Abstract
Study objective
The aim of this study was to compare the effect of dexmedetomidine versus ketamine when added to caudal bupivacaine on the incidence of emergence delerium (ED), postoperative sedation, and analgesia in pediatric patients undergoing inguinal hernia repair under sevoflurane anesthesia.
Methods
Eighty seven pediatric patients who underwent elective inguinal hernia repair under sevoflurane anesthesia were randomly distributed into one of three equal groups. Group B (bupivacaine, n = 29), group BK (bupivacaine ketamine, n = 29), and group BD (bupivacaine dexmedetomidine, n = 29). Patients of group B received caudal injectate of 1 ml/kg bupivacaine 0.25%, while group BK patients received caudal injectate of 1 ml/kg bupivacaine 0.25% mixed with ketamine 0.5 mg/kg, and group BD patients received caudal injectate of 1 ml/kg bupivacaine 0.25% mixed with dexmedetomidine 1 μg/kg. Primary outcome measure was the assessment of the incidence of postoperative ED. Secondary outcomes included the postoperative sedation scores and postoperative Face, Legs, Activity, Cry, and Consolability (FLACC) pain scores, time to 1st postoperative analgesic, and total postoperative analgesic consumption. Also, the incidence of perioperative complications were assessed.
Results
The incidence of ED was significantly lower in group BD and BK compared with group B (P < 0.05) with no significant difference between group BD and BK (P > 0.05). Postoperative sedation scores were significantly higher in group BK and BD compared with group B (P < 0.05) at the 1st 30 min and 1st 2 h postoperative respectively; they were also significantly higher in group BD compared with group BK at (10 min–2 h) postoperative (P < 0.05). The duration of analgesia was significantly longer, and the total postoperative paracetamol consumption was significantly lower in group BD and BK compared with group B (P < 0.05); they were also significantly lower in group BD compared with group BK (P < 0.05). There was no intergroup significant difference as regards the incidence of perioperative complications.
Conclusions
Both dexmedetomidine (1 μg/kg) and ketamine (0.5 mg/kg) added to pediatric caudal block were effective to control pediatric ED after sevoflurane anesthesia. Patients received caudal dexmedetomidine had longer time to 1st postoperative analgesia and less postoperative analgesic consumption but longer postoperative sedation when compared with ketamine with no significant difference between both drugs as regards the incidence of perioperative adverse events.
Similar content being viewed by others
Background
Emergence delerium (ED) is still remaining as a major problem during the early stage of recovery from general anesthesia in children. It is characterized by psychomotor agitation and perceptual disturbances (e.g., hallucinations, agitation, and confusion), which are often expressed as inconsolable crying, moaning, restlessness, and thrashing in bed (Moore & Anghelescu, 2017). The exact etiology of ED is still unidentified but several risk factors that have been noted to increase the predilection for emergence agitation such as preschool age, preoperative anxiety, certain surgical procedures like otolaryngologic or ophthalmologic surgeries, anesthesia technique (inhalational vs. intravenous agent or regional block), and postoperative pain (Kanaya, 2016).
Sevoflurane is commonly used for pediatric general anesthesia due to its lack of airway irritation, hemodynamic characteristics, and lower pungency (Brioni et al., 2017). However, emergence delirium (ED) in pediatrics after sevoflurane anesthesia is common, with a reported incidence up to 80% (Cravero et al., 2000).
Several investigators have compared different anesthetic techniques to assess their influence on incidence and severity of emergence delirium. Regional caudal blocks have been shown to reduce the incidence of sevoflurane-induced ED in clinical studies in children (Aouad et al., 2005a).
Multiple drugs were tried to prevent or control ED; these include opioids especially fentanyl, propofol, and benzodiazepines. Among these drugs is dexmedetomidine with its highly selective α2 adrenoreptor agonist activity which provides better sedation criteria, analgesic, and antiemetic effects, with no respiratory depression (Xiang et al., 2013). Ketamine is an N-Methyl-D-aspartate (NMDA) receptor antagonist that possesses a sedative and analgesic effect in pediatric patients and has been reported to decrease the incidence of postoperative ED (Sabbar et al., 2009).
The aim of the current study is to compare the effect of addition of dexmedetomidine versus ketamine as adjuvants to caudal bupivacaine on incidence of ED, postoperative sedation, and postoperative analgesia in pediatric patients undergoing congenital inguinal hernia repair under sevoflurane anesthesia.
Methods
After obtaining approval of institutional research ethical committee and patients’ guardian written informed consents, the current prospective randomized study was conducted on 87 pediatric patients scheduled to undergo elective inguinal hernia repair through the period from September 2020 to June 2021.
