Abstract
Background
Over the years, sevoflurane has been proven to be the most effective inhalational anesthetic for induction and maintenance of general anesthesia in pediatrics. However, one of the downsides of utilizing sevoflurane anesthesia in pediatrics is emergence agitation (EA). A variety of drugs have been evaluated for prophylaxis against the occurrence of EA. Both magnesium and ketamine were evaluated in controlling this phenomenon; however, the usefulness of using intraoperative magnesium and ketamine infusions in preventing EA is still debatable.
Methods
Fifty-two children aged 4–7 years who were having elective lower abdominal or pelvic surgeries under sevoflurane anesthesia were enrolled then allocated randomly into one of two groups (n = 26 each). The magnesium group (M) included 26 patients who received intravenous (IV) loading dose of magnesium 15 mg/kg before the surgical incision followed by IV infusion 10 mg/kg/h over the duration of surgery, while the ketamine group (K) included 26 patients who received an IV loading dose of ketamine 1 mg/kg before the surgical incision, then IV infusion 1 mg/kg/h over the duration of surgery. For each patient, the agitation score (Pediatric Anesthesia Emergence Delirium [PAED] scale) and pain score, as well as the time to endotracheal tube (ETT) removal, regain of mental orientation, and transfer from the post-anesthesia care unit (PACU) to ward were all documented.
Results
Intraoperative magnesium sulfate infusion showed a lower PAED score on immediate arrival to PACU than intraoperative ketamine infusion, with less time to tracheal extubation, recovery of mental orientation, and discharge from PACU with a P value below 0.001, while there was no statistical significance between both groups as regards PAED score after 30 min in PACU, pain score, or perioperative vital data.
Conclusions
Magnesium sulfate was found superior to ketamine in lowering the severity of the postoperative agitation in pediatric patients undergoing abdominal or pelvic surgeries under sevoflurane anesthesia. Also, patients restored their consciousness and mental orientation faster in the magnesium group compared to ketamine. This has increased the PACU stay in the ketamine group.
Similar content being viewed by others
Background
Emergence agitation (EA) is a postoperative phenomenon which is observed in children after sevoflurane anesthesia, with an occurrence rate of up to 80% (Abdelhalim and Alarfaj, 2013). It presents with behavioral changes such as irritability, agitation, inconsolable sobbing, mental confusion, some psychotic traits such as hallucinations and delusions, and cognitive impairment (Abu- Shahwan and Chowdary, 2008). Emergence agitation is diagnosed by a final composite score of greater than or equal to 10 on the Pediatric Anesthesia Emergence Delirium Scale (PAED) (Appendix, Table 4), a scale which measures 5 emergence behaviors in children, and each of which is assessed on a 5-point scale (Stamper et al., 2014, Sikich and Lerman, 2004).
Although its exact causes are unknown, the cause of EA may be multifactorial with patient-related, anesthesia-related, and surgical factors (Lee and Sung, 2020). One of the postulated anesthetic causes of EA is sevoflurane use (Vlajkovic and Sindjelic, 2007). Sevoflurane is a volatile anesthetic agent preferred in the initiation and maintenance of general anesthesia in pediatric patients due to its rapid onset and termination of action as well as its low pungency and non-irritating effect on the airways (Kawai et al., 2019).
Effective treatment of EA due to sevoflurane use requires an understanding of all the possible mechanisms underlying its causation (Bae et al., 2010). One of the proposed treatments for EA is the use of opioids; however, it carries the risk of an extended Post Anesthetic Care Unit (PACU) stay resulting in parents’ discomfort and added costs (Alghamdi et al., 2020). Therefore, analgesic adjuvants with NMDA (N-methyl-d-aspartate) receptor antagonist functions, such as ketamine (Chen et al., 2013) and magnesium sulfate (Abdulatif et al., 2013), have been tried to control this phenomenon in children.
