Abstract
Background
High-fat diet (HFD) has been linked to oxidative stress, dyslipidaemia, obesity and cardiovascular diseases. Studies have shown that medicinal plants have antioxidant properties and may have protective effects against oxidative stress and dyslipidaemia induced by high-fat diet. Chrysophyllum albidum (white star apple) and Irvingia gabonensis (African bush mango) are very useful medicinal plants common in the tropical and subtropical regions of the world. This study was aimed at investigating the protective effect of methanol leaf extracts of Chrysophyllum albidum and Irvingia gabonensis against dyslipidaemia and oxidative stress in Wistar rats promoted by the consumption of HFD as well as characterize active compounds in the extracts. Thirty-six male Wistar rats were assigned into six groups of six animals each and respective groups received normal fat diet (NFD), HFD, HFD + Chrysophyllum albidum (250 mg/kg/ 500 mg/kg), HFD + Irvingia gabonensis (250 mg/kg/ 500 mg/kg) for fifty six days. Dietary intake, body weight, lipid profile and indicators of oxidative stress were measured.
Results
Administration of plant extracts did not change the pattern of food intake of the animals. There was a significant inhibition (p < 0.05) of increase in the body weight of rats treated with plant extracts in comparison with those rats that consumed HFD only. There was significant increase (p ˂ 0.05) in total cholesterol, triacylglycerol, low-density lipoprotein cholesterol, very low-density lipoprotein cholesterol and malondialdehyde concentrations in rats fed with HFD only when compared with NFD control and extract treated groups. However, HFD control produced significantly lower (p < 0.05) high-density lipoprotein cholesterol, reduced glutathione and glutathione peroxidase than normal control and extract treated groups. Gas chromatography flame ionization detector analysis of these extracts revealed the abundance of kaempferol, quercetin, luteolin, myricetin and naringenin and tannic acid.
Conclusions
The observed antioxidant and anti-dyslipidaemic properties of leaves of Chrysophyllum albidum and Irvingia gabonensis may be attributed to the presence of flavonoids and tannic acid.
Similar content being viewed by others
Background
High-fat diet (HFD) is an important risk factor for several ailments such as obesity, dyslipidaemia, cardiovascular disease, type II diabetes, non-alcoholic fatty liver, non-alcoholic steatohepatitis and cancer (Vargas-Robles et al. 2015). Many reasons, such as high-fat, caloric-dense diets and sedentary lifestyles, are the contributing factors for the development of metabolic syndrome (McLaren 2007). The mechanisms for increased oxidative stress in metabolic syndrome include increased fatty acid oxidation, mitochondrial dysfunction and augmented NADPH oxidase activity (Lee 2013). HFD provoked dyslipidaemia is linked to oxidative stress, an accumulation of several transition metals as well as increased free radicals (Charradi et al. 2013).
Drugs used in the management of lipid disorders have been associated with adverse effects such as gastrointestinal, kidneys and heart problems (Rucker et al. 2007). Specifically, they have been known to cause abdominal pain, bloating, flatulence, diarrhoea, acute kidney injury, increase in blood pressure, increase in pulse rate and palpitation (Beyea et al. 2012; Kang and Park 2012). Therefore, there is a need to search for safer alternative treatments for these disorders. Chrysophyllum albidum (white star apple) and Irvingia gabonensis (African bush mango) have several medicinal properties. Reports have shown that their extracts have hepatoprotective, antibacterial, antioxidant and anti-hyperlipidaemic properties (Ainge and Brown 2001; Adewoye et al. 2010; Ibukun and Omoregie 2018). Olorunnisola et al. (2008) reported that the roots, bark and the leaf of C. albidum are used as natural remedy for sprain, bruise and wound in southern Nigeria and also for inhibition of microbial growth of wound contaminants. The high saponin content of C. albidum leaves and roots justifies the use of the extracts in control of cardiovascular diseases and reduction of blood cholesterol (Ogunleye et al. 2020). In addition, the bark of C. albidum has been used in treatment of yellow fever, fibroids and malaria, while the leaf is used as emollient and for the treatment of skin eruption, stomach ache and diarrhoea (Adewoye et al. 2010). Similarly, Irvingia gabonensis has several medicinal uses. The stem bark is used to treat hernias, yellow fever and dysentery (George and Zhao 2007). The antibiotic properties of the bark help heal scabby skin, and the boiled bark relieves tooth pain (Ainge and Brown 2001). Long-term anti-diabetic and anti-hyperlipidaemic effects of aqueous stem bark extract of I. gabonensis in streptozotocin-induced diabetic rats have been reported by Omonkhua et al. (2014). Emejulu et al. (2014) have also reported that the fruit juice of I. gabonensis possess hypolipidaemic effect on sodium fluoride induced dyslipidaemia in rats. Leaves of I. gabonensis are used locally to treat diarrhoea (Unaeze et al. 2017) and as antidote against poison (Hubert et al. 2010). Leaf extract of I. gabonensis has been found to be nephroprotective (Ewere et al. 2016) and attenuates cadmium-induced haematological derangements in rats (Ewere et al. 2017).
Most research on C. albidum and I. gabonensis has been focused on the fruits rather than leaves. Also, there is paucity of information on the effect of leaves from these plants on dyslipidaemia and oxidative stress in rats, despite the fact that they are rich in several phytochemicals such as saponins, flavonoids, alkaloids, tannins and steroids (Ojemekele et al. 2017). These phytochemicals have been suggested to possess antioxidant and anti-dyslipidaemic properties (Krishnaiah et al. 2007; Mohamed et al. 2014). Therefore, this study was aimed at evaluating the protective effects of methanol leaf extracts of C. albidum and I. gabonensis on dyslipidaemia and oxidative stress induced by high-fat diet in Wistar rats.