Inclusion criteria
Pediatric patients with aged between 2 and 6 years old with American Society of Anesthesiologist (ASA) physical status I or II were included in the current study.
Exclusion criteria
Exclusion criteria were guardian refusal for consent, ASA grade III and IV, cardiac dysrhythmia or conduction problems, mental retardation, or developmental delay which could interfere with observational pain intensity assessment and contraindication for caudal block (infection at the site of block, bleeding diathesis, pre-existing neurological or spinal disease, or abnormalities of the sacrum) or those with known allergy to the study drugs.
Anesthesia technique
Preoperative ASA fasting guidelines were followed in all patients who were premedicated with 0.5 mg/kg oral midazolam 30 min before the procedure. Following transfer to the operating room, standard monitoring including electrocardiography (ECG), noninvasive blood pressure (NIBP), pulse oximetry were started. General anesthesia induction was started with inspired sevoflurane 8% in 100% oxygen. After loss of consciousness, an intravenous cannula was placed and laryngeal mask airway (LMA) of appropriate size was inserted under adequate anesthetic depth.
General anesthesia was maintained using 50% oxygen-air mixture with end-tidal sevoflurane 2% while the patients spontaneous ventilation was maintained via Jackson-Rees modification of Ayre’s T-piece breathing circuit that was manually assisted to maintain the end tidal CO2 of 30 to 35 mmHg. An infusion of ringer solution was started at a rate of 4 ml/kg/h which was administered for the first 10 kg of weight, 2 ml/kg/h for the next 10 kg of weight, and 1 ml/kg/h for any weight over 20 kg.
The patients were placed in lateral decubitus position with both legs flexed 90° at hip joints and 90° at knee joints for caudal anesthesia. After proper sterilization of the patients back and under strict aseptic precautions done; sacral hiatus was identified by palpating sacral cornua then single dose caudal epidural injection was performed using 23-G needle which penetrated the sacroccocygeal ligament at 90° until a pop is felt then angled down to 30° and slightly advanced cephally then after negative aspiration to blood or cerebrospinal fluid (CSF) the drugs was injected incrementally into the caudal epidural space (1 ml every 5 s) with careful inspection of soft tissues to rule out subcutaneous injection.
Patients were randomly divided into three study groups via computer-generated random numbers: group B (bupivacaine, n = 29), group BK (bupivacaine ketamine, n = 29), and group BD (bupivacaine dexmedetomidine, n = 29). Patients of group B received caudal injectate of 1 ml/kg bupivacaine 0.25%, while group BK patients received caudal injectate of 1 ml/kg bupivacaine 0.25% mixed with ketamine 0.5 mg/kg, and group BD patients received caudal injectate of 1ml/Kg mixed with dexmedetomidine 1 μg/kg. The caudal injectate was prepared by an anesthesiologist rather than the observing anesthesiologist who was blinded to the caudal injected solutions.
Surgery was started 15 min after the caudal injection was performed. Heart rate (HR) and mean arterial blood pressure (MAP) were recorded every 5 min till the end of surgery. If a rise of more than 20% increase in the baseline HR or MAP was encountered at the start or during surgery, it was considered as caudal block failure and rescue dose of intravenous fentanyl (1 μg/kg) was received with exclusion of the patient from the study. Bradycardia (above 20% decrease in the HR when compared with baseline) was treated with intravenous atropine 0.01 mg/kg. Hypotension (above 20% decrease in the MAP when compared with baseline) was treated by fluid bolus and ephedrine (0.1–0.2 mg/kg/dose) if required.
At the end of surgery, inhalational anesthesia was stopped and LMA was removed .Oxygen supplementation via face mask was applied by the anesthetist till the patient were able to maintain a patent airway with adequate tidal volume after which they were discharged to post-anesthesia care unit (PACU) for spontaneous recovery of their consciousness without any stimulation and under continuation of the standard monitoring with the attendance of the responsible PACU nurse throughout their PACU stay. After 2 h of PACU stay, patients were shifted to the ward at which they stayed for further 22 h postoperative observation period. All patients were observed for any postoperative side effects such as vomiting, hypotension, bradycardia, and respiratory depression (SpO2 dropped to < 93% requiring supplementary oxygen) and treated accordingly.
The primary outcome of this study was the incidence of postoperative ED in the study groups which was evaluated using Watcha four points scale (Bajwa et al., 2010): (0 = asleep, 1 = calm, 2 = crying but consolable, 3 = crying but inconsolable, 4 = agitated and thrashing around) which was recorded upon arrival to PACU then at 10, 20, 30, 45, and 60 min later. Patient with a Watcha score of ≥ 3 was considered to have ED. Any patient suffered postoperative ED was tried to be consoled by one of their parents (preferably the mother), if the child is inconsolable for 10 min; it was treated with propofol 1 mg/kg which could be repeated after 10 min if delirium persisted.
