Abstract
Background
Assessing fluid responsiveness is the key to successful resuscitation of critically-ill sepsis patients. The use of IVC variation is favored among the dynamic methods of fluid responsiveness assessment in the ICU because it is non-invasive and inexpensive; moreover, it does not demand a high level of training. The aim of this study is to determine the value of the IVC respiratory variability for predicting fluid responsiveness in spontaneously breathing sepsis patients with acute circulatory failure.
Results
In this prospective observational study, fifty-eight spontaneously breathing sepsis patients admitted in the ICU were enrolled after the approval of the departmental Research Ethical Committee, and the informed written consent had been taken from the patients. Ultrasonographic and echocardiographic parameters were measured “IVC parameters and stroke volume (SV)” with calculation of the inferior vena cava collapsibility index (IVCCI) and cardiac output. These values were obtained before (baseline) and after volume expansion with a fluid bolus. The study showed that twenty-nine patients (50%) were considered to be responders, with an increase in CO by 10% or more after fluid challenge. There was a significant difference between responders and non-responders in baseline IVCCI (p value < 0.001). There were no significant differences between responders and non-responders in terms of demographic and baseline clinical characteristics. Also, there was statistically significantly larger maximum (IVC max) and minimum (IVC min) inferior vena cava diameters before volume expansion in non-responders than in responders with p value 0.037 and 0.001 respectively. The suggested cut off value regarding baseline IVCCI to predict response to fluid infusion is 0.32 with a high chance of response above this figure (a sensitivity of 72.41% and a specificity of 82.76%).
Conclusions
Inferior vena cava collapsibility index assessment can be a sensitive and a good predictor of fluid responsiveness, being based on a safe and a non-invasive technique compared to other methods such as central venous pressure (CVP) measurement and pulmonary artery catheter insertion.
Similar content being viewed by others
Background
In the intensive care unit (ICU), hypovolemia is a very common clinical situation and is primarily treated with volume expansion. Unfortunately, in response to volume expansion, only 40–70% of critically ill patients with acute circulatory failure show a significant increase in their cardiac output (Airapetian et al., 2015).
Sepsis is defined as life-threatening organ dysfunction provoked by a dysregulated host response to an infection. Many scoring systems were established to identify sepsis patients, such as the systemic inflammatory response syndrome (SIRS) criteria (Bone et al., 1992) and the newly introduced sequential organ failure assessment (SOFA) score in Sepsis-3 (Singer et al., 2016). However, the SIRS criteria were still used by many health institutes because of their higher sensitivity when used as a screening tool for sepsis (Dykes et al., 2019).
Sepsis is characterized pathophysiologically by vasoplegia with loss of arterial tone, venodilation with blood sequestration in the unstressed blood compartment, changes in ventricular function with decreased compliance, and hence decreased responsiveness to the preload contributing to a pathological condition called acute circulatory failure which precedes the state of shock. In addition, almost all the fluids administered are sequestered in tissues, resulting in severe vital organ edema and thereby raising the risk of organ dysfunction (Marik & Bellomo, 2016). There is an increasing body of evidence that over-resuscitation can be detrimental to patients with septic shock and acute respiratory distress syndrome. So the conservative fluid strategy results in an increased number of ventilator-free days and a reduced length of ICU stay relative to the liberal approach or standard care strategy (Silversides et al., 2017).
Assessing fluid responsiveness is crucial to the effective resuscitation of critically ill patients. Simple traditional fluid status assessment approaches, such as vital signs, may be used by some clinicians; however, they do not identify fluid responders reliably. On the other hand, complex techniques have been used, such as pulmonary artery catheters (PAC) and non-invasive cardiac output measuring (NICOM) devices. The use of a pulmonary artery catheter is invasive, condemns patients to potential harm, and has debatable efficacy. In several trials, NICOM has been validated against the PAC and generates comparable hemodynamic data compared to stroke volume (SV) variation; however, its clinical application is limited to resource-rich practice environments (Corl et al., 2017).