Methods
Chemicals and reagents
1,1-diphenyl-2-picrylhydrazyl radical (DPPH), 2,4,6-tripyridyl-S-triazine (TPTZ), ferric chloride, methanol, hexane, dichloromethane, ethyl acetate, ferrous sulphate, sodium chloride, tannic acid, Folin–Denis reagent, 5',5'-dithiobis-2-nitrobenzoic acid (DTNB), sodium acetate, acetic acid, thiobarbituric acid, trichloroacetic acid, hydrochloric acid, sodium azide, reduced glutathione, sodium carbonate, aluminium chloride, ethanol, quercetin, gallic acid, Folin–Ciocalteu reagent, hydrogen peroxide, sodium dihydrogen phosphate and disodium hydrogen phosphate were obtained from Sigma-Aldrich (USA). Cholesterol, triacylglycerol and high-density lipoprotein cholesterol kits were obtained from Randox laboratory (UK), with catalogue number CH200, TR 210 and CH203, respectively. All chemicals and reagents used were of analytical grade.
Animals
Male Wistar albino rats weighing 130–150 g obtained from animal house of University of Benin, Edo state, Nigeria, were used for the study. The animals were allowed to acclimatize for 14 days before beginning the experiment. They were housed in polypropylene cages at a temperature of 25–30 °C, relative humidity of 35–45% and 12 h for both light and dark cycles. They had free access to the formulated diet and water. The animals were handled with great care so as to minimize pain, and all experiments were conducted in strict compliance with internationally accepted principles for laboratory animals’ use and care (Olfert et al. 1993).
Collection of plant materials and identification
Leaves of Chrysophyllum albidum and Irvingia gabonensis were collected from a private farm at Ewu, Edo State, Nigeria (6.8015oN, 6.2495oE). The fresh leaves were identified by Prof. M.E. Osawaru at the Department of Plant Biology and Biotechnology of the University of Benin, Benin City, Nigeria. Herbarium specimens with voucher numbers UBHc362 and UBHi153 for Chrysophyllum albidum and Irvingia gabonensis, respectively, were deposited at the Herbarium.
Preparation of plant extracts
The leaves were rinsed properly with water and air-dried away from direct sunlight. They were then pulverized using hands, soaked in methanol for 72 h and filtered with a double-layered muslin cloth (100 µm). Each filtrate was separately concentrated using a rotary evaporator (RE-52A, Lanphan, China) at 40 °C and stored in air-tight sterile bottles.
Preparation of experimental diet
NFD and HFD were formulated using a slightly modified method of Cha and Jones (1998) as follows:
NFD Casein (15%), corn starch (60%), sucrose (10%), soya bean oil (4%), vitamins and minerals mix (4.5%), cellulose (6.5%).
HFD Casein (15%), corn starch (40%), sucrose (10%), soya bean oil (4%), beef tallow (20%) vitamins and minerals mix (4.5%), cellulose (6.5%).
Experimental design
Thirty-six male Wistar rats were randomly assigned into six groups consisting of six animals each. High-fat diet (20% w/w beef tallow) was used to induce dyslipidaemia and oxidative stress in the experimental rats in groups 2–6.
The groups were treated as follows:
Group 1 Represented the normal fat diet control. The animals were fed normal fat diet (NFD) and orally administered the vehicle (carboxymethyl cellulose 0.5% solution) once daily for eight weeks.
Group 2 Served as the high-fat diet control. The rats were fed with HFD and given the vehicle orally, once daily for eight weeks.
Group 3 The rats were orally treated (once daily) with the methanol extract of leaf of Chrysophyllum albidum (250 mg/kg) along with the HFD for eight weeks.
Group 4 The rats were orally treated (once daily) with the methanol leaf extract of Chrysophyllum albidum (500 mg/kg) along with HFD for eight weeks.
Group 5 The rats were orally treated (once daily) with the methanol leaf extract of Irvingia gabonensis (250 mg/kg) along with HFD for eight weeks.
Group 6 The rats were orally treated (once daily) with the methanol leaf extract of Irvingia gabonensis (500 mg/kg) along with HFD for eight weeks.
During the period of the study, body weight (g) of animals was recorded on a weekly basis and feed intake was recorded daily. At the end of the experiment (56 days), the rats were subjected to overnight fasting and then killed by cervical dislocation. Blood samples were collected via cardiac puncture into sterile tubes and allowed to stand for 30 min at 20–25 °C. The clear serum was separated at 2500 g for 15 min using a centrifuge.
Liver and heart samples were harvested and placed in containers with normal saline and stored at 4 °C. A sample of liver tissue (1 g) was homogenized in 9 mL of cold normal saline (0.9%), while 0.5 g of heart was homogenized in 4.5 mL of ice cold normal saline. The homogenates were centrifuged at 1000 g for 15 min, and the supernatant was stored for subsequent analyses.
Estimation of serum lipid profile
Total cholesterol concentration Allain et al. (1974).
Triacylglycerol Concentration (Tietz 1990).
High-Density Lipoprotein Cholesterol (HDL-C) Concentration (Tietz 1990).
