Abstract
Background
Intravenous dexmedetomidine and lidocaine have been shown to decrease perioperative surgical pain and analgesic consumption and facilitate the return of bowel function, decreasing post-operative hospitalization.
Results
On the first post-operative day, VAS score and total consumption of narcotics were statistically insignificant between the two groups. Heart rate and mean arterial pressure were significantly lower in the dexmedetomidine group than in the Lidocaine group all through the surgery (p value < 0.001). Post-operative nausea and vomiting were statistically higher in group D than in group L (p value 0.001).
Conclusions
The administration of either lidocaine or dexmedetomidine did not show superiority in post-operative analgesia or perioperative narcotics consumption. However, lidocaine infusion showed less drug-related side effects from the aspect of intraoperative hemodynamics stability, post-operative ileus, nausea, and vomiting.
Similar content being viewed by others
Background
Pain is one of the most distressing symptoms within the perioperative period and adequate management becomes more challenging to the anesthesiologist than before (Ljungqvist et al. 2017). Opioids are the gold standard to provide adequate analgesia in major abdominal surgery, although they have many adverse effects, including post-operative nausea and vomiting, respiratory distress, and paralytic ileus, which delay early ambulation and hospital dischage (Xu et al. 2017). Many protocols have been introduced toward Enhanced Recovery After Surgery (ERAS) guidelines that promote opioid-sparing multimodal analgesia (Zeltsman et.al., 2020) such as dexmedetomidine, non-steroidal anti-inflammatory drugs (NSAIDs), ketamine, magnesium and local anesthetic (lidocaine) to replace opioids (Salomé et al. 2021). The strategy of multimodal analgesia depends on the synergistic effect of multiple drugs which requires an adequate communication between the anesthesiologists, and surgeons with pre-operative patient education regarding the post-operative pain to improve satisfaction and reduce overall costs (Ramirez et al. 2020).
Dexmedetomidine is a potent, highly selective centrally acting α2-adrenoceptor agonist with sedative, anxiolytic, hypnotic, perioperative sympatholytic, and anesthetic sparing effects that reduce central sympathetic outflow and attenuate the stress response associated with surgery providing good perioperative hemodynamic stability (Li et al. 2016; Ibrahim et al. 2021). Intravenous lidocaine was introduced as an opioid-sparing systemic analgesic with additional anti-inflammatory and anti-hyperalgesic properties, making it a useful adjunct medication in multimodal analgesia in the early post-operative pain (Kramer et al. 2019; Oliveira and Eipe 2020).
Methods
After receiving approval from Ain Shams University, Faculty of Medicine, Research Ethics Committee (REC), FWA 000,017,585 (FAMASU M D 236/2019) and obtaining written informed consent, the interventional, randomized, and double-blinded trial was conducted in the institute hospital. Registration number is PACTR202109892575291.
One hundred and forty patients with American Society of Anesthesiologists (ASA) I and II, aged 21–55 years scheduled for elective open abdominal surgery having body mass index (BMI) between 18 and 25 kg/m2 with adequate cognitive state (able to understand and collaborate). The study exclusion criteria were hypersensitivity to any of the used drugs, pregnancy, and lactating mothers, chronic pain requiring daily use of opioid medication or chronic alcohol abuser, sleep apnea syndrome, bradycardia (heart rate of less than 50 beats per min at rest), or patients on beta-blockers or alpha 2 agonists, epilepsy or chronic daily use of psychiatric medication, patients received any other regional anesthesia technique (epidural, transverse abdominis plane (tap) or plexus block), and re-exploration cases were also excluded from the study.
Study groups
The patients were randomized into two groups by using computer-generated random sequences to prevent skewing or deliberate manipulation of results. Patients were given a random number from 1 to 140 and were allocated into one of two parallel groups with a ratio of 1:1 between the two groups. The dexmedetomidine group (group D) received 1 μg/kg loading dose over 10 min, followed by 0.5 μg/kg/h. and the lidocaine group (group L) received 1.5 mg/kg loading dose over 5 min (lidocaine 2%), followed by 1 mg/kg/h. The infusions were continued 24 h post-bolus dose. Blinded solutions were prepared by the pharmacist to be of the same volume and to the targeted doses of which is started immediately after intubation.
After full history was obtained, including age, history of chronic diseases, previous surgeries, drug allergies, and regular drug intake, the patients were fully examined, and vital data were measured and recorded.