There are two ways to assess volume status; static and dynamic methods. The dynamic parameters are based on varying cardiac output (CO) without delivering fluids to anticipate clinical response. Many of these methods are based on the lung-heart interaction. Transpulmonary pressure variations with respiration cause CO variation, which is assessed using one of the following methods: SV variation, pulse pressure variation, superior vena cava (SVC) diameter variation, and inferior vena cava (IVC) diameter variation (Furtado & Reis, 2019). Among those dynamic methods, the use of IVC variation is preferred because it is non-invasive, inexpensive, and simple as it does not require a high level of training. Moreover, complementary echocardiographic evaluation, both quantitative and qualitative, leads to a better overall clinical evaluation (Boyd et al., 2016).
Central venous pressure (CVP) measurement is one of the commonly used static methods of assessment and often involves an invasive procedure to measure the right atrial pressure which is used as a surrogate for left ventricle volume. Its value is altered by intrathoracic pressure and both left and right ventricular contractility; intravascular volume measurement may be affected by these factors. The inferior vena cava parameters may be affected by the same factors, but they can be useful and non-invasive substitutes to guide a larger spectrum of critically ill patients’ intravenous fluid resuscitation without posing any risks or complications (Garg et al., 2016).
Methods
In this prospective observational study, fifty-eight spontaneously breathing sepsis patients admitted in the ICU (between May 2017 and April 2019) were enrolled in the non-probability consecutive sampling after the approval of the departmental Research Ethical Committee, and the informed written consent had been taken from the patients.
Patients had met two or more of the following criteria of SIRS (Bone et al., 1992): {Tachycardia (heart rate > 90 beats/min), tachypnea (respiratory rate > 20 breaths/min), fever or hypothermia (temperature > 38 or < 36 °C), and leukocytosis, leukopenia, or bandemia (white blood cells > 12,000/mm3, < 4000/mm3 or bandemia ≥ 10%)}. In addition to confirmed or suspected source of infection.
The patients had clinical signs of acute circulatory failure (systolic blood pressure < 90 mmHg, urine output < 0.5 ml/kg, tachycardia > 100 bpm, mottled skin) and/or oligo-anuria (diuresis below 20 ml/h or 0.5 ml/kg/h) and/or acute kidney failure; and/or serum markers suggesting organ hypoperfusion (acidosis with a serum pH < 7.3 or lactic acid > 2 meq/l).
Patients with the following criteria were excluded from the study: clinical signs of hemorrhage, arrhythmia and cardiogenic or obstructive shock. Also, we excluded subjects in whom there was inability to postpone administration of fluid for several minutes or if the clinical staff feared that the patient had active pulmonary edema or there could be a clinical risk from further intravenous fluids.
All patients included in the study were assessed by patients’ demographic data (age, gender, and body weight “if possible”), detailed medical and surgical history, complete clinical examination, routine laboratory investigations, and serum lactate level; and standard 12 lead electrocardiogram (ECG) and echocardiogram. Standard monitoring was applied, including non-invasive arterial blood pressure, electrocardiography, and pulse oximetry using the multichannel monitor.
Technique and measurement
Each patient in the study was positioned in a semi-recumbent position for baseline measurements. Echocardiography and the curvilinear probe ultrasonography were used to assess CO and the IVC diameters, respectively. A 500 ml of normal saline solution was given intravenously over a 15-min period. Then the same measurements were taken again.
An echocardiography was done in a parasternal two-dimensional (2D) view. The aortic diameter (AoD) was measured at the aortic valve annulus. The aortic area (AA) was calculated using the formula: AA = π × (AoD2 / 4). We measured the aortic blood flow using pulsed Doppler at the aortic annulus in the apical five-chamber view so we could calculate the velocity-time integral (VTI) for aortic blood flow. The following formulas (Blanco et al., 2015) were used to measure stroke volume (SV) and CO: SV = VTI × AA and CO = SV × heart rate. The aortic area was assessed only at baseline, since it was thought to be constant throughout the test.
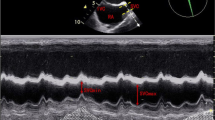
In a longitudinal view, the IVC was examined at the subcostal area using the ultrasonography and the IVC diameters were measured in M-mode coupled to 2D mode 3 cm before the IVC joined the right atrium to ensure that IVC diameter measurements are accurate and that the inter- and intra-observer variability of IVC can be minimized (Bortolotti et al., 2018; Caplan et al., 2020). The IVC collapsibility index (IVCCI) was calculated as follows: (maximum diameter on expiration (IVC max) – minimum diameter on inspiration (IVC min))/ IVC max.
