Abstract
Background
Ectopic bronchial artery and non-bronchial systemic arteries may be the culprit vessels of hemoptysis. The main cause of clinical failure of bronchial artery embolization is incomplete embolization caused by the misidentification of the culprit arteries by conventional angiography. Multidetector computed tomography angiography is useful for visualizing the culprit arteries.
Case presentation
An 82-year-old man was admitted with hemoptysis. Preprocedural multidetector computed tomography angiography revealed an ectopic bronchial artery branching from the right thyrocervical trunk. Superselective embolization of the ectopic bronchial artery was performed using gelatin sponge particles and metallic coils. Hemoptysis was controlled by this procedure without any associated complications.
Conclusions
Ectopic bronchial arteries originating from the thyrocervical trunk are rare. Preprocedural multidetector computed tomography angiography is useful for visualizing the culprit arteries of hemoptysis, especially if a patient has an ectopic bronchial artery or an ectopic non-bronchial systemic artery.
Similar content being viewed by others
Introduction
Massive hemoptysis is one of the fatal respiratory symptoms and is most frequently bronchogenic. Bronchial artery embolization (BAE) is widely used to manage massive hemoptysis (Yoon et al. 2002). However, ectopic bronchial artery (BA) and non-bronchial systemic arteries (NBSAs), such as the subclavian, internal mammary, or inferior phrenic arteries, may be the culprit vessels of hemoptysis. The main cause of clinical failure of BAE is incomplete embolization caused by the misidentification of the culprit arteries by conventional angiography (Zhao et al. 2017). Multidetector computed tomography (MDCT) angiography is useful for visualizing the ectopic origin of the BA and NBSAs during BAE that can be easily missed by conventional angiography (Li et al. 2019). We report a rare case of an ectopic BA that originated from the right thyrocervical trunk. It was detected by preprocedural MDCT angiography and was completely embolized to control massive hemoptysis.
Case presentation
An 82-year-old man with hemoptysis was admitted to our hospital. He had a history of bronchiectasis, but this was the first time he had ever developed hemoptysis. He had first gone to a neighboring hospital and received antibiotics intravenously. He was referred to our hospital for further evaluation and management. Despite conservative management, including aggressive antimicrobial treatment, the patient experienced massive hemoptysis on the third day after admission and underwent emergency BAE.
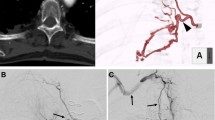
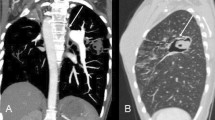
Before emergency BAE, he underwent preprocedural evaluation by contrast-enhanced computed tomography (CT) scan with a 128-slice scanner (Siemens SOMATOM Drive, Siemens Healthineers, Tokyo, Japan), with the arterial phase 30 s after, and the delayed phase 90 s after intravenous contrast administration (100 mL Oypalomin 370 mg/mL, Fuji Pharma, Tokyo, Japan) at 3 mL/s. The chest CT lung window imaging showed bilateral bronchiectasis and a large bulla with surrounding consolidation in the right lower lobe, which was thought to be the source of hemoptysis (Fig. 1). Furthermore, computed tomography angiography revealed an ectopic BA that arose from the right thyrocervical trunk, supplying the right lower lobe in addition to the normal right BA (Fig. 2). This ectopic BA was significantly hypertrophied compared to the normal right BA, suggesting that the ectopic BA was the culprit vessel of the hemoptysis (Fig. 3).
Superselective embolization of the ectopic BA was performed with gelatin sponge particles and metallic coils (Tornado, Cook Medical, Bloomington, USA) with a 1.9–2.9 Fr microcatheter (Breakthrough, Boston Scientific Japan, Tokyo, Japan) (Fig. 4A, B). After superselective embolization of the ectopic BA, the normal right BA was selectively embolized with gelatin sponge particles (Fig. 5A, B).
Hemoptysis was controlled by this procedure without any procedural complications. He was discharged on day 5 of hospitalization with no complications.