The secondary outcome measures included
-
Assessment of postoperative sedation by a four-point sedation score (Tewari et al., 2014) based on eye-opening (eyes open spontaneously = 0, eyes open in response to verbal stimulation = 1, eyes open in response to physical stimulation = 2, unarousable = 3) which was recorded upon arrival to PACU then at 10, 20, 30, 45, 60, and 90 min and at 2 h postoperative.
-
The Face, Legs, Activity, Cry, and Consolability (FLACC) scale (Table 1) (Merkel et al., 1997) was used for postoperative pain assessment which was recorded on arrival to PACU then at 2, 4, 6, 8, 10, 12, 15, 18, 21, and 24 h postoperative. This scale ranges from 0 to 10 where 0 represents no pain and 10 represents worst possible pain. Rescue analgesia (intravenous paracetamol 15 mg/kg infusion) was given if FLACC score was ≥ 4.
-
The postoperative analgesia duration (time between the caudal drug injection till the administration of first postoperative rescue analgesic).
-
Postoperative rescue paracetamol analgesic consumption in the 1st 24 h.
-
The incidence of perioperative complications.
Statistical analysis
On the bases of previous study (Kannojia et al., 2017), sample size calculation was done using PASS program 11 software program (Power Analysis and Sample Size calculation; NCSS, LLC, Chicago, USA) to be 29 cases per group (87 total) which was required to detect an expected difference of 20% in the incidence of postoperative ED (primary outcome) was calculated with 80% power, 95% confidence interval, and 5% alpha error taken into consideration a potential 20% dropout rate. Patients’ data analysis was done using SPSS version 16.0 computer software (Chicago, IL, USA). Quantitative parametric data were presented as means ± standard deviation while quantitative non parametric data as median (range). Comparison of quantitative parametric data between the study groups was done one-way analysis of variance (ANOVA). Post hoc analyses were carried out as appropriate. The Mann–Whitney U test was used for analysis of difference of means for quantitative non-parametric data (pain and sedation scores). Qualitative data were presented as number of cases (percentage). The comparison of qualitative data between the study groups was performed by chi-square or Fisher’s exact test. P values less than 0.05 were considered statistically significant.
Results
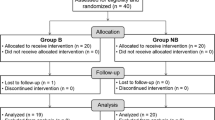
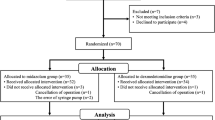
A total of 93 were initially enrolled in this study of which six patients was excluded (3 patients guardians refuse to participate and 3 patients did not meet the inclusion criteria) and the remaining 87 patients (29 patients in each group) were randomized and allocated to intervention. Three cases (1 patient in each group) were further excluded due to failed caudal block and 84 patients (28 patients in each group) were followed up all the study procedure and included in the final data analysis (Fig. 1).
Demographic data
There were no statistically significant differences between the three study groups as regards the demographic data (P > 0.05) (Table 2).
Procedure characteristics
The duration of surgeries and anesthesia were comparable between the three study groups (P > 0.05) (Table 3).
Recovery characteristics
Postoperative sedation scores
Sedation scores recorded upon arrival to PACU were significantly higher in group BD and BK when compared with group B (p < 0.05) with no significant difference between group BD and BK (P > 0.05). At (10 min–30 min) postoperative; they were significantly higher in group BD and BK when compared with group B (p < 0.05) and in group BD when compared with group BK (p < 0.05). In all the subsequent recordings, they were significantly higher in group BD when compared with group B and BK (p < 0.05) with no significant difference between group B and BK (P > 0.05) (Table 4).
Postoperative agitation scores
There was no intergroup significant difference as regards the watcha scores recorded on arrival to PACU (P > 0.05). After 10 min, they were significantly lower in group BD when compared with group B (p < 0.05) with no significant difference between group BK and B (P > 0.05) and group BD and BK (P > 0.05). At 20 and 30 min postoperative, they were significantly lower in group BD and Bk when compared with group B (p < 0.05) with no significant difference between group BD and BK (P > 0.05). At 45 and 60 min postoperative, they were significantly lower in group BD when compared with group B and BK (p < 0.05) with no significant difference between group B and BK (P > 0.05) (Table 5). None of the patients had watcha score of 3 or more in group BK and group BD while eight patients in group B had watcha score of 3; 6 patients of them was consoled after reuniting the child with the parent while 2 children received single dose of propofol as rescue injection to control their agitation episode. The incidence of ED was (28.5%) in group B vs (0%) in both BD and BK groups (p < 0.05).