As a sedating analgesic, ketamine was found to be successful in the management of EA in children but was associated with a delay in recovery (Chen et al., 2013). Also, according to Ng et al. (2019), the recommendations for ketamine use for the prevention of EA in children may be limited by a lack of confidence in the evidence.
Aim of the study
The aim of this study was to investigate the effectiveness of intraoperative ketamine versus magnesium sulfate infusions on reducing the incidence as well as the severity of emergence agitation that follows anesthesia using sevoflurane immediately and after 30 min in PACU.
We hypothesized that the intraoperative magnesium sulfate would be superior to ketamine in reducing the incidence and severity of emergence agitation in pediatric patients undergoing pelvic and abdominal operations under sevoflurane anesthesia.
Methods
The current study was approved by the Ethical Committee of Faculty of Medicine, Ain Shams University—FM ASU MD 302/2019 and retrospectively registered by PACTR, PACTR202109686219888. Registered 14 September 2021—http://www.pactr.org/PACTR202109686219888. It was conducted in Pediatric Surgery Operating Theatre at Ain Shams University Hospitals (El Demerdash Hospital).
Study design
This is a single-blinded randomized clinical trial. Sample size was calculated using PASS®11 (PASS, 2019) assuming that the mean PAED score in the magnesium sulfate group = 5 ± 1 (Abdulatif et al., 2013) and in the ketamine group = 4 ± 1.5 (Chen et al., 2013); sample size of 26 patients in each group achieves 80% power to detect this difference at 0.05 significance. A computer-generated randomization with closed envelopes was used in this study to divide 52 patients into two equal groups.
All participants’ parents or legal guardians submitted an informed written consent. Patients aged 4 to 7, with an American Society of Anesthesiologists (ASA)—Physical Status Classification System of I-II, planned for abdominal or pelvic surgeries lasting less than 2 h, such as inguinal hernia repair, circumcision, appendicectomy, or hydrocele repair were included in this study.
Behavioral changes, cerebral palsy, and other neurological abnormalities, any type of physical and/or developmental challenge, patients on sedatives or anticonvulsant medications, those with coexisting renal or cardiovascular diseases, and legal guardian refusal were all considered exclusion factors.
An anesthesiologist who was not engaged in the trial opened the sealed envelope to prepare the medication solution according to randomization. The doctor who administered the given drug as well as monitored the patient was blinded to the treatment group. In addition, the information was gathered by the same anesthesiologist, who was oblivious of the grouping.
Preoperative assessment and clinical examination were done which included auscultation of the chest for any signs of chest infection or bronchospasm, Blood pressure, heart rate, and temperature were recorded before surgery, as well as the children’s behavior. Routine tests were performed, including a complete blood count (CBC).
The patients were not given any premedication beforehand. All the patients in the study had fasted for 6 h.
Electrocardiography (ECG), non-invasive blood pressure (NIBP), and pulse oximetry (SpO2) altogether are part of standard monitoring, and in the operating room, basal measures of NIBP, SPO2, and heart rate were taken.
Anesthesia was induced with 8% sevoflurane until the patient lost consciousness, then an intravenous cannula (22G) was introduced, and 10 mL/kg lactated Ringer’s solution was given over a 20-min period, followed by fluid adjustments according to body weight. For facilitation of tracheal intubation, 1 μg/kg IV fentanyl and 0.5 mg/kg atracurium were administered. All patients were mechanically ventilated, with ventilator settings adjusted to achieve normocarbia and keep end-tidal sevoflurane between 2.5 and 3.5% while keeping heart rate and blood pressure around 20% of reference values.
Following induction, the blinded drug, being ketamine or magnesium sulfate according to previous allocation of patients, was given as a loading dose over 10 min before surgical incision, then continued as IV infusion for the entire duration of surgery.