Hemodynamic parameters “heart rate (HR), systolic and diastolic blood pressure, and mean arterial pressure (MAP)” were monitored before and after fluid administration.
Sample size calculation
A sample of 58 cases produces a two-sided 95% confidence interval with a width of 0.3 when the sample area under the curve (AUC) in receiver operating characteristic (ROC) analysis is 0.5 (Krzanowski & Hand, 2009).
The patients were classified as responders (in whom CO increased by ≥ 10% of the baseline value after volume) and non-responders (in whom CO increased by < 10%, unchanged or even decreased).
Statistical analysis
Student’s T and chi-squared tests were used to analyze the data. Absolute values of cardiac output (CO) and IVC diameters at baseline and after fluid expansion, the correlation between these variables and their value for predicting an increase in CO; all were calculated by plotting a ROC curve. The statistical significance threshold was set to p < 0.05.
Aim of the study
The primary aim of the present study is to determine the value of the IVC respiratory variability for predicting fluid responsiveness in spontaneously breathing sepsis patients with acute circulatory failure. Also, we examined the value of other parameters of IVC (the minimum and maximum diameters) as complementary predictors of the clinical response (secondary outcome of the study).
Results
Demographic data
Statistical analysis of the demographic data showed that there was no statistically significant difference found between responders and non-responders regarding age, sex, and risk factors (Table 1).
Vital data
When observing heart rate and blood pressure of the studied patients, we found no statistically significant difference between responders and non-responders (Table 2).
Cardiac output values
there was no statistically significant difference between the two studied groups regarding baseline CO, while there was statistically significant increase in CO after volume expansion in responders than in non-responders with p-value < 0.001 (Table 3).
Inferior vena cava parameters
When comparing the two groups as regard to the IVC diameters, we found that there were significantly larger baseline (before fluid bolus) minimum and maximum diameters in non-responders than in responders with p value = 0.001 and 0.037 respectively. Also, there was statistically significantly low baseline IVCCI in non-responders than in responders with p value < 0.001. The table also shows that there was statistically significant increase in IVC max. and IVCCI after volume expansion in non-responders than in responders (p value = 0.001 and 0.027 respectively) (Table 4).
Our statistical analysis of the collected data revealed that the suggested cut off value regarding baseline IVCCI to predict responder cases was 0.32 with a sensitivity of 72.41%, a specificity of 82.76%, and an AUC of 82.9% (Figs. 1 and 2).
Discussion
This prospective study was done in the ICU and included fifty-eight sepsis patients. Two types of response to fluid administration were revealed. Responders who responded to fluid bolus by increase in cardiac output by 10% or more, and non-responders who had less than 10% increase in cardiac output.
The echocardiographically measured cardiac output (derived from the VTI value) is equivalent to that measured by the transpulmonary thermodilution technique (Desai & Garry, 2018). An increase in stroke volume (SV) by more than 10% after a 500 ml crystalloid bolus over 10–15 min is the standard definition of fluid-responsiveness (Messina et al., 2017).
Based on the percentage of velocity-time integral (VTI) increase, we found a positive and significant correlation between the baseline inferior vena cava collapsibility index (IVCCI) and the percentage of VTI increase after volume expansion. This result is an interpretation of the Frank-Starling mechanism and indicates that the greater the percentage of inspiratory collapse of the inferior vena cava, the bigger cardiac output response after administration of fluid. This phenomenon means that the baseline IVCCI is capable of quantifying the preload dependency magnitude.
The dynamic tests of fluid responsiveness are based on varying cardiac output (CO) without delivering fluids to anticipate clinical response. The transpulmonary pressure variations with respiration cause CO variation, which is assessed using one of the following: stroke volume (SV) variation, pulse pressure variation, and inferior vena cava (IVC) diameter variation. This is what we call the lung-heart interaction (Bennett et al., 2018).