Discussion
The BAs are the source of massive hemoptysis in > 90% of cases (Lorenz et al. 2012). Since the report published in 1973 by Remy et al., BAE has been revealed as an effective technique for the control of massive hemoptysis. However, interventional radiologists who perform BAE should keep in mind that the BA may show anatomical variations in terms of origin, branching pattern, and course (Yoon et al. 2002). Furthermore, a minority of massive hemoptysis result from NBSAs or pulmonary arteries (Lorenz et al. 2012).
BAs originating apart from between the T5 and T6 vertebrae are considered ectopic, and the incidence of ectopicity has been reported to range from 8.3% to 36% (Michimoto et al. 2020). Ectopic BAs can be distinguished from NBSAs by their course along the major bronchi. On the other hand, NBSAs enter the pulmonary parenchyma through the adherent pleura or by way of the pulmonary ligament, and their course is not parallel to that of the bronchi (Sancho et al. 1998). In the present case, the branches of the right thyrocervical trunk ran along the main bronchus; therefore, this vessel was determined to be an ectopic BA rather than an NBSA.
Ectopic BAs originating from the thyrocervical trunk are rare. Summarizing several studies on MDCT angiography (Michimoto et al. 2020; Hartmann et al. 2007; Battal et al. 2011; Yener et al. 2015), only 9 of 624 patients (1.4%) had BAs originating from the thyrocervical trunk. This rate was lower than those of the subclavian (29/624; 4.6%) or internal mammary (11/624; 1.8%) artery origin. To our knowledge, most of the candidates for BAE for hemoptysis from a BA originating from the thyrocervical trunk have been reported to have a history of cystic fibrosis (Yoon et al. 2002; Hartmann et al. 2007; Battal et al. 2011; Yener et al. 2015). Furthermore, BAE was performed several times in these cases. In contrast, the patient in the present case had no history of cystic fibrosis and underwent BAE for the first time.
The rate of hemoptysis recurrence after BAE ranges from 9%–55% (Michimoto et al. 2020), and Zhao et al. reported that the main cause of clinical failure of BAE is incomplete embolization caused by misidentification of the culprit arteries by conventional angiography, especially for ectopic BAs and NBSAs (Zhao et al. 2017). A systematic review by Panda et al. (2017) reported that inadequate technique or incomplete embolization due to failure to detect all culprit arteries leads to early recurrence of hemoptysis within 3 months of BAE.
Many studies have reported that MDCT angiography is not only able to identify the source of bleeding and the underlying disease of hemoptysis, but also precisely detect the origins and courses of culprit arteries before BAE, which is especially advantageous for visualizing the ectopic origin of BAs and NBSAs, which are easily missed by conventional angiography during BAE (Zhao et al. 2017; Li et al. 2019; Lorenz et al. 2012; Michimoto et al. 2020; Panda et al. 2017). Li et al. (2019) suggested that preprocedural MDCT angiography can reduce the recurrence rate of hemoptysis after BAE, and recommended MDCT angiography as a routine examination before BAE in patients with hemoptysis as far as possible. In the present case, visualization of the ectopic BA from the right thyrocervical trunk on preprocedural MDCT allowed us to perform a successful BAE. We recommend that preprocedural MDCT be performed so that the thyrocervical trunk can also be evaluated. In this case, the region proximal to the ectopic BA was occluded, the patient was discharged, and no rebleeding was observed. However, Yoon et al. (2002) argue that a coil should not be used for BAE because re-embolization is precluded if hemoptysis recurs. Therefore, there was an option to not use proximal coiling of the ectopic BA.
Conclusion
We performed BAE in a patient with an ectopic BA of the right thyrocervical trunk. Preprocedural MDCT angiography is useful for visualizing the culprit arteries of hemoptysis, especially if a patient has an ectopic origin of BAs or NBSAs.
Availability of data and materials
Not applicable.
