may reflect a less protective effect on the bone, leading to earlier and more pronounced bone destruction, as occurs in RA. The amount of RANKL in synovial tissue is higher in individuals with active RA than in individuals with controlled RA and individuals with SpA and OA [18]. The RANKL/OPG ratio is significantly higher in rheumatoid synovial tissue than in the synovial tissues of other chronic inflammatory arthropathies, especially reactive arthritis [28].
We found a positive correlation between the SF cell count and the RANKL level and RANKL/OPG ratio in RA. In fact, the joint infiltrate of inflammatory cells is greater in active RA than in less active disease or other inflammatory arthropathies [10]. The presence of cells in the SF, such as neutrophils and B and T cells, induces increased RANKL expression, which stimulates activation, proliferation and interaction with dendritic cells, contributing to bone remodeling [29, 30].
In the joint analysis of the 3 groups, we found that the CRP level was negatively correlated with OPG and positively correlated with RANKL, suggesting the important role of this marker in the pathophysiology of synovial inflammation. Denosumab, a monoclonal antibody that specifically binds to and subsequently inactivates RANKL, was evaluated in patients with RA [31,32,33]. Hu et al., in a meta-analysis with a total of 1758 patients evaluating the presence of bone erosion and joint space reduction, showed that denosumab significantly increased the percentage of bone mineral density (BMD) and reduced the radiographic damage score [34].
Our results showed a strong positive correlation between the RANKL/OPG ratio and the CRP level in patients with osteoarthritis. High levels of synovial RANKL were found in individuals with OA; within this group, the levels were higher in patients with erosive nodal OA than in patients with nonerosive OA and were correlated with the presence of Heberden’s and Bouchard’s nodes, the Kellgren-Lawrence score and erosive changes found in both hands [26]. Pilichou et al. also demonstrated a correlation between the RANKL/OPG ratio and disease severity in primary OA, but only in serum, not in the SF [19].
Regarding systemic inflammation, we found elevated and similar values of serum CRP and ESR among the groups. OA group had higher frequencies of obesity and diabetes mellitus. Obesity in women is a well-known condition related to elevation of serum CRP due to a low-grade systemic inflammation [35], resulting in values above 1.0 mg/dL. The low-grade inflammation due to diabetes mellitus is associated with serum CRP elevations [36] and may have contributed to our results. Regarding ESR, values of OA group similar to those of RA and SpA can be associated to the totality of women in this group and the mean age considerably higher. Female gender and age are two conditions known to elevate ESR [37]. Besides, we should mention that we enrolled patients with Kellgren-Lawrence score III or IV and this long-term augments the chance of relevant synovial inflammation [8].
In patients with SpA, we did not find correlations between the RANKL and OPG levels and the clinical and radiological parameters evaluated. The expression of OPG and RANKL was similar to that of patients with RA, despite the marked differences between the pattern of bone involvement, with extensive new bone formation in the former and marked focal bone erosions in the latter [38]. This similarity was also observed by Vandooren et al. when analyzing the synovial tissue of patients with PsA, other SpAs and RA [14]. Our data are in agreement with this study and may suggest that the differences in the pattern and extent of bone erosions between these diseases are not related to the synovial expression of these proteins. These results should be analyzed with caution due to the heterogeneity of the samples in both studies.
Data on AS are conflicting. In 2019, Chen et al., in a meta-analysis with 1592 patients and 1064 healthy controls, concluded that the serum levels of OPG and RANKL and the RANKL/OPG ratio were significantly higher in AS patients than in healthy controls [39]. A subgroup of patients with high inflammatory activity marker levels, ESR and CRP levels, shorter disease duration and high disease activity indexes had higher serum levels of OPG and RANKL. The authors concluded that OPG, RANKL and the RANKL/OPG ratio can be used as potential biomarkers for AS [40].
