Abstract
Background
Hemoptysis is a life-threatening complication due to bleeding either from hypertrophied bronchial arteries or enlarged non-bronchial systemic collaterals, having multiple etiologies. Bronchial artery embolization (BAE) is a minimally invasive modality of management that can effectively manage moderate-to-severe hemoptysis.
Case presentation
We report the case of a 25-year-old female with moderate-to-severe hemoptysis. There was prior history of tuberculosis and treatment with anti-tubercular therapy 6 months back. There was also a background of tetralogy of Fallot(TOF) with symptoms of chronic breathlessness and palpitations. Imaging evaluation with X-ray and HRCT thorax revealed a cavity in the left upper lobe with dependent soft tissue, implying a diagnosis of aspergilloma in an old tubercular cavity. TOF and right-sided aortic arch were noted. CT bronchial angiography showed dilated and tortuous left bronchial artery, as well as non-bronchial systemic collaterals from the ipsilateral internal mammary artery. Endovascular management was achieved by super-selective catheterization and embolization of the involved branch of the left bronchial artery and selective embolization of non-bronchial systemic collaterals from the ipsilateral internal mammary artery.
Conclusions
BAE has a high clinical success rate and is recommended as first-line therapy in the management of massive hemoptysis. The CT pulmonary angiography, as well as the pre-embolization angiogram, is very important to detect the source of hemoptysis from the non-bronchial systemic circulation, increasing the success rate and decreasing the incidence of recurrence. BAE is effective even in presence of underlying vascular anomalies such as Fallot of Tetralogy.
Similar content being viewed by others
Background
Hemoptysis due to bleeding from enlarged and hypertrophied bronchial arteries and non-bronchial systemic collaterals is common and could be life threatening [1]. Most common etiologies are tuberculosis, pulmonary neoplasms, cystic fibrosis, bronchiectasis, chronic pulmonary aspergillosis [2] and congenital conditions like Tetralogy of Fallot (TOF) and pulmonary agenesis [3]. Along with the medical management of the underlying disease, bronchial artery embolization (BAE) is safe and effective therapy for moderate-to-severe hemoptysis [4].
Case presentation
We report a case of a 25-year-old female who came to our hospital with complaints of mild fever and recurrent moderate-to-severe hemoptysis (around 3 episodes over a week, approximately 350–500 ml blood loss). She was treated for active pulmonary tuberculosis and completed anti-tubercular treatment 6 months back. She was a known case of TOF and had related symptoms of chronic breathlessness and palpitations.
On physical examination, she was a malnourished, anemic (Hemoglobin- 11 g/dL) and had digital clubbing. BP at the time of admission was of 120/74 mm of Hg, a pulse of 110 beats per minute, and oxygen saturation of 90%. On respiratory examination, there were left upper lobe crepitations with reduced air entry in the left upper zone. A systolic murmur was also appreciated on cardiac auscultation. Rest of the systemic examination was unremarkable. Coagulation profile (Platelets—1.2 lacs, prothrombin time-13 s, international normalized ratio-1.2) was normal.
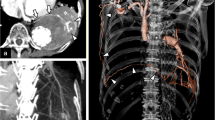
Chest radiography showed left upper lobe cavity with dependent soft tissue and associated pleural thickening indicating aspergilloma in old tubercular cavity. High-resolution computed tomography (HRCT) and CT angiogram were done which confirmed the findings of aspergilloma in tuberculous cavity with dilated and tortuous left bronchial artery(3.2 mm), arising as a branch of the left intercosto-bronchial trunk (Fig. 1). Also, some tortuous vessels were seen reaching the pleural thickening from the ipsilateral internal mammary artery. There was no evidence of Rasmussen aneurysm. Tetralogy of Fallot and right-sided aortic arch configuration was noted. In view of significant hemoptysis and dilated and tortuous bronchial arteries, endovascular embolization of bronchial arteries and non-bronchial systemic collaterals was planned. Using Sim 1 Catheter and Terumo J-shaped guidewire, selective angiography of left bronchial system was done. Findings revealed intercosto-bronchial trunk on the left side giving rise to the dilated and hypertrophied left bronchial artery (unusual anatomical variant). Super-selective catheterization of that branch was done using Progreat microcatheter (Terumo Interventional Systems, NJ, USA), and embolization was performed using 350u polyvinyl alcohol particles mixed with formed gelatin sponge slurry (Fig. 2). Similarly, selective cannulation of left subclavian artery was done using 5F picard catheter, followed by Progreat microcatheter (Terumo Interventional Systems, NJ, USA) to enter the left internal mammary artery, and embolization was performed using 350u polyvinyl alcohol particles mixed with formed gelatin sponge slurry embolization of non-bronchial systemic collaterals from the left internal mammary artery (Fig. 3). The patient’s postoperative course was uneventful. She was hemodynamically stable (blood pressure: 116/80 mmHg, heart rate around 72 bpm) and was discharged after 2 days with improving blood parameters (hematocrit 34%, hemoglobin 11.2 g/dL). She was then referred to cardiothoracic surgery for management of congenital heart disease.