Preoperatively, patients were taught how to evaluate their pain intensity using the VAS score, scored from 0 to 10 (where 0 = no pain and 10 = worst imaginable pain) (Cachemaille et al. 2020).
On arrival at the operating room, two intravenous lines were inserted in both upper limbs and all patients were premeditated with 2 mg of IV midazolam. Intraoperative monitoring included standard ASA monitors; electrocardiography (ECG), non-invasive blood pressure (NIBP), arterial oxygen saturation (SpO2), end-tidal carbon dioxide (EtCO2), body temperature using nasopharyngeal probe. Blood loss assessment was done throughout the surgeries as well.
All patients received general anesthesia, induction was done by 2 μg/kg fentanyl, 2–3 mg/kg propofol and endotracheal intubation was facilitated by 0.5 mg/kg atracurium.
All weight-based dosing was calculated on ideal body weight (IBW).
Anesthesia was maintained by 1–1.5 MAC isoflurane (according to the patient’s age) in 50% oxygen/air mixture (no nitrous oxide), and 0.1 mg/kg atracurium every 20 min, and ventilation parameters that maintain normocapnia (CO2 between 35 and 40 mmHg). [volume control mode, tidal volume 6–8 ml/kg, RR 12–14 b/min, peak respiratory pressure < 30 mmhg]. IV crystalloids (in the form of ringer lactate) were used for maintenance and to replace the deficit as needed according to a fluid chart. Fentanyl (0.5–1.5 μg/kg) was used as a rescue for pain suggested by A 20% increase in heart rate and /or arterial blood pressure from the preoperative baseline.
At the end of the surgery, reversal of muscle relaxant was done by using neostigmine (0.04 mg/kg) and atropine (0.01 mg/kg). After extubation, all patients were transmitted to the post-anesthesia care unit (PACU). Patients were discharged from the recovery unit based on modified Aldrete score.
Post-operative analgesia was in form of:
-
Regular dose of Ketorolac 30 mg per dose for a maximum 60 mg per day.
-
Pethidine 50 mg intravenous bolus once the pain is expressed by the patient or if VAS was ≥ 3.
Outcome’s measurements
All patients were followed up and assessed at baseline (time of administration of studying drug intraoperatively, (on arrival to PACU, 1 h, 2 h, 4 h, and every 6 h up to 24 h post-operatively for hemodynamics (blood pressure, heart rate), VAS score, total consumption of narcotics and post-operative occurrence of side effect as episodes of nausea and vomiting and time of the return of bowel movement. While the primary outcome was total dose consumption of intraoperative opioids.
Sample size calculation
Using G power program, setting alpha error at 5% and power at 80% after reviewing of literature, no previous similar study was done comparing between the two study groups (dexmedetomidine and lidocaine in 24 h) as regards the mean post-operative dose of analgesia. So, assuming an effect size of 0.5 (Cohen d) between the two groups regarding mean dose produced a sample size of 64 cases per group (128 total) taking an account 10% drop out rate, Sample Size will be 140 patients (70 patients in each group.
Data management and analysis
Data were analyzed using Statistical Package for Social Science (SPSS) version 18.0. Quantitative data were expressed as mean ± standard deviation (SD). Qualitative data were expressed as frequency and percentage.
The following tests were done:
-
Independent-samples ANOVA-test of significance was used when comparing between two means and variance.
-
Chi-square (χ2) test of significance will compare used to compare proportions between two qualitative parameters.
-
Probability (p value): p value < 0.05 is considered significant.
Results
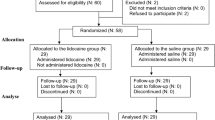
All the one hundred and forty patients successfully completed the study. The demographic data of both groups were statistically comparable, as shown in Table 1.
No significant differences were observed intraoperatively in terms of hemodynamic parameters regarding the heart rate at the baseline readings. However, patients in the dexmedetomidine group had a significantly lower heart rate at 60, 120, 180, and 140 min during the surgery, as shown in Table 2 and Fig. 1. There was no statistically significant difference as regards HR in the post-operative period as well, as shown in Table 2 and Fig. 2.
Table 3 and Fig. 3. Shows no significant differences regarding the intraoperative mean arterial pressure at the baseline readings. Additionally, it was significantly decrease in dexmedetomidine group at 60, 120, 180, and 240 min. There was no statistically significant difference as regards mean arterial pressure in the post-operative period as well, as shown in Table 3 and Fig. 4.