Low-Density Lipoprotein Cholesterol ((LDL-C) Concentration
The low-density lipoprotein (LDL) cholesterol concentration was estimated using the formula by Friedewald et al (1972)
Very Low-Density Lipoprotein Cholesterol (VLDL-C) Concentration
Very low-density lipoprotein (VLDL) cholesterol concentration was estimated using the formula by Friedewald et al (1972).
Estimation of indicators of oxidative stress
Glutathione peroxidase activity, malondialdehyde and reduced glutathione concentrations were measured in the liver and heart using established protocols as follows:
Glutathione peroxidase
In this procedure, the rate of oxidation of glutathione by hydrogen peroxide is used to measure glutathione peroxidase activity. Glutathione leftover in the solution at a given time is estimated by its reaction with DTNB (5',5'-dithiobis-(2-nitrobenzoic acid)) (Rotruck et al. 1973).
Malondialdehyde
This method depends on the formation of malondialdehyde (MDA) as an end product of lipid peroxidation which reacts with thiobarbituric acid (TBA) producing a pink chromatogen which can be measured spectrophotometrically at 532 nm (Buege and Aust 1978).
Reduced glutathione
The reduced form of glutathione comprises in most instances the bulk of cellular non-protein sulfhydryl groups. This method is based upon the development of a relatively stable (yellow) colour when 5',5'-dithiobis-(2-nitrobenzoic acid) (Ellman’s reagent) is added to sulfhydryl compounds. The chromophoric product resulting from the reaction of Ellman’s reagent with the reduced glutathione, 2-nitro-5-thiobenzoic acid possesses a molar absorption at 412 nm (Ellman 1959).
Fractionation of the extracts and determination of the most active fraction
The crude methanol extract of leaves of C. albidum and I. gabonensis (10 g) was dissolved separately in a 100 mL mixture of methanol and water (4:1). Then, it was extracted successively with different organic solvents of increasing polarity: hexane, dichloromethane and ethyl acetate to obtain their respective fractions. They were concentrated by rotary evaporator (Hossain et al. 2014).
The various fractions obtained were subjected to phytochemical screening (qualitative and quantitative). Quantification of phytochemicals included total phenols (Cicco et al. 2009), total flavonoids (Miliauskas et al. 2004) and total tannins (Polshettiwar and Ganjiwale 2007), which was carried out to determine the phytochemicals present in the extracts that may also be responsible for their in vivo activities.
Evaluation of antioxidant potential of fractions
The various fractions (hexane, dichloromethane and ethyl acetate fractions from C. albidum and I. gabonensis) were evaluated in vitro for antioxidant property using the follow assays:
Ferric reducing antioxidant potential
This assay is based on electron transfer to reduce Fe3+ to Fe2+using 2,4,6-tripyridyl-S-triazine (TPTZ), forming an intense blue iron two-ion TPTZ complex that is read at 593 nm.
Procedure
1.5 mL of freshly prepared FRAP solution (25 mL of 300 mM acetate buffer (pH 3.6), 2.5 mL of 10 mM 2,4,6-tripyridyl-S-triazine (TPTZ) in 40 mM HCl and 2.5 mL of 20 mM ferric chloride (FeCl3.6H2O) solution) was added to 1 mL of the sample (1 mg/mL). The reaction mixtures were incubated at 37 °C for 30 min, and the absorbance at 593 nm was measured. FeSO4 was used for the calibration curve and ascorbic acid served as the positive control. FRAP values (expressed as mg Fe (II)/g of the extract) for the extracts were then extrapolated from the standard curve (Benzie and Strain 1996).
1,1-Diphenyl-2-picrylhydrazyl radical (DPPH) scavenging activity
The free radical scavenging capacity of the leaf extracts against 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical was determined by the method of Brand-Williams et al (1995).
The assay is based on the rate at which a compound donates electron or hydrogen atom to DPPH which results in change of colour from deep violet to golden yellow which can be read at 570 nm.
Procedure
Briefly, 0.5 mL of 0.3 mM DPPH solution in methanol was added to 2 mL of various concentrations (0.01 – 0.2 mg/mL) of the sample. The reaction tubes were shaken and incubated for 15 min at room temperature in the dark, absorbance read at 517 nm. All tests were performed in triplicates. Ascorbic acid was used as the standard control, with similar concentrations as the test samples prepared. A blank containing 0.5 mL of 0.3 mM DPPH and 2 mL of methanol was prepared and treated as the test samples. The radical scavenging activity was calculated using the following formula:
DPPH radical scavenging activity (%) = [(A0 − A1)/(A0)] × 100.
Where:
A0 = Absorbance of DPPH radical + methanol.
A1 = Absorbance of DPPH radical + sample or standard.
The 50% inhibitory concentration (IC50) value was calculated as the effective concentration of the sample that is required to scavenge 50% of the DPPH free radicals. This was extrapolated from a straight line graph of percentage inhibition of DPPH against sample concentration (Brand-Williams et al. 1995).
The fraction that has the highest concentration of phytochemicals and antioxidant property was regarded as the most active fraction and was subjected to gas chromatography flame ionization detector (GC-FID) analysis.
Statistical analysis
The results were analysed using the statistical package for social sciences (SPSS) version 23 for windows. One-way analysis of variance (ANOVA) was used to compare standard error of mean. Data were termed significant at p < 0.05. Inter-group comparisons were done by the Tukey’s post hoc test.
Results
Feed consumption of rats
Figure 1 represents the effect of C. albidum and I. gabonensis extracts on feed intake by rats. There was no significant (p > 0.05) difference in the daily feed consumption in rats treated with the extracts in comparison with the controls.