A Digital subtraction angiography (DSA) image in arterial phase showing left intercosto-bronchial trunk (white arrow) with hypertrophied left bronchial artery and normal first intercostal artery. B Digital subtraction angiography (DSA) image in parenchymal phase showing marked parenchymal blush in region of left upper zone (white arrow). C Digital subtraction angiography (DSA) image after embolization with PVA particles shows complete disappearance of parenchymal blush
A Digital subtraction angiography (DSA) image after super-selective catheterization of the left internal mammary artery shows contrast blush representing collateral channels supplying the bronchial circulation (white arrow). B Post-embolization after PVA particles, the DSA images show complete absence of contrast blush (black arrow)
Discussion
Chronic pulmonary aspergillosis can result in moderate-to-significant hemoptysis which could be life threatening and needs emergent treatment [5]. Patients with acyanotic congenital heart disease are more susceptible to common infections like pulmonary tuberculosis(TB) [6]. However, few case reports are available in the literature describing the increased incidence of mycobacterium tuberculosis in cyanotic heart diseases like tetralogy of Fallot (TOF) [6]. Our patient was a diagnosed case of TOF with right-sided aortic arch and left-sided intercosto-bronchial trunk which is a very rare anomaly [3]. Aspergilloma is a form of chronic pulmonary aspergillosis that develops in a pre-existing lung cavity usually secondary to tuberculosis, sarcoidosis or bronchiectasis. The prevalence of aspergilloma in patients with pulmonary cavities secondary to pulmonary tuberculosis is 1.2 million worldwide [5] and is more common in Africa and south east Asia. Although the affected patients are mostly asymptomatic, however, moderate-to-massive hemoptysis has been reported predominantly due to hypertrophied bronchial artery and rarely secondary to pulmonary artery pseudo-aneurysm [5]. BAE is considered as first-line therapy in emergency management of massive hemoptysis with high clinical success and low complication rates [4]. The findings that make our study unique are the combination of cyanotic heart disease(TOF), right-sided aortic arch, left intercosto-bronchial trunk causing challenges in BAE in a patient with pulmonary tuberculosis complicated by aspergilloma. BAE can be complicated by non-target embolization resulting in major side effects like spinal cord ischemia, transient dysphagia, aortic dissection or cortical blindness, however, no such complication was encountered in our case [4]. On 3 months follow-up, our patient did not have any episode of hemoptysis and is planned for surgery for congenital heart disease.
Conclusions
TOF with right-sided aortic arch and left intercosto-bronchial trunk is a rare congenital anomaly. BAE has high clinical success rates and is recommended as a first-line therapy in the management of massive hemoptysis along with embolization of non-bronchial systemic circulation as diagnosed on pre-embolization angiograms.
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BAE:
-
Bronchial artery embolization
- TOF:
-
Tetralogy of Fallot
- HRCT:
-
High resolution computed tomography
References
Haponik EF, Fein A, Chin R (2000) Managing life-threatening hemoptysis: has anything really changed? Chest 118:1431–1435
Yoon W, Kim J, Kim Y, Chung T, Kang H (2002) Bronchial and nonbronchial systemic artery embolisation for life threatening hemptysis: a comprehensive review. Radiographics 22(6):1395–1409
Almeida J, Leal C, Figueiredo L (2020) Evaluation of the bronchial arteries: normal findings, hypertrophy and embolization in patients with hemoptysis. Insights Imaging 11(1):70
Sopko DR, Smith TP (2011) Bronchial artery embolization for hemoptysis. Semin Intervent Radiol 28(1):48–62
Ding WY, Chan T, Yadavilli RK, McWilliams R (2014) Aspergilloma and massive haemoptysis. Case Rep 2014:bcr2013200019
Van der Merwe PL, Kalis N, Schaaf HS, Nel EH, Gie RP (1995) Risk of pulmonary tuberculosis in children with congenital heart disease. Pediatr Cardiol 16:172–175
Acknowledgements
Not applicable.
Funding
The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
JK collected and interpreted the patient data, including the clinical and imaging details. VA performed the endovascular procedure and was a major contributor in writing the manuscript. AB had assisted in the data collection and image interpretation. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Varghese, J.K., Agarwal, V. & Batra, A. Endovascular management of hemoptysis in a known case of tetralogy of fallot and tuberculosis complicated with aspergilloma: a case report. Egypt J Radiol Nucl Med 53, 140 (2022). https://doi.org/10.1186/s43055-022-00816-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-022-00816-x