Postoperative analgesia
Postoperative FLACC scores recordings
There was no intergroup significant difference as regards the FLACC scores recorded Upon arrival to PACU and 2 h postoperative (P > 0.05). Postoperative FLACC scores were significantly lower in group BK and BD compared with group B at 4–8 h and at 4–12 h respectively (p < 0.05), they were also significantly lower in group BD compared with group BK at 8–12 h postoperative (p < 0.05). In all the subsequent recordings (15–24 h) postoperatively, there was no intergroup significant difference as regards the FLACC scores (P > 0.05) (Table 6).
The time to 1st postoperative rescue analgesia
The time to 1st postoperative analgesic demand was significantly longer in group BD and group BK when compared with group B (P value < 0.05). It was also significantly longer in group BD when compared with group BK (P value < 0.05) (Table 7).
The postoperative paracetamol consumption
The total dose of postoperative paracetamol consumption was significantly lower in group BD and group BK when compared with group B (P value < 0.05). It was also significantly lower in group BD when compared with group BK (P value < 0.05) (Table 8).
Perioperative complications in the study groups
There was no intergroup significant difference as regards the incidence of perioperative complications (Table 9).
Discussion
This study was conducted to compare the effect of addition of dexmedetomidine versus ketamine to caudal bupivacaine on the incidence of ED in patients undergoing inguinal hernia repair under sevoflurane anesthesia. Both dexmedetomidine and ketamine were used as adjuvants to caudal bupivacaine analgesia in multiple previous studies in different doses. In this study, we used caudal dexmedetomidine (1 μg/kg) and ketamine (0.5 mg/kg) due to the documented safety and efficacy of these doses when compared with higher doses of both drugs (Fares et al., 2014; Meenakshi Karuppiah et al., 2016; Panjabi et al., 2004; Khoshfetrat et al., 2018).
In this study, we observed that postoperative sedation scores recorded in group BK were significantly higher than those of group B in the initial 30 min of PACU arrival; they were also significantly higher in group BD than those of group B throughout their PACU stay. Similar findings was reported by Ahuja et al. (Ahuja et al., 2015) and Aliena et al. (Aliena et al., 2018) who reported a higher sedation scores in the early postoperative period when ketamine (0.5 mg/kg) was added as an adjuvant to bupivacaine for caudal analgesia. The higher sedation scores and longer sedation time observed in dexmedetomidine group was also reported in multiple previous studies (Fares et al., 2014; Saadawy et al., 2009; El-Feky & Abd El-Aziz, 2015; Salama et al., 2016; Tandale et al., 2017; Nasreen et al., 2019) when dexmedtomidine (1 μg/kg) was used as an adjuvant to bupivacaine for caudal analgesia. Both ketamine and dexmedetomidine are well known for their sedative properties. The sedative effect of ketamine could be attributed to blockade of central NMDA and hyperpolarization-activated cyclic nucleotide channels (HCN1) receptors (Sleigh et al., 2014; Gao et al., 2016) while the sedative effect of dexmedetomidine is mainly caused by its agonist effect on α2 receptors causing hyperpolarization of excitable cells in locus ceruleus of brainstem which is the primary site in modulating wakefulness (Afonso J& Reis F., 2012).
Although that postoperative pain is thought to be important contributing factor to ED, many children experienced emergence agitation during recovery after sevoflurane anesthesia either in a non-painful procedures or despite the use of effective techniques such as caudal analgesia for postoperative pain control (Weldon et al., 2004; Aouad et al., 2005b). In this study all patients of the study groups received caudal analgesia for postoperative pain control and 8 patients developed ED in group B (28.5%) vs no patients in group BK (0%) and group BD (0%) which was statistically significant. It could be difficult for the preschool children to cope with a strange unfamiliar environment upon rapid emergence from general anesthesia with inhalational agents with low blood gas solubility coefficient like sevoflurane (Weldon et al., 2004; Aouad et al., 2005b; Dahmani et al., 2010). A meta-analysis by Dahmani et al. (Dahmani et al., 2010) reported that analgesics alone are unlikely to be associated with a low incidence of emergence agitation, and that sedation during emergence from anesthesia could reduce the incidence of emergence agitation. Therefore, it is assumed that the lower incidence of ED observed in patients of group BK and group BD when compared with group B could be attributed to better postoperative sedation scores and longer sedation time which allowed a slower and calmer anesthesia emergence in both groups when compared group B.