Abbreviations
- BAE:
-
Bronchial artery embolization
- BA:
-
Bronchial artery
- NBSAs:
-
Non-bronchial systemic arteries
- MDCT:
-
Multi-detector computed tomography
- CT:
-
computed tomography
References
Battal B, Akgun V, Karaman B, Bozlar U, Tasar M (2011) Normal anatomical features and variations of bronchial arteries: an analysis with 64-detector-row computed tomographic angiography. J Comput Assist Tomogr 35(2):253–259. https://doi.org/10.1097/RCT.0b013e3182073c27
Hartmann IJ, Remy-Jardin M, Menchini L, Teisseire A, Khalil C, Remy J (2007) Ectopic origin of bronchial arteries: assessment with multidetector helical CT angiography. Eur Radiol 17(8):1943–1953. https://doi.org/10.1007/s00330-006-0576-8
Li PJ, Yu H, Wang Y, Jiang FM, Wang W, Li XO, Wang Y, Liang ZA (2019) Multidetector computed tomography angiography prior to bronchial artery embolization helps detect culprit ectopic bronchial arteries and non-bronchial systemic arteries originating from subclavian and internal mammary arteries and improve hemoptysis-free early survival rate in patients with hemoptysis. Eur Radiol 29(4):1950–1958. https://doi.org/10.1007/s00330-018-5767-6
Lorenz J, Sheth D, Patel J (2012) Bronchial artery embolization. Semin Intervent Radiol 29(03):155–160. https://doi.org/10.1055/s-0032-1326923
Michimoto K, Takenaga S, Matsui Y, Enoki K, Nozawa Y, Higuchi T, Kano R, Kimura T (2020) Ectopic origin of bronchial arteries: still a potential pitfall in embolization. Surg Radiol Anat 42(11):1293–1298. https://doi.org/10.1007/s00276-020-02495-7
Panda A, Bhalla AS, Goyal A (2017) Bronchial artery embolization in hemoptysis: a systematic review. Diagn Interv Radiol 23(4):307–317. https://doi.org/10.5152/dir.2017.16454
Sancho C, Escalante E, Domínguez J, Vidal J, Lopez E, Valldeperas J, Montañá XJ (1998) Embolization of bronchial arteries of anomalous origin. Cardiovasc Intervent Radiol 21(4):300–304. https://doi.org/10.1007/s002709900265
Yener Ö, Türkvatan A, Yüce G, Yener AÜ (2015) The normal anatomy and variations of the bronchial arteries: evaluation with multidetector computed tomography. Can Assoc Radiol J 66(1):44–52. https://doi.org/10.1016/j.carj.2014.07.001
Yoon W, Kim JK, Kim YH, Chung TW, Kang HK (2002) Bronchial and nonbronchial systemic artery embolization for life-threatening hemoptysis: a comprehensive review. RadioGraphics. 22(6):1395–1409. https://doi.org/10.1148/rg.226015180
Zhao T, Wang S, Zheng L, Jia Z, Yang Y, Wang W et al (2017) The Value of 320-Row Multidetector CT bronchial arteriography in recurrent hemoptysis after failed transcatheter arterial embolization. J Vasc Interv Radiol 28:533–541.e1. https://doi.org/10.1016/j.jvir.2017.01.006
Acknowledgments
None.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
SC, KK, YoH, and YaH performed the computed tomography angiography evaluations and developed a strategy for bronchial artery embolization. SC and YoH performed the treatment. YM performed pre- and post-procedure interventional follow-ups. SC, NY, and YaH drafted the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures were in accordance with the ethical standards of the institution and with the 1964 Helsinki declaration. Informed consent was obtained from the patient in this case.
Consent for publication
Written informed consent was obtained from the patient for the publication of this case report and any accompanying images.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cho, S., Kubota, K., Hirose, Y. et al. Massive hemoptysis treated with embolization of an ectopic bronchial artery arising from the right thyrocervical trunk: a case report. CVIR Endovasc 5, 6 (2022). https://doi.org/10.1186/s42155-022-00285-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42155-022-00285-3