Table 4 and Fig. 5 show the difference in VAS score rated during the first 24 post-operatively. There was no significant difference at any point in time between the two groups. Additionally, the analgesic need in both groups shows insignificant changes, as shown in Table 5.
As regards the post-operative side effect, the difference was significantly lower in group L as shown in Table 6. There was no significant difference either in the time of passage of flatus, or the time of ambulation in the two groups, as shown in Table 6.
Discussion
There is a limited data regarding the comparison between lidocaine and dexmemotidine infusion in elective open abdominal surgery as the majority of current papers top the scope of laparoscopic surgeries, which have less pain score. In our study, we found that there is no statically difference between the two groups during the 1st 24 h post-operative as regards VAS score and total consumption of perioperative of opioids. Additionally, our results were supported by SI-QI XU et al. (Xu et al. 2017), who reported the same outcome on women undergoing abdominal hysterectomy who received either dexmedetomidine (0.5 μg/kg loading followed by 0.4 μg/kg/h infusion) or Lidocaine 1.5 mg/kg loading followed by 1.5 mg/kg maintenance) during the 48 h post-operatively when compared to the control group.
However, dexmedetomidine showed an improvement in post-operative outcomes when used as a part of ERAS protocols (Li et al., 2021; Kaye et al. 2020). Previous studies have shown significant improvement in post-operative pain in patients undergoing abdominal surgeries who received dexmedetomidine (Tseng et al. 2021) or lidocaine (Sridhar et al. 2015; Harvey et al. 2009) when compared to control groups.
Perioperative opioids produce unfavorable adverse effects as post-operative nausea and vomiting (PONV) and post-discharge nausea and vomiting (PDNV) which are the most common complications following surgery which slow the speed of recovery and prolong hospitalization.
In our study, the episodes of PONV were significantly lower in the lidocaine group in comparison to the dexmedetomidine group, which might have been derived from the anti-inflammatory effects of lidocaine.
The result of the present study are in agreement with those done by (Sridhar et al. 2015), in open abdominal surgery, the authors reported a lesser incidence of PONV when lidocaine was used during the operation.
Significant findings by (S. Xu et al. 2021), observed that there was no significant difference in PONV in patients either receiving lidocaine or Dexmedetomidine when compared to control groups. However, they found that co-administration of lidocaine and dexmedetomidine resulted in a lower incidence of PONV.
Patients were assigned to lidocaine in the study done by (Farag et al. 2013). There was no difference between the two groups regarding post-operative nausea or vomiting, perhaps the distinction might be made on the difference in surgical type, lidocaine dose, and duration of administration (the study was approximately 8 h).
Regarding the analysis of mean arterial pressure, our study found that there was a significant reduction in mean arterial pressure in the dexmedetomidine group in comparison to the lidocaine group. This can be explained by the sympatholytic effect of dexmedetomidine. In contrary to our results, the study done by (Ge et al. 2015) administrated a continuous dose of dexmedetomidine (0.4 mg/kg/h) in a patient undergoing abdominal colectomy; they did not see any significant changes in the mean arterial pressure, this was explained by using a continuous dose with an omission of a loading dose.
Also according to a double-blinded, randomized control study by (Weinberg et al. 2017) reported that intraoperative infusion of lidocaine reduces mean arterial pressure during open radical prostatectomy in comparison to the control group, although the maintenance dose is as high as 1.5 mg/kg/h.
Our study observed significant bradycardia in the dexmedetomidine group, which requiring intervention by atropine in comparison to the lidocaine group.
In dis-concordance to our results, Ibrahim et al. (Ibrahim et al. 2021), demonstrated that a dexmedetomidine infusion without loading dose does not produce significant bradycardia that requires intervention by atropine. Li et al. (2016) studied the patients anesthetized with dexmedetomidine in elective open gastrectomy. The patients did not show any episodes of bradycardia during the operation. This may be explained by using a moderate maintenance dose of dexmedetomidine (0.4 μg/kg/h).
Return of bowel function is important to discharge the patient from the hospital. Our study showed no significant difference in both groups regarding recovery of bowel movement as well as earlier time in first flatus passages.
Similarly, (Staikou et al. 2014), found no statistical difference between intravenous lidocaine and the other group using lumbar epidural with lidocaine as an analgesic in patients undergoing major abdominal surgery. Also, (Ho et al. 2018), reported no significant difference between lidocaine and the control group in speed in return of bowel function.