In our sample, 59 of the 65 patients with immune-mediated rheumatic disease were regularly using DMARDs, which may have interfered with our results. In a study with 50 patients with RA, Vassev et al. evaluated the effect of anti-TNF therapy in combination with methotrexate on bone remodeling and osteoclastogenesis. After 15 months of treatment, they observed a reduction in resorption markers and RANKL/OPG ratio and an increase in bone formation markers, regardless of the type of anti-TNF drug used [40]. The high frequency of treatments with bDMARDs (51%) in our cohort may explain the similarity on SF levels of OPG and RANKL we found.
Our study has some limitations, such as the nonconcomitant evaluation of serum RANKL and OPG levels. The heterogeneity of the sample with respect to disease duration and treatments is another limitation, since less than one third of the patients with immune-mediated arthropathy had early disease and less than 10% were treatment naive. This could explain some conflicting results such as the RANKL negative co-relation with DAS-28/ASDAS-CRP in SpA. Finally, the lack of statistical difference between patients with active versus non-active RA and SpA may be due to a type 2 error due to the small sample size of the subgroups.
Conclusion
In conclusion, the synovial levels of RANKL and OPG as well as the RANKL/OPG ratio were not suitable for differentiating the three groups of individuals with chronic arthropathy studied. The synovial RANKL/OPG ratio was correlated with the SF cell count in patients with RA and with serum CRP in patients with OA.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
References
Tateiwa D, Yoshikawa H, Kaito T. Cartilage and bone destruction in arthritis: pathogenesis and treatment strategy: a literature review. Cells. 2019;8:818.
Walsh NC, Crotti TN, Goldring SR, Gravallese EM. Rheumatic diseases: the effects of inflammation on bone. Immunol Rev. 2005;208:228–51.
Scherer HU, Häupl T, Burmester GR. The etiology of rheumatoid arthritis. J Autoimmun. 2020;110:102400.
Ritchlin C, Adamopoulos IE. Axial spondyloarthritis: new advances in diagnosis and management. BMJ. 2021;372:m4447.
van den Bosch MHJ. Osteoarthritis year in review 2020: biology. Osteoarthritis Cartilage. 2021;29:143–50.
Schett G, Hayer S, Zwerina J, Redlich K, Smolen JS. Mechanisms of Disease: the link between RANKL and arthritic bone disease. Nat Clin Pract Rheumatol. 2005;1:47–54.
Hu Y, Wang Y, Chen T, Hao Z, Cai L, Li J. Exosome: function and application in Inflammatory Bone Diseases. Oxid Med Cell Longev. 2021;2021:6324912.
Martel-Pelletier J, Barr AJ, Cicuttini FM, Conaghan PG, Cooper C, Goldring MB, et al. Osteoarthr Nat Rev Dis Primers. 2016;2:16072.
Levescot A, Chang MH, Schnell J, Nelson-Maney N, Yan J, Martínez-Bonet M, et al. IL-1β-driven osteoclastogenic Tregs accelerate bone erosion in arthritis. J Clin Invest. 2021;131:e141008.
Maeda K, Yoshida K, Nishizawa T, Otani K, Yamashita Y, Okabe H, et al. Inflammation and bone metabolism in rheumatoid arthritis: molecular Mechanisms of Joint Destruction and pharmacological treatments. Int J Mol Sci. 2022;23:2871.
Sun Y, Li J, Xie X, Gu F, Sui Z, Zhang K, et al. Recent advances in osteoclast biological behavior. Front Cell Dev Biol. 2021;9:788680.
Mercatali L, Ricci M, Scarpi E, Serra P, Fabbri F, Ricci R, et al. RANK/RANK-L/OPG in patients with bone metastases treated with anticancer agents and zoledronic acid: a prospective study. Int J Mol Sci. 2013;14:10683–93.
Infante M, Fabi A, Cognetti F, Gorini S, Caprio M, Fabbri A. RANKL/RANK/OPG system beyond bone remodeling: involvement in breast cancer and clinical perspectives. J Exp Clin Cancer Res. 2019;38:12.