Effect of methanol leaf extracts of C. albidum and I. gabonensis on feed intake of rats fed with HFD. Values are expressed as mean ± SEM (n = 6). The daily food consumption of experimental rats in various groups was measured. The plant extracts did not have a significant effect on the amount of food consumed by the rats in comparison with the NFD control and HFD control
Body weight
Figure 2 shows the effect of C. albidum and I. gabonensis extracts on weekly body weight of rats fed with different diets. From week 4 to 8, the mean body weight of rats fed with high-fat diet alone was significantly higher (p < 0.05) when compared to rats in the extracts treated groups and NFD control (Fig. 2).
Effect of C. albidum and I. gabonensis extracts on the weekly body weight of rats fed with HFD. Values are expressed as mean ± SEM (n = 6). Points bearing asterisk * = Extracts and NFD control are significantly different from HFD control. At weeks 0, 1, 2 and 3 there was no significant difference (p > 0.05) in the body weights of all groups. However, from week 4 to 8 the body weight of rats in HFD control was significantly higher (p ˂ 0.05) than the body weights of rats in the extracts treated groups and NFD control
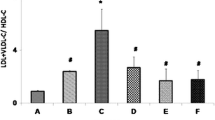
Effect of C. albidum and I. gabonensis extracts on lipid profile of rats
Total cholesterol, triacylglycerol, LDL-C and VLDL-C concentrations were significantly higher (p < 0.05) in HFD control than NFD control and extract treated groups. However, HDL-C concentration was significantly lower (p < 0.05) in HFD control than NFD control and extract treated groups (Table 1).
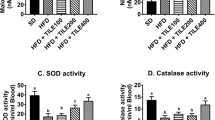
Effect of C. albidum and I. gabonensis extracts on antioxidant indices of rats
The concentration of GSH, MDA and activity of GPx in rats’ liver and heart homogenates are depicted in Table 2. The concentration of GSH in the liver of extract treated groups was significantly higher (p ˂ 0.05) than that of high-fat diet control. The GPx activity in the liver and heart of extract treated groups was significantly higher (p ˂ 0.05) than that of high-fat diet control. The liver and heart MDA concentrations were significantly (p ˂ 0.05) lower for the extract treated groups than the high-fat diet control.
Phytochemical constituents and in vitro antioxidant capacity of fractions from leaf extracts of C. albidum and I. gabonensis
The phytochemical screening (qualitative) results of n-hexane, dichloromethane, ethyl acetate and hydromethanol fractions from leaf extracts of C. albidum and I. gabonensis are shown in Tables 3 and 4, respectively.
Quantitatively, the total phenols, total flavonoids and total tannins contents of the ethyl acetate fraction of both C. albidum and I. gabonensis were significantly higher (p < 0.05) than those of hexane, dichloromethane and hydromethanol fractions (Tables 5 and 6).
The results of the DPPH radical scavenging activities of the various fractions from C. albidum and I. gabonensis are shown in Figs. 3 and 4, while their corresponding IC50 values are presented in Tables 7 and 8, respectively. The ethyl acetate fractions of both extracts had significantly lower (p < 0.05) IC50 values and therefore better DPPH radical scavenging ability than the other fractions. Only the positive control, ascorbic acid had significantly lower (p < 0.05) IC50 value than ethyl acetate fraction.
DPPH radical scavenging activity of fractions from leaf extract of C. albidum. Values are expressed as mean ± SEM, n = 3. Different alphabets on same grouped bars show that values for the fractions are significantly different (p < 0.05) from each other. This figure shows the ability of different fractions obtained from leaf extract of C. albidum to scavenge DPPH radical at different concentrations
DPPH radical scavenging ability of fractions from leaf extract of I. gabonensis. Values are expressed as mean ± SEM, n = 3. Different alphabets on same grouped bars show that values for the fractions are significantly different (p < 0.05) from each other. This figure shows the ability of different fractions obtained from leaf extract of I. gabonensis to scavenge DPPH radical at different concentrations
Figures 5 and 6 indicate the ferric reducing antioxidant potential (FRAP) of the fractions from leaf extracts of C. albidum and I. gabonensis, respectively. Also, the ethyl acetate fraction of both plant extracts had the highest FRAP values in comparison with the other fractions.
Ferric reducing antioxidant potential (FRAP) of fractions from leaf extract of C. albidum. Values are expressed as mean ± SEM, n = 3. Different alphabets on bars show that values for the fractions are significantly different (p < 0.05) from each other. The ethyl acetate fraction from leaf extract of C. albidum had significantly higher (p < 0.05) FRAP value in comparison with the other fractions. However, ascorbic acid standard had significantly higher (p < 0.05) FRAP value than ethyl acetate fraction
Ferric reducing antioxidant potential (FRAP) of fractions from leaf extract of I. gabonensis. Values are expressed as mean ± SEM, n = 3. Different alphabets on bars show that values for the fractions are significantly different (p < 0.05) from each other. The ethyl acetate fraction from leaf extract of I. gabonensis had significantly higher (p < 0.05) FRAP value in comparison with the other fractions. However, ascorbic acid standard had significantly higher (p < 0.05) FRAP value than ethyl acetate fraction
Phytochemical components in the ethyl acetate fraction of methanol leaf extracts of C. albidum and I. gabonensis identified by GC-FID
Based on result of quantitative phytochemical screening and in vitro antioxidant property, the ethyl acetate fraction was considered the most active fraction as it had higher amount of phytochemicals and antioxidant property than all the other fractions. Therefore, the ethyl acetate fractions of C. albidum and I. gabonensis extracts subjected to gas chromatography flame ionization detector (GC-FID) analysis revealed the phytochemical components shown in Tables 9 and 10, respectively.