Drugs were given in the following doses:
For the magnesium group (M): 26 patients received IV magnesium as a loading dose 15 mg/kg diluted in 0.9% NaCl given over 10 min following induction of anesthesia and before the surgical incision followed by 10mg/kg/h IV infusion for the entire duration of surgery. Concentration of solution did not exceed 1 gm/25 mL (40 mg/mL). For the ketamine group (K): 26 patients received intravenous (IV) ketamine 1mg/kg diluted in 0.9% NaCl as a loading dose over 10 min following induction of anesthesia and before the surgical incision, then 1mg/kg/h IV infusion throughout the duration of surgery.
At the end of surgery, the infusions of both medications and sevoflurane were stopped. For neuromuscular reversal, patients were administered neostigmine and atropine at a dose of (0.05 mg/kg) and (0.01–0.02 mg/kg), respectively, and then permitted to breathe on their own. After regaining consciousness, patients who were obeying orders, hemodynamically stable, normothermic, with positive gag reflex, spontaneous breathing of satisfactory tidal volumes, rate of respiration below 20 breaths/min, normocarbic, and showing clinical evidence of neuromuscular reversal were extubated after endotracheal and oral suctioning. Patients were observed for signs of laryngeal spasm or respiratory distress, which would necessitate additional attention and therapy.
Patients were then moved to the PACU safely. Patients were observed in the PACU for 30 min before being discharged to ward if they met the modified Aldrete score (Appendix, Table 5) (Aldrete, 1995).
The PAED score was recorded just after transfer to PACU and after 30 min. In addition, the time required to extubate, regain mental orientation, and discharge from PACU, as well as the postoperative pain score according to the Faces Pain Scale (Fig. 1) were all recorded.
Faces pain scale (Belville et al 2005)
Statistical analysis
Statistical analysis was conducted by the aid of IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp.
The mean, standard deviation (SD), percentage, median, and interquartile range (IQR) were used to describe the data. In quantitative data, the unpaired Student’s t-test was employed to compare two groups. The chi-square test and Fisher’s exact test were used to study categorical variables. The PAED scores and the objective pain score were compared using the Mann-Whitney U test.
P value ≤ 0.05 is considered statistically significant.
Results
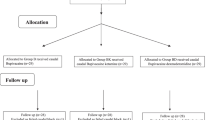
Fifty-two patients were screened to identify their eligibility for the trial. None of the patients were excluded from the trial. Each group had 26 patients who were followed up on (Fig. 2). At the start of the trial, demographic data such as age, gender, and weight were collected (Table 1).
No statistically significant difference was recorded between both groups as regards duration of surgery as shown in (Table 1).
The PAED score was obtained upon arrival to PACU and after 30 min, and the data was statistically compared between both groups, as shown in (Fig. 3). On admission to PACU, a statistically significant difference is observed between both groups (P < 0.001). However, after 30 min, both magnesium and ketamine demonstrated a decreased PAED score with no statistically significant difference (Fig. 3).
Figure 4 illustrates the difference in the percentage of agitated patients, described as PAED score ≥ 10, and non- agitated patients, described as PAED score < 10, between both groups on arrival to PACU and after 30 min.
Table 2 shows a statistical difference between both groups as regards time to tracheal extubation, return of mental orientation, and discharge from the PACU.
Table 3 shows no statistically significant difference between both groups as regards perioperative vital data.
Postoperative pain was assessed using the Faces Pain Scale. There was no statistically significant difference between both groups as shown in (Fig. 5).
There was no respiratory depression, laryngeal spasm, or postoperative vomiting recorded in both groups.
Discussion
This study revealed that intraoperative magnesium sulfate infusion resulted in a lower PAED score on immediate arrival to PACU than intraoperative ketamine infusion, with less time for tracheal extubation, resumption of mental orientation, and discharge from PACU, whereas no statistically significant difference was observed between the tested groups as regards PAED score after 30 min in PACU, pain score, or perioperative vital data.