In agreement with the results of our study, Saadawy et al. (Aliena et al., 2018) and Kannojia et al. (Kannojia et al., 2017) reported a lower incidence of ED when dexmedetomidine (1 μg/kg) was added to pediatric caudal analgesia. A similar dose of dexmedetomidine was used by Al-Zaben et al. (Al-Zaben et al., 2016) which was efficient for control of ED when used by both caudal and intravenous route, giving superiority to the caudal route due to longer postoperative analgesia, less postoperative analgesic consumption and less incidence of bradycardia and hypotension when compared with intravenous route .The efficacy of caudal ketamine (0.5 mg/kg) in prevention of ED was reported by Sinha and Sood (Sinha & Sood, 2012) in pediatric patients undergoing elective subumbilical surgery. Similarly, Abdel-Ghaffar et al. (Abdel-Ghaffar et al., 2017) observed no incidence of postoperative agitation when ketamine was added to bupivacaine caudal or topical analgesia for pediatric patients undergoing inguinal herniotomy. A recent met-analysis by Rao et al. (Rao et al., 2020) which evaluated the effect of dexmedetomidine on ED in pediatric patients reported that dexmedetomidine significantly decreased the incidence of post-anesthesia ED compared with placebo, midazolam, and opioids. However, dexmedetomidine did not exhibit this superiority when compared with propofol and ketamine.
In this study, postoperative FLACC scores were significantly lower in group BK and BD compared with group B at 4–8 h and at 4–12 h respectively, they were also significantly lower in group BD compared with group BK at 8–12 h postoperative with significantly longer time to 1st postoperative analgesic requirement and significant reduction in the total postoperative analgesic consumption in group BD and BK versus group B and in group BD versus group BK. The results of this study run in accordance with multiple previous studies (Xiang et al., 2013; Kannojia et al., 2017; Fares et al., 2014; Meenakshi Karuppiah et al., 2016; Tandale et al., 2017; Nasreen et al., 2019; Neogi et al., 2010; Schnabel et al., 2013; Bharti et al., 2014; Tong et al., 2014; Al-Zaben et al., 2015; Mavuri et al., 2017; Sayed et al., 2018; Trifa et al., 2018; Tu et al., 2019; Tobias, 2007) in which the addition of dexmedetomidine (1 μg/kg) to LA for pediatric caudal block resulted in longer duration of postoperative analgesia and less postoperative analgesic consumption without an increase in the incidence of remarkable side effects. The potentiating of postoperative analgesia observed when dexmedtomidine was added to bupivacaine caudal block could be explained by the ability of dexmedetomidine to inhibit the Ad and C fibers (nocioceptive fibers) and to diffuse to CSF to exert an agonist activity on the α2 receptors located in the superficial lamina of dorsal horn of the spinal cord which attenuates substance P and glutamate release from the nocioceptive afferent terminals and suppresses the nocioceptive signals transmission. After its systemic absorption or diffusion to CSF, it can exert a supra-spinal analgesic activity via its agonist effect on central alpha α2 receptors in the locus ceruleus at the brain stem and the descending noradrenergic pathway of the spinal cord to the presynaptic membrane, inhibiting the release of nociceptive peptides and thereby inhibiting the transmission of angular noxious stimuli, which in turn terminates the signaling of pain (Ishii et al., 2008; Mantz et al., 2011; Zhang & Bai, 2014; Konakci et al., 2008).
The prolongation of caudal bupivacaine analgesia observed in this study with caudal ketamine addition was also reported when ketamine (0.5 mg/kg) was used as an adjuvant to LA for pediatric caudal block (Panjabi et al., 2004; Khoshfetrat et al., 2018; Ahuja et al., 2015; Aliena et al., 2018; Somasundran & Garasia, 2008; Choudhuri et al., 2008; Locatelli et al., 2008; Odes & Erhan OL& Demirci M., 2010; Kaur & Anand, 2016; Chandramohan & D’ Sauza, 2016) which could be attributed mainly to its antagonist effect on NMDA receptors located at the central nervous system including substantia gelatinosa of rolandi in the spinal cord involved in nocioceptive transmission. Also, it has an agonist effects on mu-opioid receptors (De Beer & Thomas, 2003; Ivani et al., 2003; Vadivelu et al., 2010).
In this study, it was observed that the postoperative analgesia duration was even longer and the postoperative analgesic consumption was less in the dexmedetomidine group compared with ketamine group. Similar findings were noted in the study conducted by Abd El-Aziz and Abd-Allah (AAA & Abd-Allah, 2015) which evaluated the effects of dexmedetomidine and ketamine added to caudal bupivacaine in patients undergoing inguinoscrotal surgeries. In their study, the time to 1st postoperative analgesic was significantly longer in dexmedetomidine group (19.6 ± 1.4 h) compared with ketamine group (11.4 ± 1.2 h) with significantly lower postoperative analgesic consumption in dexmedetomidine group.
Finally, there was no detectable difference as regards the incidence of perioperative complications between the study groups which run in accordance with the results of the previous studies that documented the safety of caudal ketamine and dexmedetomidine in the doses used in this study.