The analysis showed the presence of various flavonoids. The most abundant flavonoids present in the ethyl acetate fraction of both C. albidum and I. gabonensis were kaempferol, quercetin, luteolin, myricetin and naringenin as highlighted in Tables 9 and 10, respectively.
Discussion
This present study evaluated the impact of leaf extracts of Chrysophyllum albidum and Irvingia gabonensis on dyslipidaemia and oxidative stress induced by HFD in Wistar rats. A link between HFD, lipid abnormalities and oxidative stress has been recognized for long, and HFD is considered to be a reliable method in induction of lipid abnormalities in experimental rats (Bais et al., 2014). Long-term feeding with HFD which has a high content of saturated fatty acids may lead to a decrease in the levels of polyunsaturated fatty acids (PUFAs) in hepatocyte cells, causing oxidative stress and inflammatory conditions. These conditions cause sterol regulatory element-binding protein (SREBP-1c) activation (Wali et al. 2020). This is a transcription factor that regulates genes that play a function in the synthesis of free fatty acids (FFAs) and increases the absorption of FFAs, cholesterol and triglycerides by stimulating the expression of fatty acid synthase (FAS) and acetyl-CoA carboxylase (ACC) (Ruiz et al. 2014). It has been suggested that the administration of HFD can increase the expression of FAS and ACC proteins. ACC protein plays a role in inhibiting fatty acid transport to the mitochondria, and thereby, fatty acids will be esterified into a large proportion of triglycerides and stimulate de novo lipogenesis. Furthermore, triglycerides are converted into VLDL. VLDL will then become LDL, resulting in an increase in LDL concentration followed by a decrease in HDL concentration (Zhang et al. 2020).
Research has also shown that prolonged consumption of a high-saturated fat diet induces oxidative stress, because it attenuates the hepatic antioxidant enzyme system and raises the concentration of lipid peroxidation products in the liver and plasma (Oliveros et al. 2004). Plant-derived polyphenols and polyphenol metabolites have long been recognized for their prominent antioxidative benefits, with minimal adverse effects. Antioxidant therapy with phytochemicals has been said to be effective in suppressing multiple oxidative stress pathways (Rodrigo and Gil-Becerra 2014).
Dyslipidaemia has been characterized as a major risk factor for cardiovascular risk, including atherosclerosis (Martins and Redgrave 2004). In this study, administration of C. albidum and I. gabonensis leaf extracts to rats at doses of 250 mg/kg and 500 mg/kg with HFD significantly lowered (p < 0.05) the levels of total cholesterol, VLDL-C, triacylglycerol, LDL-C and increased the level of HDL-C in comparison with HFD control. Similar results were obtained by Ghasi et al (2000), where treatment of rats with HFD and crude extract of a medicinal plant led to an increased serum HDL-C and decreased levels of total cholesterol, LDL-C, VLDL-C and triacylglycerol.
In this study, the capacity of the extracts in ameliorating oxidative stress in rats was determined using measurement of MDA, GSH concentration and activity of GPx in the liver and heart. GSH is a tripeptide, non-enzymatic biological antioxidant present in the liver which protects cellular proteins against reactive oxygen species produced from a number of processes (Arivazhagan et al. 2000). Decreased level of GSH is associated with increased lipid peroxidation (Arivazhagan et al. 2000). GPx also functions in detoxifying hydroperoxides. It catalyses the reaction of hydroperoxides with reduced glutathione to form glutathione disulphide (GSSG) and the reduction product of hydroperoxide (Alam et al. 2012). MDA is a by-product of lipid peroxidation. Increased levels of lipid peroxidation determined by measurement of MDA have been associated with various disease conditions and pathological states (Wang et al. 2004).
The results from the study revealed significantly higher (p < 0.05) concentration of liver GSH in the extracts treated groups when compared to the rats in the HFD control. The results also showed significantly higher (p < 0.05) liver and heart GPx activities in the extracts treated groups when compared to the HFD control. The liver and heart MDA levels in the extract treated groups were significantly lower (p < 0.05) than that of the rats exposed to the high-fat diet without treatment. The relative decrease in the activity of GPx and GSH concentration in the rats induced with HFD could be attributed to their excessive utilization in mopping up the free radicals generated due to the HFD (Ma et al. 2011). In addition, the high MDA concentration in the HFD control indicates evidence of lipid peroxidation as a result of the HFD.
The observed ameliorative effect of methanol leaf extracts of C. albidum and I. gabonensis against the aforementioned oxidative stress indices supports the work of Olorunnisola et al (2012), who also reported that oxidative stress induced by high-fat diet was ameliorated by a plant extract (T. violacea). In their study, administration of crude extracts of T. violacea rhizomes to rats fed with HFD protected against HFD induced reduction in GSH, reduction in GPx activity and increase in MDA in liver, heart and aorta. Also in this present study, leaf extracts of C. albidum and I. gabonensis inhibited HFD induced lowering of GSH, lowering of GPx and rise in MDA in liver and heart of rats fed.