In the current study, magnesium showed significantly lower PAED scores on immediate arrival to PACU compared to ketamine. The findings are supported by the study of Abdullatif and others, in 2013, which stated that magnesium sulfate infusion reduces the frequency and intensity of EA in pediatric patients who underwent adenotonsillectomy under general anesthesia using sevoflurane. This lowered EA was assumed to be due to the neuroprotective and anticonvulsant effects of magnesium sulfate. However, the prevalence of EA in Abdullatif et al. was 36%, which is higher than the current study (11.5%); this can be attributed to the type of surgery and the presence of obstructive sleep apnea in children presented for adenotonsillectomy. Furthermore, Xie et al. (2017) mentioned that intraoperative magnesium sulfate infusions can significantly reduce EA in patients undergoing surgical intervention for esophageal carcinoma under sevoflurane anesthesia, without delayed muscle recovery or electrolyte impairment.
Also, a study published in 2017 in Elsersy et al. (Elsersy, Metyas, Elfeky & Hassan, 2017) discovered that giving magnesium intraoperatively to patients having endoscopic sinus surgery reduced postoperative agitation compared with a control group. In addition, Lee et al. in 2020 (Lee et al. 2020) found that using intraoperative magnesium sulfate infusion considerably reduced the occurrence of EA in children after ambulatory ophthalmic operations from 77.3% (control group) to 57.1% (magnesium group).
As regards pain management and analgesic requirements, magnesium sulfate was found to help alleviate postoperative pain, shorten PACU stay, and decrease the intensity of postoperative EA. This was documented in a study by Hussein et al. in 2019 (Hussein, Mohamed & Mahmoud, 2019) where intraoperative use of magnesium sulfate in patients who undergo nasal operations reduced postoperative EA, pain severity, and pethidine administration in the postoperative phase compared to the control group.
In contrast, Apan et al. (2010) reported that magnesium sulfate has no effect on sevoflurane-induced EA, this can be explained by the study methodolgy as they used a bolus of magnesium rather than infusion. The same results were interpreted by Lee et al. in 2020, concluding that magnesium administration during anesthesia had no considerable impact on the frequency of EA or postoperative pain in pediatrics who did strabismus surgery. In addition, a study published by Khatiwada et al. (2020) indicated that intraoperative magnesium sulfate does not reduce EA in children between the ages of 3 to 12 years receiving general anesthesia for hernia and hydrocele operations.
As regards delayed recovery and postoperative side effects, both studies by Abdulatif et al. (2013) and Lee et al. in 2020 (Lee et al. 2020) found that no perioperative side effects or delayed emergence were linked to giving magnesium sulfate, which was consistent with the current study results.
However, this was opposed by a study performed by (Bondok & Ali, 2014) where patients who took Magnesium had a considerably longer emergence time compared with the control group receiving saline.
No studies were found to the recent knowledge comparing between the effect of magnesium sulfate and ketamine on incidence and severity of EA.
Regarding ketamine, Chen and others in 2013 conducted a study involving 84 children who underwent strabismus surgery under sevoflurane anesthesia and were given either saline, dexmedetomidine, or ketamine (1 mg/kg IV then 1 mg/kg/h IV infusion). The incidence of EA was found to be lower with ketamine (22%) compared to saline (44%). These results were consistent with the current study.
In children undergoing elective eye surgery, Kim and colleagues (2016) found that the EA was considerably lower with ketamine (24.2%) than midazolam, which is also consistent with the results of the current study.
Another finding by Chen et al. in 2013 was that the average times for regain of mental orientation in ketamine groups were considerably prolonged compared with those in the placebo group, which was consistent with the results of the current trial.
On the contrary, as previously mentioned, according to Ng et al. (2019), the recommendations for ketamine use for the prevention of EA in children may be limited by a lack of confidence in the evidence.