Limitations of the present study
First, it is a single-center study. Second, this study was limited to patients who underwent inguinal hernia repair surgery only but we tried to standardize the severity of surgical trauma which may impact the pain severity and/or the incidence of postoperative ED. Third, different doses of each study drug were not used in order to compare their effects. Lastly, the cost implications for the studied drugs should be considered.
Conclusions
From this study, we concluded that both dexmedetomidine (1 μg/kg) and ketamine (0.5 mg/kg) added to pediatric caudal block were effective to control pediatric ED after sevoflurane anesthesia. Patients received caudal dexmedetomidine had longer time to 1st postoperative analgesic and less postoperative analgesic consumption but longer postoperative arousable sedation when compared with ketamine with no significant difference between both drugs as regards the incidence of perioperative adverse events.
Availability of data and materials
The data sets generated during and/or analyzed during the current study are not publicly available due to restrictions based on privacy regulations and informed consent of the participant’s guardians, but are available from the corresponding author on reasonable request.
Abbreviations
- ASA:
-
American Society of Anesthesiologist
- CSF:
-
Cerebrospinal fluid
- ECG:
-
Electrocardiography
- ED:
-
Emergence delerium
- HCN1:
-
Hyperpolarization-activated cyclic nucleotide channels
- HR:
-
Heart rate
- MAP:
-
Mean arterial pressure
- NIBP:
-
Non-invasive blood pressure
- NMDA:
-
N-methyl-D-aspartate
- PACU:
-
Post-anesthesia care unit
- SpO2 :
-
Peripheral oxygen saturation
References
AAA AE-A, Abd-Allah WA (2015) Comparative study between caudal dexmedetomidine with bupivacaine versus ketamine with bupivacaine for postoperative analgesia after inguinoscrotal surgery in pediatric patients. Ain-Shams J Anaesthesiol 8:634–638
Abdel-Ghaffar HS, Moeen SM, Moeen AM (2017) Topical versus caudal ketamine/bupivacaine combination for postoperative analgesia in children undergoing inguinal herniotomy. Saudi J Anaesth 11:41–48
Afonso J, Reis F (2012) Dexmedetomidine: current role on anaesthesia and intensive care. Rev Bras Anestesiol 62:118–133
Ahuja S, Yadav S, Joshi N et al (2015) Efficacy of caudal fentanyl and ketamine on post-operative pain and neuroendocrine stress response in children undergoing infraumbilical and perineal surgery: A pilot study. J Anaesthesiol Clin Pharmacol 31:104–109
Aliena SP, Lini C, Chirayath JJ (2018) Comparison of postoperative analgesic effect of caudal bupivacaine with and without ketamine in Pediatric subumbilical surgeries. J Anaesthesiol Clin Pharmacol 34:324–327
Al-Zaben KR, Qudaisat IY, Abu-Halaweh SA et al (2015) Comparison of caudal bupivacaine alone with bupivacaine plus two doses of dexmedetomidine for postoperative analgesia in pediatric patients undergoing infra-umbilical surgery: A randomized controlled double-blinded study. Pediatr Anaesth. 25:883–890
Al-Zaben KR, Qudaisat IY, Abu-Halaweh SA et al (2016) The effects of caudal or intravenous dexmedetomidine on postoperative analgesia produced by caudal bupivacaine in children: a randomized controlled double-blinded study. J Clin Anesth 33:386–394
Aouad MT, Kanazi GE, Siddik-Sayyid SM et al (2005a) Preoperative caudal block prevents emergence agitation in children following sevoflurane anesthesia. Acta Anaesthesiol Scand. 49(3):300–304
Bajwa SA, Costi D, Cyna AM (2010) A comparison of emergence delirium scales following general anesthesia in children. Paediatr Anaesth 20:704–711
Bharti N, Praveen R, Bala I (2014) A dose–response study of caudal dexmedetomidine with ropivacaine in pediatric day care patients undergoing lower abdominal and perineal surgeries: a randomized controlled trial. Pediatr Anesth 24:1158–1163
Brioni JD, Varughese SH, Ahmed RA et al (2017) A clinical review of inhalation anesthesia with sevofurane: from early research to emerging topics. J Anesth 31:764–778
Chandramohan D, D’ Sauza SA (2016) Preservative- free racemic ketamine with bupivacaine : a desirable option for extended caudal analgesia in pediatric surgery. Ain- Shams J Anesthesiol 9:426–431
Choudhuri AH, Dharmani P, Kumarl N et al (2008) Comparison of caudal epidural bupivacaine with bupivacaine plus tramadol and bupivacaine plus ketamine for postoperative analgesia in children. Anaesth Intensive Care 36:174–179