In this study, the findings on weight gain in HFD fed rats suggest that the extracts significantly inhibited weight gain. In a previous study, Bias et al. (2014) speculated that treatment of HFD fed rats with methanol leaf extract of Moringa oleifera for 49 days resulted in a significant reduction in body weight compared to the HFD control. They therefore suggested that leaf extracts of Moringa oleifera have weight-reducing potential. These findings from this research are in line with the report by Bias et al. (2014). In our study, the extracts probably acted to inhibit weight gain by lowering fat accumulation in the body. This can be seen from the significant reduction in the serum concentration of triacylglycerol in rats treated with the extracts in comparison with the HFD control.
To ascertain the effect of the plant extracts on appetite of rats, food consumption was measured daily. It was observed that administration of C. albidum and I. gabonensis extracts did not affect feed consumption. This indicates that the extracts produced antioxidant and anti-obesity effects, in HFD rat model, without affecting appetite.
In a recent study by Zalar et al. (2022), HFD was used to induce dyslipidaemia in rats. However, treatment of the rats with an alkaloid extract from Colchicum autumnale L. significantly reduced plasma levels of total cholesterol, LDL-C and triacylglycerol while increasing HDL-C level in comparison with the HFD control. Also, they reported that HFD lowered GSH concentration and increased MDA concentration, signifying induction of oxidative stress. However, administration of extract reduced oxidative stress in the rats. Similarly, in another recent research by Labban et al. (2021), Garcinia mangostana and Curcuma longa ameliorated oxidative stress and dyslipidaemia in rats fed with HFD. Reports from these studies are also consistent with our findings in this present study.
Upon analysis of the various fractions resulting from the extracts using solvents with increasing polarities (hexane < dichloromethane < ethyl acetate), ethyl acetate fractions of both extracts had higher amounts of phytochemicals (phenols, flavonoids and tannins) than the other fractions. The ethyl acetate fractions also showed significantly higher (p < 0.05) antioxidant property than the other fractions and were therefore suggested to be the most active fraction. The higher antioxidant property of ethyl acetate fractions may be due to the presence of higher amount of phytochemicals in comparison with the other fractions.
GC-FID analysis of the ethyl acetate fraction from crude extract of C. albidum and I. gabonensis revealed the presence of twenty-eight known flavonoids, which consisted of high concentrations of kaempferol, quercetin, luteolin, myricetin and naringenin. The remaining flavonoids were less than 1 mg/100 g in the extracts. Tannic acid was also detected in abundant amounts in the extracts. Kaempferol, quercetin, luteolin, myricetin and naringenin are flavonoids present in different plant species (especially vegetables and fruits). Tannic acid is a polyphenol which is water soluble and has the ability to complex macromolecules and metal ions (Leal et al. 2015). These phytochemicals have several properties including strong antioxidant properties and anti-dyslipidaemic properties (Brusselmans et al. 2005; Fang et al. 2008; Ilhami et al. 2010). Specifically, flavonoids have been reported to lower lipid levels by inhibiting lipid absorption, lipogenesis and stimulating lipolysis (Al Shukor et al. 2016). Therefore, these phytochemicals may be responsible for the protective effects of the plant extracts against dyslipidaemia and oxidative stress induced by HFD as observed in this study.
Conclusions
The study has shown that methanol leaf extracts of C. albidum and I. gabonensis have significant weight gain inhibitory activity in rats without affecting feed consumption. The extracts also exhibited protective effect against dyslipidaemia and oxidative stress induced by high-fat diet in rats. These desirable properties may be due to the presence of several bioactive compounds in the extracts. However, there is need for further preclinical trials and thorough investigation of their mode of action, bioavailability and pharmacokinetics.
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- HFD:
-
High-fat diet
- NFD:
-
Normal fat diet
- GC-FID:
-
Gas chromatography flame ionization detector
- FRAP:
-
Ferric reducing antioxidant power
- DPPH:
-
1,1-Diphenyl-2-picrylhydrazyl
- GPx:
-
Glutathione peroxidase
- GSH:
-
Reduced glutathione
- MDA:
-
Malondialdehyde
References
Adewoye EO, Salami AT, Taiwo VO (2010) Antiplasmodial and toxicological effects of methanolic bark extract of Chrysophyllum albidum in albino mice. J Physiol Pathophysiol 1(1):1–9
Ainge L, Brown N (2001) Irvingia gabonensis and Irvingia wombolu. In: A state of knowledge report undertaken for the central african regional program for the environment. Oxford Forestry Institute. Department of Plant Sciences. University of Oxford, United Kingdom.