Some of the study limitations are the lack of recording of the magnesium-related side effects as well as improving the efficiency of NMB through employing a train of four monitoring and evaluating serum magnesium levels. We recommend the use of the bispectral index in future research for monitoring the depth of anesthesia, and pain would be eliminated as a confounder in studies via caudal or other regional blocks. If replicated, these results could pave the way for the use of more available medications for emergence agitation prevention practices.
Conclusions
Magnesium sulfate was found superior to ketamine in reducing the severity of the postoperative agitation in pediatric patients undergoing abdominal or pelvic surgeries under sevoflurane anesthesia. The patients also experienced faster restoration of consciousness and orientation in the magnesium group compared to ketamine. This has increased the PACU stay in the K-group.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available due [Publishing the clinical data about any study conducted in our hospitals and approved by the institutional ethical committee is against the policy of the Faculty of medicine, Ain Shams university unless there is a reasonable request] but are available from the corresponding author on reasonable request.
Abbreviations
- ASA:
-
American Society of Anesthesiologists
- Bpm:
-
Beats per minute
- CBC:
-
Complete blood count
- EA:
-
Emergence agitation
- ECG:
-
Electrocardiography
- ETT:
-
Endotracheal tube
- HR:
-
Heart rate
- IQR:
-
Interquartile range
- K:
-
Ketamine
- M:
-
Magnesium sulfate
- mmHg:
-
Millimeter of mercury
- NaCl:
-
Sodium chloride
- NIBP:
-
Non-invasive blood pressure
- NMB:
-
Neuromuscular blockade
- NMDA:
-
N-Methyl-d-aspartate
- OIH:
-
Oblique inguinal hernia
- PACTR:
-
Pan-African Clinical Trial Registry
- PACU:
-
Post-anesthesia care unit
- PAED:
-
Pediatric Anesthesia Emergence Delirium Scale
- SD:
-
Standard deviation
- SPO2 :
-
Pulse oximetry
References
Abdelhalim AA, Alarfaj AM (2013) The effect of ketamine versus fentanyl on the incidence of emergence agitation after sevoflurane anesthesia in pediatric patients undergoing tonsillectomy with or without adenoidectomy. Saudi J Anesth 7(4):392
Abdulatif M, Ahmed A, Mukhtar A, Badawy S (2013) The effect of magnesium sulfate infusion on the incidence and severity of emergence agitation in children undergoing adenotonsillectomy using sevoflurane anesthesia. Anesthesia 68(10):1045–1052
Abu-Shahwan I, Chowdary K (2008) Ketamine is effective in decreasing the incidence of emergence agitation in children undergoing dental repair under sevoflurane general anesthesia. Paediatr Anaesth 17:846–850
Aldrete JA (1995) The post-anesthesia recovery score revisited. J Clin Anesth 7(1):89–91
Alghamdi F, Roth C, Jatana KR, Elmaraghy CA, Rice J, Tobias JD, Thung AK (2020) Opioid-sparing anesthetic technique for pediatric patients undergoing adenoidectomy: a pilot study. J Pain Res 13:2997
Apan A, Aykac E, Kazkayasi M, Doganci N, Tahran FD (2010) Magnesium sulphate infusion is not effective on discomfort or emergence phenomenon in pediatric adenoidectomy/ tonsillectomy. Int J Pediatr Otorhinolaryngol 74:1367–1371
Bae JH, Koo BW, Kim SJ, Lee DH, Lee ET, Kang CJ (2010) The effects of midazolam administered postoperatively on emergence agitation in pediatric strabismus surgery. Korean J Anesthesiol 58(1):45
Belville RG, Seupaul RA (2005) Pain measurement in pediatric emergency care: a review of the faces pain scale-revised. Pediatric emergency care 21(2):90-93.