Cravero J, Surgenor S, Whalen K (2000) Emergence agitation in paediatric patients after sevoflurane anaesthesia and no surgery: a comparison with halothane. Paediatr Anaesth 10:419–424
Dahmani S, Stany I, Brasher C et al (2010) Pharmacological prevention of sevoflurane and desflurane related emergence agitation in children: a meta-analysis of published studies. Br J Anaesth 104:216–223
De Beer DAH, Thomas ML (2003) Caudal additives in children: solutions or problems? Br J Anaesth 90:487–498
El-Feky EM, Abd El-Aziz AA (2015) Fentanyl, dexmedetomidine, dexamethasone as adjuvant to local anesthetics in caudal analgesia in pediatrics: A comparative study. Egypt J Anesth. 31:175–178
Fares KM, Othman AH, Alieldin NH (2014) Efficacy and safety of dexmedetomidine added to caudal Bupivacaine in pediatric major abdominal cancer surgery. Pain Physician 17:393–400
Gao M, Rejaei D, Liu H (2016) Ketamine use in current clinical practice. Acta Pharmacol Sin 37:865–872
Ishii H, Kohno T, Yamakura T et al (2008) Action of dexmedetomidine on the substantia gelatinosa neurons of the rat spinal cord. Eur J Neurosci 27:3182–3190
Ivani G, Vercellino C, Tonetti F (2003) Ketamine: a new look to an old drug. Minerva Anestesiol 69:468–471
Kanaya A (2016) Emergence agitation in children: risk factors, prevention, and treatment. J Anesth 30(2):261–267
Kannojia UK, Meena RK, Paswan AK et al (2017) Comparison of caudal dexmedetomidine and fentanyl combined with bupivacaine in pediatric patients undergoing urogenital surgery. Anaesth Pain Intensive Care 21(2):204–211
Kaur D, Anand S (2016) Comparison between caudal bupivacaine and bupivacaine with ketamine for postoperative analgesia in children: a prospective randomized clinical study. Anesth Essays Res 10:488–489
Khoshfetrat M, Davoodi R, Keykha A (2018) Comparing the effects of three different doses of caudal ketamine plus bupivacaine on pain control after paediatric surgery. Biomed Res Ther 5(8):2572–2580
Konakci S, Adanir T, Yilmaz G et al (2008) The efficacy and neurotoxicity of dexmedetomidine administered via the epidural route. Eur J Anaesthesiol 25:403–409
Locatelli BG, Frawley G, Spotti A et al (2008) Analgesic effectiveness of caudal levobupivacaine and ketamine. Br J Anaesth 100:701–706
Mantz J, Josserand J, a Hamada S. (2011) Dexmedetomidine: new insights. Eur. J. Anaesthesiol 28:3–6
Mavuri G, Jain P, Chakraborty S et al (2017) A randomized double-blinded comparison between dexmedetomidine and clonidine as an adjuvant to caudal ropivacaine in children for below umbilical surgery. J Clin Sci 14:157–161
Meenakshi Karuppiah NP, Shetty SR, Patla KP (2016) Comparison between two doses of dexmedetomidine added to bupivacaine for caudal analgesia in paediatric infraumbilical surgeries. Indian J Anaesth 60:409–414
Merkel SI, Voepel-Lewis T, Shayevitz JR et al (1997) The FLACC: a behavioral scale for scoring postoperative pain in young children. Paediatr Nurs 23:293–297
Moore AD, Anghelescu DL (2017) Emergence delirium in pediatric anesthesia. Paediatr Drugs. 19(1):11–20
Nasreen F, Khalid A, Rashid H (2019) Comparison of 0.125% levobupivacaine with dexmedetomidine and 0.25% levobupivacaine in ultrasonography-guided pediatric caudal block: A prospective, randomized, double-blinded study. Indian. J Pain 33:86–93
Neogi M, Bhattacharjee DP, Dawn S et al (2010) A comparative study between clonidine and dexmedetomidine used as adjuncts to ropivacaine for caudal analgesia in paediatric patients. J Anaesthesiol Clin Pharmacol. 26:149–153
Odes R, Erhan OL& Demirci M. (2010) Effects of ketamine added to ropivacaine in pediatric caudal block. Agri. 22(2):53–60
Panjabi N, Prakash S, Gupta P et al (2004) Efficacy of three doses of ketamine with bupivacaine for caudal analgesia in pediatric inguinal herniotomy. Reg Anesth Pain Med 29:28–31
Rao Y, Zeng R, Jiang X et al (2020) The effect of dexmedetomidine on emergence agitation or delirium in children after anesthesia—a systematic review and meta-analysis of clinical studies. Front. Pediatr. 8:329
Saadawy I, Boker A, Elshahawy MA et al (2009) Effect of dexmedetomidine on the characteristics of bupivacaine in a caudal block in pediatrics. Acta. Anaesthesiol. Scand. 53:251–256