Al shukor N, Raes K, Smagghe G, Camp JV, (2016) Flavonoids: evidence for inhibitory effects against obesity and their possible mechanisms of action. RPMP 40:496–514
Alam N, Bristi NJ, Rafiquzzaman, (2012) Review on in vivo and in vitro methods of evaluation of antioxidant activity. SPJ 21(2):143–152
Allain CC (1974) Enzymatic determination of total serum cholesterol. Clin Chem 20:470–475
Arivazhagan S, Balasenthil S, Nagini S (2000) Garlic and neem leaf extracts enhance hepatic glutathione and glutathione dependent enzymes during N-methyl- N nitrosoguanidine (MNNG)- induced gastric carcinogenesis. Phytother Res 14:291–293
Bais S, Singh GS, Sharma R (2014) Anti-obesity and Hypolipidemic activity of Moringa oleifera leaves against high fat diet-induced obesity in rats. Adv Biol 10:1–9
Benzie IF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of ”antioxidant power”: the FRAP assay. Anal Biochem 239(1):70–76
Beyea MM, Garg AX, Weir MA (2012) Does orlistat cause acute kidney injury? Ther Adv Drug Saf 3(2):53–57
Brand-Williams W, Cuvelier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol 28(1):25–30
Brusselmans L, Vrolix R, Verhoeven G, Swinnen JV (2005) Induction of cancer cell apoptosis by flavonoids is associated with their ability to inhibit fatty acid synthase activity. J Biol Chem 280(7):5636–5645
Buege A, Aust SD (1978) Microsomal lipid peroxidation. Methods Enzymol 52:302–310
Cha MC, Jones PJ (1998) Dietary fat type and energy restriction interactively influence plasma leptin concentration in rats. J Lipid Res 39:1655–1660
Charradi K, Elkahoui S, Limam F, Aouani E (2013) High-fat diet induced an oxidative stress in white adipose tissue and disturbed plasma transition metals in rat: prevention by grape seed and skin extract. J Physiol Sci 10:20–28
Cicco N, Lanorte MT, Paraggio M, Viggiano M, Lattanzio V (2009) A reproducible, rapid and inexpensive Folin-Ciocalteu micromethod in determining phenolics of plantmethanol extracts. Microchem J 91:107–110
Ellman GL (1959) Tissue sulphydryl groups. Arch Biochem Biophys 82:70–77
Emejulu AA, Adamma A, Alisi CS, Asiwe ES, Iheanacho KM, Onwuliri VA (2014) Hypolipdaemic effect of Irvingia gabonensis fruits juice on sodium fluoride induced dyslipidaemia in rats. Afr J Biochem Res 8:151–157
Ewere EG, Oyebadejo SA, Peter VC (2016) Ethanolic leaf extract of Irvingia gabonensis (O’ Rorke) BAILL protects against nephrotoxicity and Hepatotoxocity in cadmium-induced Wistar albino rats. Int J Pharmacol Toxicol 4:105–110
Ewere EG, Etim OE, Oyebadejo SA, Edem BV (2017) Attenuation of cadmium induced Haematological derangements in Wistar albino rats by Irvingia gabonensis O’Rorke baill ethanol leaf extract. Eur J Biomed Pharm Sci 4:169–174
Fang XK, Gao J, Zhu DN (2008) Kaempferol and quercetin isolated from euonymus alatus improve glucose of 3T3-L1 cells without adipogenesis activity. Life Sci 82(11):615–622
Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18:1530–8561
George IN, Zhao Y (2007) Pharmacological activity of 2,3,8-tri-O-methyl ellagic acid isolated from the stem bark of Irvingia gabonensis. Afr J Biotechnol 6:1910–1912
Ghasi S, Nwobodo E, Ofili JO (2000) Hypocholesterolemic effects of crude extract of leaf of Moringa oleifera Lam in high fat diet fed wistar rats. J Ethnopharmacol 69(1):21–25
Hossain MA, Al-Hdhrami SS, Weli AM, Al-Riyami Q, Al-Sabahi (2014) Isolation, fractionation and identification of chemical constituents from the leaves crude extracts of Menthapiperita L grown in sultanate of Oman. Asian Pac J Trop Biomed 4:368–372
Hubert DJ, Wabo FG, Ngameni B, Ngheguin TF, Tchoukoua A, Ambassa P (2010) In vitro Hepatoprotective and antioxidant activities of the crude extract and isolated compounds from Irvingia gabonensis. Asian J Tradit Med 5:79–88
Ibukun O, Omoregie ES (2018) Antihyperlipidaemic property of methanol leaf extract of Chrysophyllum albidum in albino rats (Wistar Strain) fed on high fat diet. JAB 11(1):2146–2154
Ilhami G, Zu H, Mahfuz E, Hassan YA (2010) Radical scavenging and antioxidant activity of tannic acid. Arab J Chem 3:43–53
Kang GJ, Park C (2012) Anti-obesity drugs: a review about their effects and safety. Diabetes Metab J 36:13–25
Krishnaiah D, Sarbatly R, Bono A (2007) Phytochemical antioxidants for health and medicine: a move towards nature. Biotechnol Mol Biol Rev 1:97–104
Labban RS, Alfawaz HA, Almnaizel AT, Al-Muammar MN, Bhat RS, El-Ansary A (2021) Garcinia mangostana extract and curcumin ameliorate oxidative stress, dyslipidemia, and hyperglycemia in high fat diet-induced obese Wistar albino rats. Sci Rep 11:7278–7289
Leal AD, de Carvalho LH, da Silva D, Nunes LC, Lopes JA (2015) Incorporation of tannic acid in formulations for topical use in wound healing: a technological prospecting. Afr J Pharm Pharmacol 9(26):662–674
Lee C (2013) The Effect of high-fat diet-induced pathophysiological changes in the gut on obesity: what should be the ideal treatment? Clin Transl Gastroenterol 4:3–9
Ma J, Qiao Z, Xiang X (2011) Aqueous extract of Astragalus mongholicus ameliorates high cholesterol diet induced oxidative injury in experimental rats models. J Med Plants Res 5:855–858