There is a negative transpulmonary pressure at the beginning of inspiration in non-ventilated spontaneously breathing patients or those under intermittent mandatory ventilation (IMV) with respiratory effort that causes a variable degree of IVC collapse as a result of its compliance. IVC shows decreased compliance and restricted collapse in patients with high right side heart pressure or excessive preload (during the flat phase of the Frank-Starling curve) and in some cases collapse may be absent. IVC compliance is high in patients with low right heart cavity pressure such as in hypovolemia (i.e., the ascending phase of the Frank-Starling curve); hence, the inspiratory collapse is significant (Furtado & Reis, 2019).
Airapetian et al. studied spontaneously breathing patients in the intensive care unit with suspected hypovolemia and discovered that IVC diameter was not a strong predictor of fluid responsiveness but respiratory variations of IVC > 42% had a high specificity to anticipate an increase of cardiac output after fluid administration (Airapetian et al., 2015).
On the other hand Orde, S. et al.’s study supports our observations regarding the maximal and minimal inferior vena cava diameters to assess fluid responsiveness. In our study, we found a statistically significantly smaller baseline IVC min. and IVC max. in responders than in non-responders with p value = 0.001 and 0.037 respectively. The Orde S. et al.’s study showed that in low blood volume states, an IVC diameter of < 10 mm is prevalent, suggesting a higher probability of response to volume expansion, while in high blood volume states, a diameter of > 25 mm is frequent and indicates a small chance of fluid responsiveness (Orde et al., 2017).
Some conditions can mimic the hypovolemic status found in sepsis, such as spinal anesthesia induced sympathectomy. From this prospective, research on patients scheduled for surgery under spinal anesthesia was done by Ceruti et al. More than a hundred patients were randomized into two groups. The inferior vena cava collapsibility index (IVCCI) was used in one group to identify patients who were fluid respondents, i.e., patients with an IVCCI > 36%. These patients received fluid boluses of 500 ml until they became fluid non-responders based on IVCCI (Ceruti et al., 2018).
Anas et al. found a strong positive association between central venous pressure (CVP) and maximal inferior vena cava diameter (IVC max) (r = 0.547, p < 0.001) where IVC max ≤ 1.73 cm can predict low CVP of less than 10 cm H2O with 71.4% sensitivity and 75.6% specificity (Shalaby et al., 2018).
With CVP use, the problem of predicting fluid responsiveness is very contentious and quite puzzling, as the shape of the Frank-Starling curve can vary from one patient to another and in the same patient from one time to another. A number of studies and meta-analyses had identified that a given value of CVP may not correlate to preload responsiveness (Bentzer et al., 2016). What is true for CVP is true for all static cardiac preload measures, such as pulmonary artery occlusion pressure, global end-diastolic volume derived from the transpulmonary thermodilution technique, and the flow time of aortic flow by esophageal Doppler (Monnet et al., 2016).
After a 500 ml fluid bolus in an intensive care unit patients, Machare-Delgado et al. compared inferior vena cava (IVC) diameter variation to pulse contour analysis and found a much stronger association between stroke volume improvement and IVC variability than the stroke volume variation in pulse contour analysis (Machare-Delgado et al., 2011).
Patients with severe acute respiratory distress syndrome (ARDS) usually require high positive end expiratory pressure (PEEP) values to maintain oxygenation, hence increasing the intrathoracic pressure with a resultant decrease in IVC compliance, leading to false negative results (Charron et al., 2006). Also, fluid administration to this type of patients may aggravate lung and tissue edema. For this reason, they were excluded from our study.
The relevance of this procedure is also debatable in ventilated patients with respiratory effort (IMV in the assisted or spontaneous mode). A negative transpulmonary pressure occurs during the respiratory stimulus, which is the opposite to that caused by IMV. As a consequence, the variance in IVC diameter among these patients cannot be reliable (Via et al., 2016). None of the patients in our study needed any mean of mechanical respiratory support. So further studies are needed to quantify IVC values in patients who need spontaneous modes of mechanical ventilation.