In dis-concordance to our result, (Dai et al. 2020) observed that lidocaine in 1.5 mg/kg/h. was superior to the control group in return of bowel movement during gastrointestinal surgery (Saadawy et al. 2010), also reported that lidocaine exhibited a rapid return of bowel function compared with control groups in a patient undergoing laparoscopic cholecystectomy.
Limitation
The main limitations were the small sample size and the usage of a subjective score (VAS). Moreover, some patients in the preoperative period were on non-opioid analgesics to control the abdominal pain which may affect our measurements. However, our results provide clues for further investigation.
Conclusions
The administration of either lidocaine or dexmedetomidine did not show superiority in post-operative analgesia or perioperative narcotics consumption. However, lidocaine infusion showed less drug-related side effects from the aspect of intraoperative hemodynamics stability, nausea, and vomiting.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ASA:
-
American Society of Anesthesiologists
- ERAS:
-
Enhanced Recovery After Abdominal Surgery
- NSAIDs:
-
Non-steroidal anti-inflammatory drugs
- BMI:
-
Body mass index
- tap:
-
Transverse abdominis plane
- VAS:
-
Visual analog scale
- SpO2 :
-
Arterial oxygen saturation
- EtCo2:
-
End-tidal carbon dioxide
- IBW:
-
Ideal body weight
- PACU:
-
Post-anesthesia care unit
- CBD:
-
Common bile duct
- PONV:
-
Post-operative nausea and vomiting
- Iv:
-
Intravenous
- SD:
-
Standard deviation
- PDNV:
-
Post-discharge nausea and vomiting
References
Alan David Kaye,David J.Chernobylsky,Pankaj Thakur,Harish Siddaiah,Rachel j.Kaye,lauren K.Eng,Monica W.Harbell,Jared Lajaunie and Elyse M.Cornett (2020).Dexmedetomidine in enhanced recovery after surgery protcolos for postoperative pain.current pain and headache reports.24:21.
Cachemaille M, Grass F, Fournier N, Suter MR, Demartines N, Hübner M, Blanc C (2020) Pain intensity in the first 96 hours after abdominal surgery: A prospective cohort study. Pain Med 21(4):803–813
Dai Y, Jiang R, Su W, Wang M, Liu Y, Zuo Y (2020) Impact of perioperative intravenous lidocaine infusion on postoperative pain and rapid recovery of patients undergoing gastrointestinal tumor surgery: A randomized, double-blind trial. J Gastrointest Oncol 11(6):1274
De Oliveira K, Eipe N (2020) Intravenous lidocaine for acute pain: a single-institution retrospective study. Drugs-Real World Outcomes 7(3):205–212
Farag E, Ghobrial M, Sessler DI, Dalton JE, Liu J, Lee JH, Zaky S, Benzel E, Bingaman W, Kurz A (2013) Effect of perioperative intravenous lidocaine administration on pain, opioid consumption, and quality of life after complex spine surgery. Anesthesiology 119(4):932–940
Ge DJ, Qi B, Tang G, Li JY (2015) Intraoperative dexmedetomidine promotes postoperative analgesia and recovery in patients after abdominal colectomy: a CONSORT-prospective, randomized, controlled clinical trial. Medicine Baltimore. 94(43):e1727
Harvey KP, Adair JD, Isho M, Robinson R (2009) Can intravenous lidocaine decrease postsurgical ileus and shorten hospital stay in elective bowel surgery? A pilot study and literature review. ANZ J Surg 198(2):231–236
Ho MLJ, Kerr SJ, Stevens J (2018) Intravenous lidocaine infusions for 48 hours in open colorectal surgery: a prospective, randomized, double-blinded, placebo-controlled trial. Korean J Anesthesiol 71(1):57
Huai-Jin Li,Chun-Jing Li, Xiao -NA Wei,Jian Hu, Dong-Liang Mu, Dong-Xin Wang (2018).Dexmedetomidine in combination with morphine improves postoperative analgesia and sleep quality in eledry patients after open abdominal surgery.a pilot randmoized control trial .PLoS ONE13(8)
Ibrahim IM, Hassan R, Mostafa RH, Ibrahim MA (2021) Efficacy of dexmedetomidine infusion without loading dose on hemodynamic variables and recovery time during craniotomy: a randomized double-blinded controlled study. Anesthesiol Pain Med. 11(2):e113410
Kramer ME, Holtan EE, Ives AL, Wall RT (2019) Perioperative Intravenous Lidocaine Infusion Adverse Reaction: a Case Report. AA Pract 13(3):96–98
Li, Y., Wang, B., Zhang, L., He, S., Hu, X., Wong, G. T. C., & Zhang, Y. (2016) Dexmedetomidine combined with general anesthesia provides similar intraoperative stress response reduction when compared with a combined general and epidural anesthetic technique.