Martins IJ, Redgrave TG (2004) Obesity and post-prandial lipid metabolism Feast or famine ? J Nutr Biochem 15(3):130–141
McLaren L (2007) Socioeconomic status and obesity. Epidemiol Rev 29:29–48
Miliauskas G, Vensketonis PR, Van- Beck TA (2004) Screening of radical scavenging of some medicinal and aromatic plant extracts. Food Chem 85:231–237
Mohamed GA, Ibrahim RM, Elkhayat ES, El Dine RS (2014) Natural anti-obesity agents. Bull Fac Pharm Cairo Univ 52:269–284
Ogunleye FA, Fapohunda O, Nwangwu O (2020) A review on medicinal uses and pharmacological activities of african star apple (Chrysophyllum albidum). ASPC 1:1–9
Ojemekele O, Irabor F, Ebohon O, Omoregie ES (2017) A Comparative Study on the Phytochemical Screening and in vitro antioxidant activity of methanol leaf extracts of Chrysophyllum albidum and Irvingia gabonensis. Haya Saudi J Life Sci 2(3):58–64
Olfert ED, Cross BM, McWilliam AA (1993) Guide to the care and use of experimental animals. Can Counc Anim Care 1:82–89
Oliveros LB, Videla AM, Giménez MS (2004) Effect of dietary fat saturation on lipid metabolism, arachidonic acid turnover and peritoneal macrophage oxidative stress in mice. Braz J Med Biol Res 37(3):311–320
Olorunnisola O, Ehigie LO, Ajayi AF (2008) Antihyperglycemic and hypolipidaemic effect of ethanolic extract of Chrysophyllum albidum seed cotyledon in alloxan induced diabetic rats. Res J Appl Sci 3:123–127
Olorunnisola S, Bradley G, Afolayan A (2012) Protective effect of T. violacea rhizome extract against hypercholesterolemia-induced oxidative stress in Wistar rats. Molecules 17:6033–6045
Omonkhua AA, Onoagbe IO, Fajimeye IA, Adekola MB, Imoru ZA (2014) Long-term antidiabetic and antihyperlipidaemic effects of aqueous stem bark extract of Irvingia gabonensis in streptozotocin-induced diabetic rats. Biokemistri 26:1–8
Polshettiwar SA, Ganjiwale RO (2007) Spectrophotometric estimation of total tannins in some ayurvedic eye drops. Indian J Pharm Sci 69(4):574–576
Rodrigo R, Gil-Becerra D (2014) Implications of polyphenols on endogenous antioxidant defense systems in human diseases. In: Watson RR, Preedy VR, Zibadi S (eds) Polyphenols in human health and disease. Academic Press, San Diego
Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG (1973) Selenium: biochemical role as a component of glutathione peroxidase. Science 179:588–590
Rucker D, Padwal R, Li SK, Curioni C, Lau DC (2007) Long term pharmacotherapy for obesity and overweight: updated meta-analysis. BMJ 335(7631):1194–1199
Ruiz R, Jideonwo V, Ahn M, Surendran S, Tagliabracci VS, Hou Y (2014) Sterol Regulatory element-binding protein-1 (SREBP-1) Is required to regulate glycogen synthesis and gluconeogenic gene expression in mouse liver. J Biol Chem 289:55–60
Tietz NW (1990) Clinical guide to laboratory tests, 2nd edn. W.B. Saunders Company, Philadelphia, pp 554–556
Unaeze BC, Nwobu RU, Ilo CE, Ejike EC (2017) Effect of methanol, n-hexane and aqueous extract of Irvingia gabonensis leaf on castor oil-induced diarrhoea in albino rats. Int J Biol Chem Sci 11:1878–1883
Vargas-Robles H, Rios A, Arellano-Mendoza M, Escalante BA, Schnoor M (2015) Antioxidative diet supplementation reverses high-fat dietinduced increases of cardiovascular risk factors in mice. Oxid Med Cell Longev 2015:467–471
Wali JA, Jarzebska N, Raubenheimer D, Simpson SJ, Rodionov RN, O’sullivan JF (2020) Cardio-metabolic effects of high-fat diets and their underlying mechanisms—a narrative review. Nutrients 12:15–25
Wang TJ, Parise H, Levy D (2004) Obesity and the risk of new-onset atrial fibrillation. JAMA 292:2471–2477
Zalar D, Pop C, Buzdugan E, Kiss B, Stefan M, Ghibu S, Crisan D, Buruiană-Simic A, Grozav A, Borda IM, Mogos CI (2022) Effects of colchicine in a rat model of diet-induced hyperlipidemia. Antioxidants 11:1–13
Zhang Y, Shanshan G, Yang Z, Li Z, Gong X, Zhang Q (2020) Disturbance of di-(2-ethylhexyl) phthalate in hepatic lipid metabolism in rats fed with high fat diet. Food Chem Toxicol 146:118–148
Acknowledgements
The authors are grateful to members of malaria research, molecular biology and toxicology unit, Department of Biochemistry, Faculty of Life Sciences, University of Benin for their support.
Funding
The authors declare that there is no funding in the manuscript.
Author information
Authors and Affiliations
Contributions
OI contributed to methodology, data analysis and drafting of the manuscript. ESO contributed to investigation, methodology, supervision and editing of the drafted manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
All procedures using animals obtained the approval of Life Sciences Institutional Animal Ethical Committee, University of Benin with LS19225 ethical number.
Consent for publication
Not applicable.
Competing interests
No competing interests were declared by the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ibukun, O., Omoregie, E.S. Methanol leaf extracts of Chrysophyllum albidum and Irvingia gabonensis protected against dyslipidaemia and oxidative stress induced by high-fat diet in Wistar rats. Bull Natl Res Cent 46, 204 (2022). https://doi.org/10.1186/s42269-022-00883-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42269-022-00883-0