The inferior vena cava (IVC) diameter measurement site can greatly affect the relationship between the caval index (i.e., IVCCI) and fluid responsiveness. Bortolotti et al. found that deep inhalation did not influence the index when IVC diameters were measured beyond 3 cm caudal to the right atrium (Bortolotti et al., 2018). Caplan M. et al.’s study endorse the clinical use of subcostal, long-axis view of (IVC) measurements in two-dimensional mode, perpendicular to the IVC wall and 4 cm caudal to the right atrium. The systematic implementation of this technique ensures that IVC diameter measurements are accurate and that the inter- and intra-observer variability of IVC can be minimized (Caplan et al., 2020).
Conclusions
Inferior vena cava collapsibility index (IVCCI) assessment can be a sensitive predictor of fluid responsiveness. Other inferior vena cava parameters (minimum and maximum IVC diameters) may also predict clinical response to fluids.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AA:
-
Aortic area
- AoD:
-
Aortic diameter
- ARDS:
-
Acute respiratory distress syndrome
- AUC:
-
Area under the curve
- CO:
-
Cardiac output
- COPD:
-
Chronic obstructive pulmonary disease
- CVP:
-
Central venous pressure
- DM:
-
Diabetes mellitus
- ECG:
-
Electrocardiogram
- HR:
-
Heart rate
- HTN:
-
Hypertension
- ICU:
-
Intensive care unit
- IHD:
-
Ischemic heart disease
- IMV:
-
Intermittent mandatory ventilation
- IVC max:
-
Maximum inferior vena cava diameter
- IVC:
-
Inferior vena cava
- IVCCI:
-
Inferior vena cava collapsibility index
- MAP:
-
Mean arterial pressure
- NICOM:
-
Non-invasive cardiac output measuring device
- PAC:
-
Pulmonary artery catheter
- PEEP:
-
Positive end expiratory pressure
- ROC:
-
Receiver operating characteristic
- SD:
-
Standard deviation
- Sig:
-
Significance
- SIRS:
-
Systemic inflammatory response syndrome
- SOFA:
-
Sequential organ failure assessment
- SV:
-
Stroke volume
- SVC:
-
Superior vena cava
- VTI:
-
Velocity time integral
References
Airapetian N, Maizel J, Alyamani O, Mahjoub Y, Lorne E, Levrard M, Ammenouche N, Seydi A, Tinturier F, Lobjoie E, Dupont H, Slama M (2015) Does inferior vena cava respiratory variability predict fluid responsiveness in spontaneously breathing patients. Crit Care 19:400
Bennett A, Aya D, Cecconi M (2018) Evaluation of cardiac function using heart-lung interactions. Ann Transl Med 6(18):356
Bentzer P, Griesdale E, Boyd J, MacLean K, Sirounis D, Ayas T (2016) Will this hemodynamically unstable patient respond to a bolus of intravenous fluids. J Am Med Assoc 316:1298–1309
Blanco P, Aguiar FM, Blaivas M (2015) Rapid ultrasound in shock (RUSH) velocity– time integral: a proposal to expand the rush protocol. J Ultrasound Med 34(9):1691–1700
Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ (1992) Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of. Crit Care Med 101(6):1644–1655
Bortolotti P, Colling D, Colas V et al (2018) Respiratory changes of the inferior vena cava diameter predict fluid responsiveness in spontaneously breathing patients with cardiac arrhythmias. Ann Intensive Care 8(1):79
Boyd H, Sirounis D, Maizel J, Slama M (2016) Echocardiography as a guide for fluid management. J Crit Care 20(1):274
Caplan M, Durand A, Bortolotti P et al (2020) Measurement site of inferior vena cava diameter affects the accuracy with which fluid responsiveness can be predicted in spontaneously breathing patients: a post hoc analysis of two prospective cohorts. Ann Intensive Care 10:168
Ceruti S, Anselmi L, Minotti B, Franceschini D, Aguirre J, Borgeat A et al (2018) Prevention of arterial hypotension after spinal anaesthesia using vena cava ultrasound to guide fluid management. Br J Anaesth 120:101–108
Charron C, Caille V, Jardin F, Vieillard-Baron A (2006) Echocardiographic measurement of fluid responsiveness. Curr Opin Crit Care 12(3):249–254
Corl A, George R, Romanoff J, Levinson T, Chheng B, Merchant C, Levy M, Napoli M (2017) Inferior vena cava collapsibility detects fluid responsiveness among spontaneously breathing critically-ill patients. J Crit Care 41:130–137