Ljungqvist O, Scott M, Fearon KC (2017) Enhanced Recovery after Surgery: a Review. JAMA Surg 152(3):292–298
Ramirez, M. F., Kamdar, B. B., & Cata, J. P. (2020) Optimizing Perioperative Use of Opioids: A Multimodal Approach Curr. Anesthesiol. Rep., 1–12.
Saadawy IM, Kaki AM, Abd El Latif AA, Abd-Elmaksoud AM, Tolba OM (2010) Lidocaine vs magnesium: effect on analgesia after a laparoscopic cholecystectomy. Acta Anaesthesiol Scand. 54(5):549–556
Salomé A, Harkouk H, Fletcher D, Martinez V (2021) Opioid-free anesthesia benefit–risk balance: a systematic review and meta-analysis of randomized controlled trials. J Clin Med 10(10):2069
Sridhar P, Sistla SC, Ali SM, Karthikeyan VS, Badhe AS, Ananthanarayanan PH (2015) Effect of intravenous lignocaine on perioperative stress response and post-surgical ileus in elective open abdominal surgeries: a double-blind randomized controlled trial. ANZ J Surg 85(6):425–429
Staikou C, Avramidou A, Ayiomamitis GD, Vrakas S, Argyra E (2014) Effects of intravenous versus epidural lidocaine infusion on pain intensity and bowel function after major large bowel surgery: a double-blind randomized controlled trial. J Gastrointest Surg 18(12):2155–2162
Tseng W, Lin W, Lai H, Chen T, Chiu Y, Chen P, Wu Z (2021) Adjunctive dexmedetomidine infusion in open living donor hepatectomy: a way to enhance postoperative analgesia and recovery. Int J Clin Pract 75(5):e14002
Weinberg L, Jang J, Rachbuch C, Tan C, Hu R, McNicol L (2017) The effects of intravenous lignocaine on depth of anaesthesia and intraoperative haemodynamics during open radical prostatectomy. BMC Res Notes 10(1):1–7
Xu S-Q, Li Y-H, Wang S-B, Hu S-H, Ju X, Xiao J-B (2017) Effects of intravenous lidocaine, dexmedetomidine and their combination on postoperative pain and bowel function recovery after abdominal hysterectomy. Minerva Anestesiol 83(7):685–694
Xu S, Wang S, Hu S, Ju X, Li Q, Li Y (2021) Effects of lidocaine, dexmedetomidine, and their combination infusion on postoperative nausea and vomiting following laparoscopic hysterectomy: a randomized controlled trial. BMC Anesthesiol. 21(1):1–10
Zeltsman DO, M., Aronsohn MD, J., Dowling PhD, O., Gerasimov MD, M., & Palleschi MD, G. (2020) Opioid-free anesthesia in bariatric surgery: a retrospective cohort study.
Acknowledgements
Not applicable.
Funding
None.
Author information
Authors and Affiliations
Contributions
F.H.I.A. revised literature, followed the patients, measured and collected demographic data preoperative, intraoperative, and post-operative data, performed the block, and critically reviewed the manuscript. S.A.H. designed the study, revised literature, performed the analysis followed the patients, measured objective pain score, and wrote the manuscript. H.M.A. designed the study, performed the analysis, and wrote and critically revised the manuscript. A.H.R. revised literature, performed the analysis, and critically reviewed the manuscript. M.S.L. revised literature, followed the patients, measured and collected demographic data preoperative, intraoperative, and post-operative data, and reviewed the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
After receiving approval from Ain Shams University, Faculty of Medicine, Research Ethics Committee (REC), FWA 000017585 (FAMASU M D 236/2019) and obtaining written informed consent, the interventional, randomized, and double-blinded trial was conducted in the institute hospital. Registration number is PACTR202109892575291.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ibrahim, F.H., Mohamed, S.A., Hamid, H.M.A.E. et al. The effect of intravenous infusion of dexmedetomidine versus lidocaine as an analgesic adjuvant to balanced general anesthesia and enhanced recovery after abdominal surgery. Ain-Shams J Anesthesiol 14, 59 (2022). https://doi.org/10.1186/s42077-022-00258-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42077-022-00258-7