Vandooren B, Cantaert T, Noordenbos T, Tak PP, Baeten D. The abundant synovial expression of the RANK/RANKL/Osteoprotegerin system in peripheral spondylarthritis is partially disconnected from inflammation. Arthritis Rheum. 2008;58:718–29.
Geusens PP, Landewé RB, Garnero P, Chen D, Dunstan CR, Lems WF, et al. The ratio of circulating osteoprotegerin to RANKL in early rheumatoid arthritis predicts later joint destruction. Arthritis Rheum. 2006;54:1772–7.
Skoumal M, Kolarz G, Haberhauer G, Woloszczuk W, Hawa G, Klingler A. Osteoprotegerin and the receptor activator of NF-kappa B ligand in the serum and synovial fluid. A comparison of patients with longstanding rheumatoid arthritis and osteoarthritis. Rheumatol Int. 2005;26:63–9.
Haynes DR, Crotti TN, Loric M, Bain GI, Atkins GJ, Findlay DM. Osteoprotegerin and receptor activator of nuclear factor kappaB ligand (RANKL) regulate osteoclast formation by cells in the human rheumatoid arthritic joint. Rheumatology. 2001;40:623–30.
Haynes DR, Barg E, Crotti TN, Holding C, Weedon H, Atkins GJ, et al. Osteoprotegerin expression in synovial tissue from patients with rheumatoid arthritis, spondyloarthropathies and osteoarthritis and normal controls. Rheumatology. 2003;42:123–34.
Pilichou A, Papassotiriou I, Michalakakou K, Fessatou S, Fandridis E, Papachristou G, et al. High levels of synovial fluid osteoprotegerin (OPG) and increased serum ratio of receptor activator of nuclear factor-kappa B ligand (RANKL) to OPG correlate with disease severity in patients with primary knee osteoarthritis. Clin Biochem. 2008;41:746–9.
Kay J, Upchurch KS. ACR/EULAR 2010 rheumatoid arthritis classification criteria. Rheumatology (Oxford). 2012;51(Suppl 6):vi5–9.
Akkoc N, Khan MA. ASAS classification criteria for axial spondyloarthritis: time to modify. Clin Rheumatol. 2016;35:1415–23.
Bannuru RR, Osani MC, Vaysbrot EE, Arden NK, Bennell K, Bierma-Zeinstra SMA, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 2019;27:1578–89.
England BR, Tiong BK, Bergman MJ, Curtis JR, Kazi S, Mikuls TR, et al. 2019 update of the American college of rheumatology recommended rheumatoid arthritis disease activity measures. Arthritis Care Res. 2019;71:1540–55.
Machado P, Landewé R, Lie E, Kvien TK, Braun J, Baker D, et al. Assessment of SpondyloArthritis international society. Ankylosing Spondylitis Disease Activity score (ASDAS): defining cut-off values for disease activity states and improvement scores. Ann Rheum Dis. 2011;70:47–53.
Schiphof D, de Klerk BM, Kerkhof HJ, Hofman A, Koes BW, Boers M, et al. Impact of different descriptions of the Kellgren and Lawrence classification criteria on the diagnosis of knee osteoarthritis. Ann Rheum Dis. 2011;70:1422–7.
Ellabban AS, Kamel SR, Ahmed SS, Osman AM. Receptor activator of nuclear factor kappa B ligand serum and synovial fluid level. A comparative study between rheumatoid arthritis and osteoarthritis. Rheumatol Int. 2012;32:1589–96.
Kong YY, Feige U, Sarosi I, Bolon B, Tafuri A, Morony S, et al. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999;402:304–9.
Fonseca JE, Cortez-Dias N, Francisco A, Sobral M, Canhão H, Resende C, et al. Inflammatory cell infiltrate and RANKL/OPG expression in rheumatoid synovium: comparison with other inflammatory arthropathies and correlation with outcome. Clin Exp Rheumatol. 2005;23:185–92.