Desai N, Garry D (2018) Assessing dynamic fluid-responsiveness using transthoracic echocardiography in intensive care. Br J Anaesth 18(7):218–226
Dykes LA, Heintz SJ, Heintz BH, Livorsi DJ, Egge JA, Lund BC (2019) Contrasting qSOFA and SIRS Criteria for Early Sepsis Identification in a Veteran Population. Fed Pract: Health Care Professionals VA DoD PHS 36(Suppl 2):S21–S24
Furtado S, Reis L (2019) Inferior vena cava evaluation in fluid therapy decision making in intensive care: practical implications. Avaliação da veia cava inferior na decisão de fluidoterapia em cuidados intensivos: implicações práticas. Rev Bras Ter Intensiva 31(2):240–247
Garg M, Sen J, Goyal S, Chaudhry D (2016) Comparative evaluation of central venous pressure and sonographic inferior vena cava variability in assessing fluid responsiveness in septic shock. Indian J Crit Care Med 20(12):708–713
Krzanowski W, Hand D (2009) ROC curves for continuous data. Boca Raton: Chapman and Hall/CRC Press
Machare-Delgado E, Decaro M, Marik P (2011) Inferior vena cava variation compared to pulse contour analysis as predictors of fluid responsiveness: a prospective cohort study. J Intensive Care Med 26(2):116–124
Marik P, Bellomo R (2016) A rational approach to fluid therapy in sepsis. Br J Anaesth 116(3):339–349
Messina A, Longhini F, Coppo C et al (2017) Use of the fluid challenge in critically ill adult patients: a systematic review. Anesth Analg 125:1532–1543
Monnet X, Marik P, Teboul J (2016) Prediction of fluid responsiveness: an update. Ann Intensive Care 6:111
Orde S, Slama M, Hilton A, Yastrebov K, McLean A (2017) Pearls and pitfalls in comprehensive critical care echocardiography. J Crit Care 21(1):279
Shalaby M, Roshdy H, Elmahdy W, Fathy A (2018) Correlation between central venous pressure and the diameter of Inferior Vena Cava by using ultrasonography for the assessment of the fluid status in intensive care unit patients. Egypt J Hosp Med 72(10):5375–5384
Silversides A, Major E, Ferguson J, Mann EE, McAuley F, Marshall C, Blackwood B, Fan E (2017) Conservative fluid management or deresuscitation for patients with sepsis or acute respiratory distress syndrome following the resuscitation phase of critical illness: a systematic review and meta-analysis. Intensive Care Med 43(2):155–170
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC (2016) The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). J Am Med Assoc (JAMA) 315(8):801–810
Via G, Tavazzi G, Price S (2016) Ten situations where inferior vena cava ultrasound may fail to accurately predict fluid responsiveness: a physiologically based point of view. Intensive Care Med 42(7):1164–1167
Acknowledgements
Not applicable.
Funding
None.
Author information
Authors and Affiliations
Contributions
AN designed the study, revised literature, performed the analysis, followed the patients, measured vital data, ultrasonographic and echocardiographic parameters; recorded the data; and wrote the manuscript. AM revised literature, performed the analysis, and critically reviewed the manuscript. AA revised literature, followed the patients, collected the data, performed the analysis, and critically reviewed the manuscript. WZ revised statistics, performed the analysis, and critically reviewed the manuscript. AF revised literature and the analysis and critically reviewed the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Approval of research ethical committee of Faculty of Medicine, Ain-Shams University was obtained (code number: FMASU M D 109 /2018) and written informed consent was obtained from the patients after description of the procedure and its potential complications.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nagi, A.I., Shafik, A.M., Fatah, A.M.A. et al. Inferior vena cava collapsibility index as a predictor of fluid responsiveness in sepsis-related acute circulatory failure. Ain-Shams J Anesthesiol 13, 75 (2021). https://doi.org/10.1186/s42077-021-00194-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42077-021-00194-y