Bondok RS, Ali RM (2014) Magnesium sulfate reduces sevoflurane-induced emergence agitation in pediatric patients. Ain-Shams J Anaesthesiol 7(3):282
Chen JY, Jia JE, Liu TJ, Qin MJ, Li WX (2013) Comparison of the effects of dexmedetomidine, ketamine, and placebo on emergence agitation after strabismus surgery in children. Can J Anesth 60(4):385–392
Elsersy HE, Metyas MC, Elfeky HA, Hassan AA (2017) Intraoperative magnesium sulphate decreases agitation and pain in patients undergoing functional endoscopic surgery: a randomised double-blind study. Eur J Anaesthesiol 34:658–664
Hussein FKA, Mohamed MIW, Mahmoud SSA (2019) The efficacy of magnesium sulphate on emergence agitation in adults following nasal surgeries. Al-Azhar Med J 48(4):467
Kawai M, Kurata S, Sanuki T, Mishima G, Kiriishi K, Watanabe T, Tanoue N (2019) The effect of midazolam administration for the prevention of emergence agitation in pediatric patients with extreme fear and non-cooperation undergoing dental treatment under sevoflurane anesthesia, a double-blind, randomized study. Drug Des Devel Ther 13:1729
Khatiwada S, Pokharel K, Subedi A (2020) Intraoperative infusion of magnesium sulphate does not reduce laryngospasm and agitation during emergence from anaesthesia in children. Kathmandu Univ Med J 71(3):223–227
Kim KM, Lee KH, Kim YH, Ko MJ, Jung JW, Kang E (2016) Comparison of effects of intravenous midazolam and ketamine on emergence agitation in children: randomized controlled trial. J Int Med Res 44(2):258–266
Lee SJ, Sung TY (2020) Emergence agitation: current knowledge and unresolved questions. Korean J Anesthesiol 73(6):471
Lee YJ, Kim BY, Park JH, Kim SY, Park HY, Do SH (2020) The Effect of intraoperative magnesium sulphate infusion on emergence agitation after ambulatory ophthalmic surgery in children. J Clin Med 9(12):4126
Ng KT, Sarode D, Lai YS, Teoh WY, Wang CY (2019) The effect of Ketamine on emergence agitation in children: a systematic review and meta-analysis. Pediatr Anesth 29(12):1163–1172
PASS (2019) Power Analysis and Sample Size Software (2020). NCSS, LLC, Kaysville ncss.com/software/pass
Sikich N, Lerman J (2004) Development and psychometric evaluation of the Pediatric Anesthesia Emergence Delirium scale. Anesthesiology 100:1138–1145
Stamper MJ, Hawks SJ, Taicher BM, Bonta J, Brandon DH (2014) Identifying pediatric emergence delirium by using the PAED Scale: a quality improvement project. AORN J 99(4):480–494
Vlajkovic GP, Sindjelic RP (2007) Emergence delirium in children: many questions, few answers. Anesth Analg 104(1):84–91
Xie M, Li XK, Peng Y (2017) Magnesium sulfate for postoperative complications in children undergoing tonsillectomies: a systematic review and meta-analysis. J Evid Based Med 10(1):16–25
Acknowledgement
Not applicable.
Funding
Self- funded.
Author information
Authors and Affiliations
Contributions
AE designed the study, revised literature, followed the patients and critically reviewed the manuscript. DA designed the study, analyse the data, wrote and critically revised the manuscript. FK revised literature, followed the patients. NA collected the data, performed the analysis and wrote the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by ethical committee of Ain Shams University with approval number (FM ASU MD 302/2019). All participants' parents or legal guardians submitted an informed written consent. This clinical trial is retrospectively registered by PACTR, PACTR202109686219888. Registered 14 September 2021 - http://www.pactr.org/PACTR202109686219888.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdelhakim, N., Hassan, A.E.M., Nasr, D.A.M. et al. Efficacy of intraoperative magnesium sulfate versus ketamine on emergence agitation in pediatric patients under sevoflurane anesthesia: a randomized clinical trial. Ain-Shams J Anesthesiol 14, 39 (2022). https://doi.org/10.1186/s42077-022-00234-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42077-022-00234-1