Poubelle PE, Chakravarti A, Fernandes MJ, Doiron K, Marceau AA. Differential expression of RANK, RANK-L, and osteoprotegerin by synovial fluid neutrophils from patients with rheumatoid arthritis and by healthy human blood neutrophils. Arthritis Res Ther. 2007;9:R25.
Anderson DM, Maraskovsky E, Billingsley WL, Dougall WC, Tometsko ME, Roux ER, et al. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature. 1997;390:175–9.
Mori Y, Izumiyama T, Kurishima H, Kamimura M, Baba K, Mori N, et al. Effect of denosumab switched from bisphosphonates in preventing joint destruction in postmenopausal rheumatoid arthritis patients with anti-cyclic citrullinated peptide antibodies. J Orthop Surg Res. 2021;16:107.
Kinoshita H, Miyakoshi N, Kashiwagura T, Kasukawa Y, Sugimura Y, Shimada Y. Comparison of the efficacy of denosumab and bisphosphonates for treating secondary osteoporosis in patients with rheumatoid arthritis. Mod Rheumatol. 2017;27:582–6.
Takeuchi T, Tanaka Y, Soen S, Yamanaka H, Yoneda T, Tanaka S, et al. Effects of the anti-RANKL antibody denosumab on joint structural damage in patients with rheumatoid arthritis treated with conventional synthetic disease-modifying antirheumatic drugs (DESIRABLE study): a randomised, double-blind, placebo-controlled phase 3 trial. Ann Rheum Dis. 2019;78:899–907.
Hu Q, Zhong X, Tian H, Liao P. The efficacy of Denosumab in patients with rheumatoid arthritis: a systematic review and pooled analysis of Randomized or Matched Data. Front Immunol. 2022;12:799575.
Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282:2131–5.
Calle MC, Fernandez ML. Inflammation and type 2 diabetes. Diabetes Metab. 2012;38:183–91.
Böttiger LE, Svedberg CA. Normal erythrocyte sedimentation rate and age. Br Med J. 1967;2(5544):85–7.
Goldring SR. Differential mechanisms of de-regulated bone formation in rheumatoid arthritis and spondyloarthritis. Rheumatology. 2016;55(Suppl 2):ii56–ii60.
Chen M, Hu X, Wu M, Yang J, Han R, Ma Y, et al. Serum levels of OPG, RANKL, and RANKL/OPG ratio in patients with Ankylosing Spondylitis: a systematic review and Meta-analysis. Immunol Invest. 2019;48:490–504.
Jura-Półtorak A, Szeremeta A, Olczyk K, Zoń-Giebel A, Komosińska-Vassev K. Bone metabolism and RANKL/OPG ratio in rheumatoid arthritis women treated with TNF-α inhibitors. J Clin Med. 2021;10:2905.
Acknowledgements
We would like to thank to Livia Maria Cordeiro Simões Ambrósio for technical assistance.
Funding
This study was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (Grant: FAPESP-CRID 2013/08216-2) (São Paulo, SP, Brazil); Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (Grant: 425075/2016-8) (Brasilia, DF, Brazil).
Author information
Authors and Affiliations
Contributions
TOQ, TAS, PLJ and RDRO conceived the study; TOQ and RDRO assessed and enrolled the patients; SCLA evaluated x-rays; RDRO analyzed and interpreted data; TOQ and RDRO wrote the manuscript draft; TOQ, SCLA, TAS, PLJ and RDRO revised the final version of the manuscript. All authors have read and agreed with this final version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The local ethics committee approved the study (CAAE: 59784122.8.0000.5440). All patients provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors have nothing to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Quaresma, T.O., de Almeida, S.C.L., da Silva, T.A. et al. Comparative study of the synovial levels of RANKL and OPG in rheumatoid arthritis, spondyloarthritis and osteoarthritis. Adv Rheumatol 63, 13 (2023). https://doi.org/10.1186/s42358-023-00294-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42358-023-00294-3