Sabbar S, Zamir AK, Khan FA (2009) Caudal ketamine with bupivacaine and bupivacaine alone for postoperative analgesia in paediatric inguino-scrotal surgeries. Med Channel 4:207–210
Salama AK, Galante D, Abdallah NM (2016) Comparison between caudal dexmedetomidine and nalbuphine in children undergoing hypospadias surgery: a prospective randomized double blind controlled study. Pediatr Anesth Crit Care J 4(1):48–54
Sayed JA, Kamel EZ, Riad MAF et al (2018) Dexmedetomidine with magnesium sulphate as adjuvants in caudal block to augment anaesthesia and analgesia in paediatric lower abdominal surgeries. Egypt J Anaesth 34:115–122
Schnabel A, Reichl S, Poepping D et al (2013) Efficacy and safety of intraoperative dexmedetomidine for acute postoperative pain in children: a meta-analysis of randomized controlled trials. Paediatr Anaesth 23:170–179
Sinha A, Sood J (2012) Caudal block and emergence delirium in pediatric patients: Is it analgesia or sedation? Saudi J Anaesth. 6(4):403–407
Sleigh J, Harvey M, Voss L et al (2014) Ketamine-more mechanisms of action than just NMDA blockade. Trends Anaesth Crit Care 4:76–81
Somasundran S, Garasia M (2008) A comparative study of ketamine and tramadol as additives to plain bupivacaine in caudal anaesthesia in children. Internet J Anaesthesiol. 17:2
Tandale SR, Patil V, Savant P et al (2017) Efficacy and safety of dexmedetomidine as an adjuvant to caudal levobupivacaine in paediatric patients. Pediatr Anesth Crit Care J 5(2):103–110
Tewari A, Dhawan I, Mahendru V et al (2014) A comparative study evaluating the prophylactic efficacy of oral clonidine and tramadol for perioperative shivering in geriatric patients undergoing transurethral resection of prostate. J Anaesthesiol Clin Pharmacol. 30:340–344
Tobias JD (2007) Dexmedetomidine: applications in pediatric critical care and pediatric anesthesiology. Pediatr Crit Care Med 8:115–131
Tong Y, Ren H, Ding X et al (2014) Analgesic effect and adverse events of dexmedetomidine as additive for pediatric caudal anesthesia: a meta-analysis. Paediatr Anaesth 24:1224–1230
Trifa M, Tumin D, Tobias JD (2018) Dexmedetomidine as an adjunct for caudal anaesthesia and analgesia in children: a review. Minerva Anestesiol. 84:836–847
Tu Z, Tan X, Li S et al (2019) The efficacy and safety of dexmedetomidine combined with bupivacaine on caudal epidural block in children: a meta-analysis. Med Sci Monit. 25:165–173
Vadivelu N, Mitra S, Narayan D (2010) Recent advances in postoperative pain management. Yale J Biol Med 83:11–25
Weldon BC, Bell M, Craddock T (2004) The effect of caudal analgesia on emergence agitation in children after sevoflurane versus halothane anesthesia. Anesth Analg. 98:321–326
Xiang Q, Huang DY, Zhao YL et al (2013) Caudal dexmedetomidine combined with bupivacaine inhibit the response to hernial sac traction in children undergoing inguinal hernia repair. Br J Anaesth 110:420–424
Zhang X, Bai X (2014) New therapeutic uses for an alpha2 adrenergic receptor agonist–dexmedetomidine in pain management. Neurosci. Lett. 561:7–12
Acknowledgements
The authors sincerely thank deeply all the members of pediatric surgery team in Ain Shams University Hospitals for their cooperation during the study period.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Corresponding author HM contributed to study conception and design, acquisition of data, analysis, and interpretation of data. The other author, MA, contributed to the drafting of manuscript and its critical revision. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The current prospective randomized double-blinded study was conducted on 87pediatric patients scheduled to undergo inguinal hernia repair at Ain Shams university hospitals through the period from September 2020 to June 2021after obtaining approval of research ethical committee (REC) of Faculty of Medicine, Ain Shams University (FMASU) in September 2020 with reference number of FMASU R56/2020 and patients’ guardian written informed consents for acceptance of participation in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fahim, H.M., Menshawi, M.A. Effect of caudal dexmedetomidine versus ketamine in prevention of emergence delirium in pediatric patients undergoing congenital inguinal hernia repair under sevoflurane anesthesia. Ain-Shams J Anesthesiol 14, 45 (2022). https://doi.org/10.1186/s42077-022-00244-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42077-022-00244-z